Abstract
Aims: To assess the impact of the composition in L- and D- of lactic acid stereo copolymers without drug elution on the in situ behaviour of prototype stents in terms of biomechanics and biocompatibility.
Methods and results: PLA50, 75, and 92 stereo-copolymer stents (L/D lactic acid ratio from 1 to 11.5) were processed using the injection moulding facilities of Arterial Remodeling Technologies (Noisy le Roi, France). The resulting 3 mm outer diameter tubes having a diameter at the desired nominal size were laser-cut and crimped on regular angioplasty balloons and chemically sterilised prior to implantation in iliac rabbit arteries. Acute recoil was higher in PLA50 and PLA75 stent-treated arteries than in those with PLA92 stents (17.4±11.4 vs. 13.5±7.6 vs. 4.1±3.8 %, respectively, p=0.001). At one month, in-stent area was higher in PLA92 than in PLA50 and PLA75 stented arteries (5.9±0.6 vs. 1.6±1.6 vs. 2.6±3.2 mm2, respectively, p<0.001). Re-endothelialisation was complete, and inflammation was mild around the struts, similar among the three stents. Late lumen loss and neointimal area were low and similar in PLA92 stent-treated arteries one and six months after angioplasty (0.2±0.2 vs. 0.3±0.2 mm, p=0.60; 0.5±0.5 vs. 0.5±0.8 mm2, p=0.72, respectively). At six months, inflammation decreased compared to one-month follow-up (1.4±0.5 vs. 0.6±0.5, p=0.006).
Conclusions: A stereo-copolymer composition strongly influences biomechanical properties of PLA bioresorbable stents in agreement with what has been known for a long time from other applications, but not biocompatibility. PLA92 stents appeared as presenting acceptable acute deployment and 6-month favourable outcome in the rabbit model despite the absence of drugs.
Introduction
Bare metal stents (BMS) have been shown to prevent acute occlusion and restenosis efficiently after percutaneous coronary intervention (PCI)1. However, the ad vitam lifetime of metallic stents is not ideal with respect to the rather short healing time of an artery wall. Ideally, coronary artery healing is relevant to transitory mechanical scaffolding (i.e., 3 to 6 months) similar to many other time-limited applications (osteosynthesis, sustained drug delivery, etc.2). Therefore, a bioresorbable stent is now perceived a priori as an attractive alternative to a permanent stent3,4. The ideal stent should incorporate biomechanical properties of sufficient radial force to maintain patency, exhibit sufficient flexibility to match the vessel geometry without overstressing the arterial wall and disturbing the blood flow, cause minimal intimal hyperplasia, and resorb, i.e., generate degradation by-products that are eliminated from the body by natural pathways or bio-assimilated, once the scaffolding is no longer required. Elimination and/or assimilation are generally currently referred to as “bioresorption”5.
Bioresorbable aliphatic polyesters of the lactic acid-based aliphatic polymers family (generic abbreviation: PLA) are by far the most extensively studied bioresorbable polymers in the biomedical field because of their good biocompatibility and the possibility of modifying their mechanical properties and degradation profiles by their stereo composition6. Indeed, lactic acid is a chiral molecule that exists under two enantiomeric forms, i.e., D-lactic acid and L-lactic acid. The combination of the lactate constitutional repeating units derived from these enantiomers in different proportions leads to a very rich family of polymers (PLAX with X standing for the percentage in L-lactate units in the polymer backbone) that have different lifetimes and physical characteristics7. PLA100 is thus composed of 100% L-lactic acid, and is already in widespread clinical use with applications such as osteosynthesis implants that require rather high mechanical properties8. It is worth noting that because of the complexity of chemical, structural, and degradation characteristics of these aliphatic polyesters, there is no single PLAX but a number of them depending on many factors8. So far, only polymers of the PLA100 type have been considered for PCI. The Igaki-Tamai PLA100 coronary stent was the first fully bioresorbable stent to be implanted in humans ten years ago9. More recently, the everolimus-eluting PLA stent, i.e., BVS scaffold, has been in clinical assessment in humans in the pilot ABSORB trial10. This trial showed the feasibility of implantation of the BVS stent and favourable biocompatibility with a low rate of restenosis. However, acute recoil was 6.85±6.96%, slightly higher than comparable metal drug-eluting stents11. These two PLA100 stents have very similar bioresorption kinetics profiles with an estimated scaffold period over 12 months and a complete resorption of the stent estimated in the range of two years after implantation from imaging extrapolation12-14. Only suitable radiolabelling can prove total resorption15,16. Ideally, the required temporary scaffolding period can be reasonably assumed to be in the range of three to six months with the aims of avoiding acute recoil and promoting constrictive remodelling. Using the influence of the stereo composition is a recognised means of modifying and adjusting the lifetime of a PLA10017.
In this article, we report a detailed in vivo investigation comparing the behaviour of prototype stents made of three different stereo compositions in the rabbit model with the goal to assess the influence of stereo copolymerisation in terms of biomechanics and biocompatibility with respect to the specifications of the application, and in particular that recommendation to avoid the use of highly cytotoxic drugs.
Methods
Description of the stent
PLA50, PLA75, and PLA92 tubular stents were synthesised by ring-opening polymerisation of mixtures of L- and D-lactides with the following L/D composition: 50/50 (ratio 1.0), 75/25 (ratio 3.0), and 92/8 (ratio 11.5), respectively using zinc lactate as the initiator18. After careful purification and characterisation, polymers were injection moldered at suitable temperatures depending on the viscosity of the tubes. These tubes were laser-cut to create struts according to a Palmer design as shown in Figure 1. The stents (with the following dimensions: 200 µm thick and 15 mm long) were crimped on standard 3.0 mm diameter balloon catheters and were not blocked by a sheath. The initial molecular weight average (Mw) and polydispersity index (I= Mw/Mn) characteristics of the PLA50, PLA75 and PLA92 polymers were respectively Mw=198 kDa; I=1.7, Mw=192 kDa; I=1.7 and Mw=175 kDa; I=1.7 according to size exclusion chromatography (SEC).
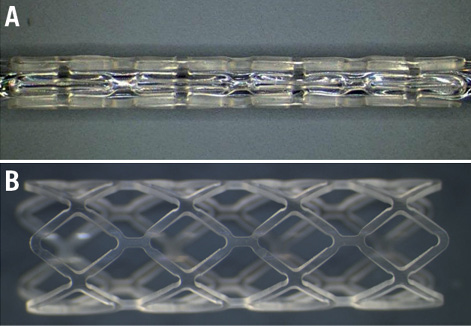
Figure 1. PLA stent. Illustration of PLA stent with a Palmaz-type design (200 µm thick and 15 mm long) processed from moulded tubes by laser cutting crimped on a balloon catheter (A), and after deployment (B).
Animal experiments and design of the study
Ten PLA50 stents, 10 PLA75 stents, and 20 PLA92 stents were randomly implanted in the iliac arteries of male New Zealand White rabbits (3.5-4 kg, n=20) in 2009. Thirty PLAX stents were harvested one month (n=10 for each PLAX), and 10 PLA92 stents six months after implantation. An additional group of four New Zealand White rabbits (3.5-4 kg) received four PLA92 stents and four paclitaxel-eluting stents (TAXUS®; Boston Scientific, Natick, MA, USA). These stents were investigated for scanning electron microscopy analysis one month after deployment.
Investigations conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institute of Health (NIH publication No. 85-23, revised 1996) and were approved by the local animal experiment committee.
The animals were anaesthetised by intramuscular injection of Xylazine (5 mg/kg) and ketamine (35 mg/kg). Arteriotomy of the right carotid artery was performed and a 6 Fr sheath was placed. Both 500 IU heparin and 80 mg aspirin were injected intra-arterially. A 6 Fr guiding catheter was positioned into the abdominal aorta. A baseline iliofemoral angiogram was performed after injection of nitrates. The PLAX stents were implanted in iliac arteries with a stent-to-artery ratio between 1.1 and 1.3. The balloon was inflated until full deployment of the stent at 3 atmospheres (ATM). Once deployed, the pressure was increased to 6 ATM. Angiography was repeated after stent expansion following intra injection of nitrates. The rabbits were treated daily by aspirin and clopidogrel administered in drinking water (25 mg and 10 mg daily, respectively) started 48 hours before surgery and maintained until the sacrifice. An example of PLA stent implantation is shown (Figure 2A-Figure 2C). Follow-up angiography was performed one and six months after stent implantation. The rabbits were sacrificed with an overdose of 50 mg IV sodium pentobarbital immediately before in vivo pressure-perfusion fixation. The iliac arteries were excised, and tissues were stored in 4% buffered formaldehyde for histomorphometry and immunohistology.
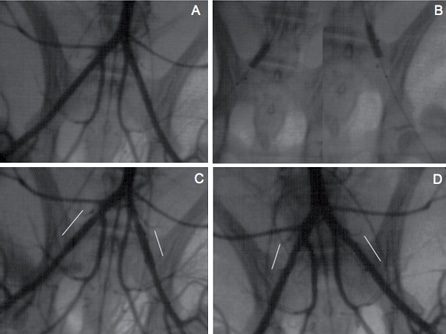
Figure 2. Angiographic analysis. Angiographic illustration of baseline iliac angiogram (A), bilateral stent deployment at 6 atm (B), immediate post-angioplasty iliac angiogram (C), and final iliac angiogram one month after stent deployment. Four radiopaque markers are present, 2 at the balloon extremities, and 2 at the PLA stent extremities (B). Immediately post stent deployment, minimal spasm is present proximal to the left stent without recoil in both stents (C). One month after stent deployment, stents are patent without thrombosis or restenosis (D). White lines indicate the arterial stented segments (C, D).
Angiography analysis
Minimal luminal diameter (MLD) was quantified before angioplasty, immediately after stent implantation, and before the sacrifice by a blinded physician by quantitative angiography. The MLD of the dilated segments was measured in the same projection before and immediately after stent deployment. Stent/artery ratio (stent diameter/MLD before angioplasty) and acute recoil (MLD post deployment/stent diameter) were also measured. Late lumen loss was evaluated as the difference between the MLDs immediately after angioplasty and before sacrifice. Percentage restenosis was defined as the ratio of the difference of MLD in the stented and reference segments, and the reference site. The reference site was defined by the arterial section upstream to the stented segment.
Histomorphometry analysis
Each stent was cut at –20°C in 8 µm cross-sections, and stained with haematoxylin-eosin and orcein to evaluate in-stent area, in-stent luminal area, and in-stent neointimal area. Sections were taken at the proximal, middle, and distal parts of the stents, and the final results were obtained by averaging the three measurements in each stented artery. In-stent areas were quantified using IPS 4.02 software (Tribvn, Chatillon, France), as previously described in non-occluded vessels19. Injury score was also evaluated, as previously described20. A stent malapposition score was defined as follows: 0: complete stent apposition; 1: incomplete stent apposition; 2: semi-circular stent expansion with limited contact between the stent and the arterial wall; 3: complete stent shrinkage into the lumen without contact between the stent and the arterial wall. Neointimal area was not measured in stents presenting complete stent shrinkage into the lumen without contact between the stent and the arterial wall (score 3).
Immunohistochemical analysis
Cell proliferation and macrophages were respectively detected by a monoclonal rabbit antibody against human Ki-67 proliferation antigen (dilution of 1/50; Dako, Trappes, France), and a monoclonal mouse anti-rabbit macrophage (RAM 11, dilution 1/50; Dako), as previously described19. Endothelial cells were identified by a mouse monoclonal antibody against CD-31 (dilution 1/10; Dako), as previously described19. A negative control (sample without primary antibody incubation) was included for each assay. Quantification of inflammation was performed using a semi-quantitative score: 0: absence of inflammatory cells; 1: scattered monocyte-macrophage infiltrates associated with struts; 2: notable monocyte-macrophage infiltrates associated with struts; and 3: monocyte-macrophages circumscribing the struts. Quantification of cell proliferation was performed using a semi-quantitative score: 0: absence of Ki-67 positive cells; 1: scattered Ki-67 positive cell infiltrates associated with struts; 2: notable Ki-67 positive cells associated with struts; and 3: Ki-67 positive cells circumscribing the struts. Finally, re-endothelialisation was evaluated using the endothelialisation score: 0: absence of endothelial cells; 1: less than 25%; 2: 25-75%; 3: greater than 75%; and 4: 100%. All these parameters were analysed by a blinded observer at the proximal, middle, and distal part of the stents, and the final results were obtained by averaging the three measurements in each stented artery.
Scanning electron microscopy
Four PLA92 and four TAXUS® stents were harvested one month after implantation for scanning electron microscopy analysis (SEM), as previously described21. For evaluation of endothelial coverage, the stented arteries were fixed in situ with 10% neutral buffered formalin after perfusion with Ringer’s lactate solution and further fixed by glutaraldehyde immersion and then dissected longitudinally for SEM analysis.
Statistical analysis
Data are expressed as mean±SD. Comparison between the three PLA stents was made by non-parametric ANOVA (Kruskal-Wallis test). Comparison between PLA92 stents harvested at one and six months was made by a Mann-Whitney test. Values were considered statistically different when p<0.05.
Results
Impact of the stereo composition of PLAX stents on biomechanics and biocompatibility
A typical stent is displayed in Figure 1 and the angiography procedure in Figure 2. Acute recoil was low in arteries treated by PLA92 stents, similar to metallic stents (i.e., TAXUS® stents, data not shown), and significantly higher than in arteries treated by PLA50 and PLA75 stents (4.1±3.8% vs. 17.4±11.4% vs. 13.5±7.6%, p=0.001, Figure 3D). Stent/artery ratio was not statistically different among the three groups (Table 1).
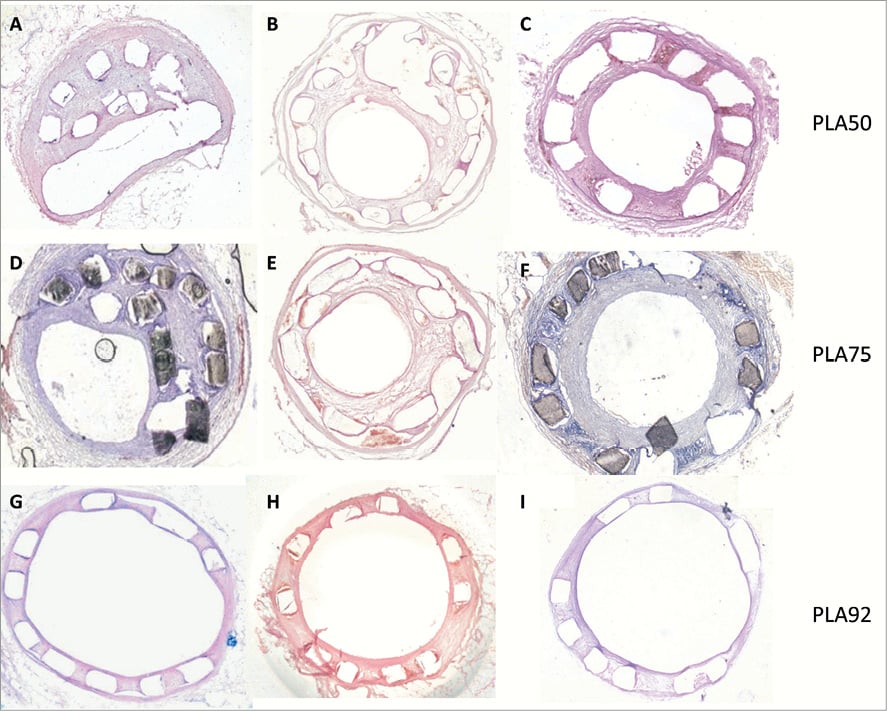
Figure 3. Biomechanical and biocompatibility analysis. (A-I) Histologic representation of PLA stents implanted into iliac rabbit arteries. (A-C) Iliac rabbit artery implanted with a PLA50 stent harvested one month after angioplasty. (D-F) Iliac rabbit artery implanted with a PLA75 stent harvested one month after angioplasty. Note stent shrinkage into the lumen without thrombosis (A, D) with a stent malapposition score of 2. (G-I) Iliac rabbit artery implanted with a PLA92 stent harvested one month after angioplasty. Note complete stent apposition with minimal neointimal hyperplasia and no restenosis. Arterial sections were stained with haematoxylin-eosin, magnification ×5 (A-I).
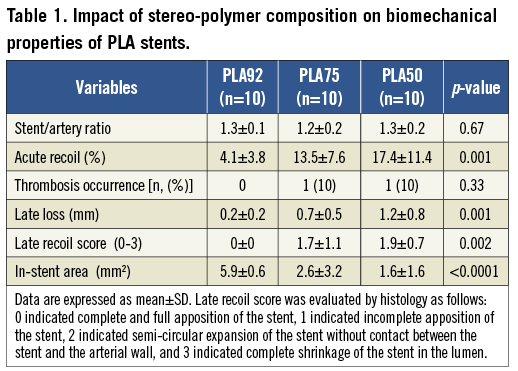
One month after deployment, late recoil frequently occurred in arteries treated with PLA50 and PLA75 stents but not in those implanted with PLA92 (Figure 3A- Figure 3I, Table 1). Restenosis at one month was significantly higher in arteries treated by PLA50 and PLA75 stents than in those with PLA92 stents (late lumen loss: 1.2±0.8 mm vs. 0.7±0.5 mm vs. 0.2±0.2 mm, p=0.001; restenosis: 36.4±16.6% vs. 23.0±21.3% vs. 4.0±3.6%, p=0.001).
Inflammation and cell proliferation were detected at a low level and predominantly around the struts without any significant difference among the three PLA stereo configurations (Table 2). Neointimal hyperplasia was very mild without any significant difference among the three PLA stereo configurations (Table 2).
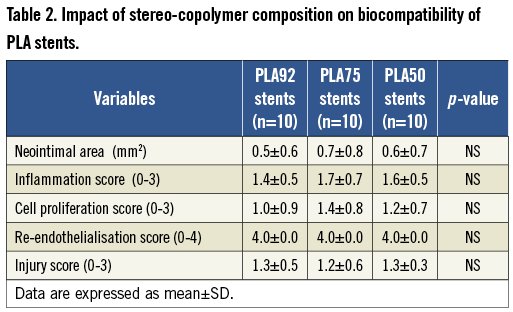
Stent re-endothelialisation was almost complete one month after stent implantation regardless of the stereo-copolymer (re-endothelialisation score: 4.0 for the 3 PLA stents; Figure 4A and Figure 4B, Table 2). Stent thrombosis did not occur with PLA92 stents, and was kept low with PLA50 and PLA75 stents despite unfavourable recoil (Table 1). Re-endothelialisation between PLA92 and TAXUS® stents by SEM analysis one month after implantation confirmed that endothelial coverage was near complete in all PLA92 stents and poor in TAXUS® stents (Figure 4C- Figure 4H).
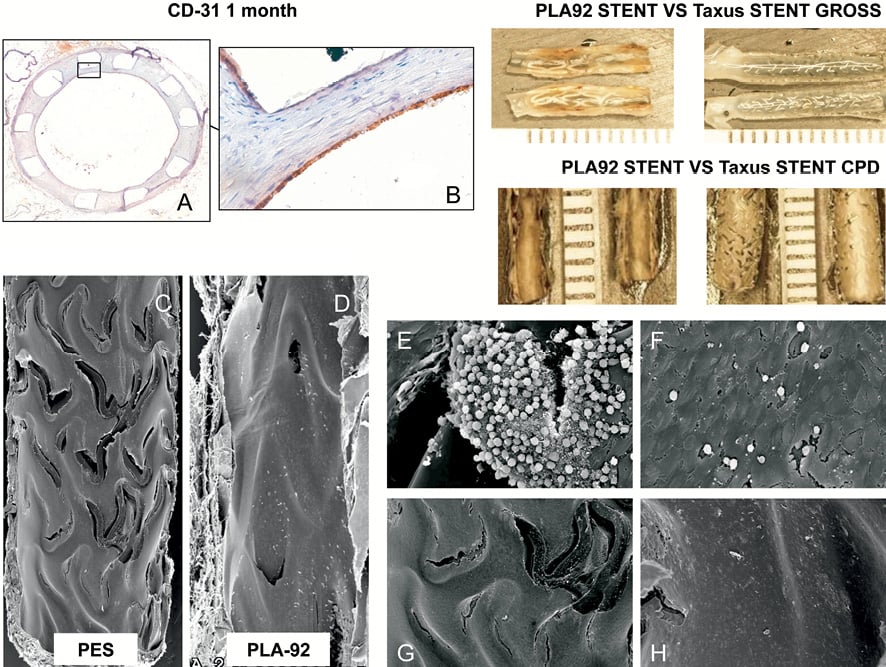
Figure 4. Re-endothelialisation analysis. (A-B) Immunostainings with an antibody against CD-31 (brown staining) evaluating re-endothelialisation of the PLA92 stent one month after stent implantation. Counterstaining with haematoxylin, magnification ×5 (A) and ×40 (B). (C-H) Scanning electron micrographs of TAXUS stent (PES) and PLA92 stent one month after implantation. The upper panels show radiographic images of each stent. The lumens are patent and struts are less discernible. Overall endothelial coverage is near complete in all PLA92 stents whereas it remains poor around PES struts (C, D). The panel insets are at higher magnification (×600) from the proximal and distal regions and show persistent uncovered struts, surface thrombi, inflammatory cells, and endothelial cells in PES (E, G) as compared to PLA92 stents (F, H).
Evaluation of PLA92 stents in the rabbit model: ONE and SIX month follow-ups (Figure 5, Table 3)
All the PLA92 stents were well deployed without late recoil at one and six months. Late lumen loss and restenosis were similar at one and six months with a persistent low rate of 6-month restenosis. This was confirmed by histomorphometry (Figure 5A-Figure 5F): neointimal area was low and did not increase from one to six months (Figure 5A-Figure 5F and Figure 5L). Despite the ongoing degradation of the stents, inflammation score remained low, and was even lower at 6 months as compared with arteries harvested at one month (0.6±0.5 vs. 1.4±0.5, p=0.006, Figure 5I-Figure 5K). Re-endothelialisation remained complete at six months, and we did not observe significant variation of cell proliferation at six months (Figure 5G, Figue 5H, and Figure 5K).
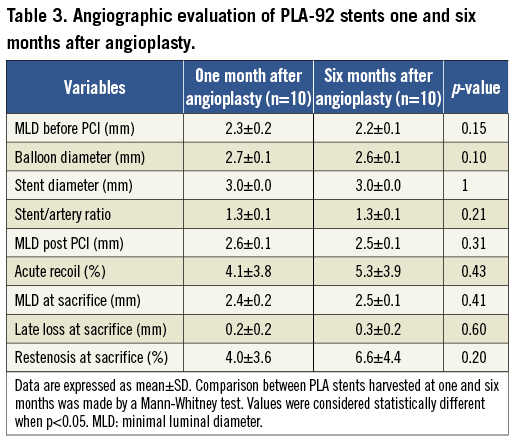
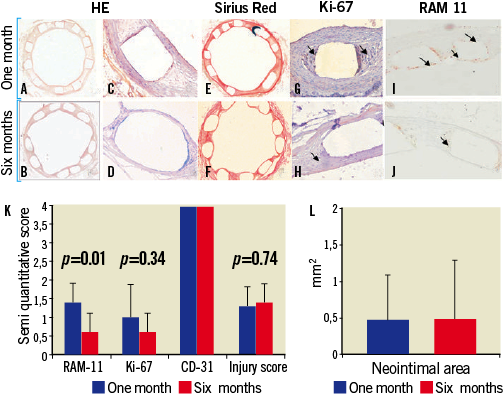
Figure 5. (A-J) Histologic representation of PLA92 stents implanted into rabbit iliac arteries harvested one (A, C, E, G, I) and six (B, D, F, H, J) months after angioplasty. Sections were stained with haematoxylin-eosin (A-D), sirius red (E, F), and antibodies against Ki-67 (G, H) and RAM-11 (I, J), in order to detect, respectively, cell proliferation and inflammatory cells. Slides were counterstained with haematoxylin (G, H). These data illustrate complete stent apposition with minimal concentric neointimal hyperplasia without thrombosis at one and six months, complete stent re-endothelialisation as soon as one month after stent deployment and minimal scattered inflammation and cell proliferation predominantly detected around the struts of PLA92 stents. Inflammation and cell proliferation were minimal and further decreased six months after stent deployment despite polymer bioresorption.(K) Bar graph representation of semi-quantitative evaluation of inflammation (RAM-11), cell proliferation (Ki-67), re-endothelialisation (CD-31), and injury score in arteries treated with PLA92 stents harvested one (blue) and six (red) months after angioplasty. Results are presented as mean±SD. Comparisons were made by a Mann-Whitney test. (I) Bar graph representation of neointimal area in arteries treated with PLA92 stents harvested one (blue) and six (red) months after angioplasty. Results are presented as mean±SD. Comparisons were made by a Mann-Whitney test.
Discussion
According to previous data, the chirality of the PLAX used to build the stents strongly influenced acute and late stent recoils but not biocompatibility. PLA degradation during the investigated period of time did not result in inflammation, cell proliferation, and restenosis despite the lack of antiproliferative cytotoxic drugs.
Biomechanical features of PLA stents
With regard to mechanical weakness, the major finding was that acute and late recoils of PLA stents depended on the stereo-copolymer composition. Previous in vivo preclinical and clinical studies demonstrated that acute recoil was significantly increased in arteries treated by the BVS PLA100 stent as compared with metallic DES11. Moreover, late recoil was also observed in humans by comparing in-stent area using IVUS immediately after implantation and at six months follow-up22. PLA50 and PLA75 stents did not offer adequate scaffolding with a high incidence of acute and late stent recoils. By contrast, PLA92 stents had more favourable biomechanical properties since stent thrombosis was absent and acute and late stent recoils were minimal. Acute and late recoils can be assigned to low initial radial force, stent resorption, and/or weakening due to water absorption that plasticises the polymer23. Indeed, it is well known that stereo copolymers of PLA with an L-lactic acid composition between 50 and 92% (PLA50 to PLA92) give amorphous materials which absorb more water than partial crystalline materials made of PLA stereo copolymer with higher L-lactic acid composition (PLA92 to PLA100). On the other hand, the present PLAX stereo copolymers were synthesised using zinc lactate as the initiator instead of stannous octoate, a choice that is known to favour early water uptake because of greater hydrophilicity18,24. Factors other than stereo composition and water uptake may play a role in accounting for insufficient mechanical properties of PLA stents. Stent recoil may be related to the occurrence of stent micro fractures during crimping and/or stent deployment24. Moreover, a too fast or a too important decrease of the initial molecular weight of PLA macromolecules during thermal processing and in vivo as well, may also affect mechanical behaviour. However, these factors have been kept under strict control and are thus not thought to be influential in this study.
Biocompatibility of PLA stents
The three types of degradable stents led to tissue reactions that allowed us to conclude to acceptable biocompatibility and limited tissue proliferation in the absence of any complementary local drug release. In contrast, marked inflammatory response with a thick layer of fibro-cellular component was reported after implantation of degradable polymer drug-eluting stents in porcine coronary arteries25. Recently, the fate of PLA drug-eluting stents was studied in both a swine model and in human. For the pig model, Vogt et al reported that degradable paclitaxel-eluting coronary PLA100 stents significantly reduced in-stent restenosis as compared to uncoated PLA stents and BMS. However, they found marked local inflammatory response26. In human, BVS stent implantation was associated with acceptable restenosis in the ABSORB trial10. The major difference with the BVS stent is that the PLA92 stent of the present study had no additional drug therapy and obtained a favourable response at 1 and 6 months without inflammatory reaction, although degradation was already well advanced as previously shown from molecular weight determinations24.
Degradation of PLA stents
Degradation is another critical issue for bioresorbable stents since it may increase inflammation, especially when degradation by-products soluble in surrounding body fluids start diffusing out of the device, as is well documented in the literature. Although it is very slow, such release may contribute to neointimal hyperplasia and late restenosis too, a possibility that is difficult to assess specifically3. Detailed discussion of the complexity of the degradation of lactic acid-based polymers can be found in the literature4.
Previous studies on PLA50 and PLA92 prototype stents had very good in vitro and in vivo correlation concerning degradation and bioresorption3,18,24. The degradations of the present more complex PLA50 and PLA75 stents were also comparable with 70% of molecular weight reduction at six months. PLA92 stents had a slower time course of molecular weight reduction in agreement with the greater X17,24. In the present study, the PLA92 degradation occurring between 1 and 6 months after stent implantation was not associated with inflammation or cell proliferation since the inflammation and cell proliferation scores decreased from one to six months and remained very low. Consequently, neointimal areas were similar at one and six months. This is a very important finding since it has occasionally been reported that degradation of bioresorbable polymer stents was associated with high rates of inflammation25,26. In contrast, Onuma Y et al reported a BVS PLA stent study in the porcine model without significant inflammatory response at two to four years, although inflammatory response might have been hidden by everolimus. Data on the BVS platform without drug elution are not available13.
In conclusion, this work confirms that the lifetime of a Palmaz-Schatz type bioresorbable stent can be varied using the chirality-related configurational structures as already shown for applications in orthopaedics and in many other therapeutic applications relevant to temporary therapeutic aids. It also shows that acute and late recoils depended significantly on the enantiomeric composition of the selected stereo copolymers whereas biocompatibility did not. Among the three tested types of stents, PLA92 appeared to be the most favourable one to proceed further towards the tailor-making of a drug-free bioresorbable stent, a critical step towards evaluation in human.
Acknowledgements
We thank F. Kolodgie for his precious help in histology, and J. Piquet for her assistance with animal care.
Conflict of interest statement
A. Lafont and M. Vert are co-founders of Arterial Remodeling Technologies (ART). The other authors have no conflicts of interest to declare.

