Abstract
Aims: The Genous™ endothelial progenitor cell (EPC) capture stent is a bioengineered R stent coated with immobilised antibodies on its stent struts to allow for the capture of circulating EPCs to promote rapid endothelisation. We assessed the impact of this stent in the primary percutaneous coronary intervention (PCI) of patients with acute ST-elevation myocardial infarction (STEMI) and examined its long term clinical outcomes.
Methods and results: All patients with acute STEMI without cardiogenic shock who underwent primary PCI between January 2005 and April 2007 and received the stent were enrolled in the study. The study endpoints were major adverse cardiac events (MACE) defined as death, MI and target vessel revascularisation (TVR) at 30 days, six months and one year. A total of 321 enrolled patients received 357 EPC capture stents during the study period. The cohort comprises 81.0% males with mean age of 54.6±11.6 years. The mean stent length used was 20.98±5.50 mm and mean stent size was 2.99±0.32mm. Ninety-four percent of patients achieved Thrombolysis in Myocardial Infarction (TIMI) 3 flow post-procedurally. The cumulative MACE rate was 8.1% at 30 days, 10.0% at six months and 12.2% at one year. There was one patient who developed acute stent thrombosis and another two with subacute stent thromboses. No late thrombosis or late cardiac mortality was observed in our cohort. The need for TVR was 4.4% at one year.
Conclusions: The use of EPC capture stents in patients who underwent primary PCI for STEMI is safe and showed good clinical outcomes, with low rates of TVR and no late stent thrombosis.
Abbreviations list
ACT: activated clotting time
DES: drug-eluting stents
EPC: endothelial progenitor cell
GP: glycoprotein
LDL: low-density lipoprotein
MACE: major adverse cardiac events
NSTEMI: non-ST elevation myocardial infarction
PCI: percutaneous coronary intervention
STEMI: ST-elevation myocardial infarction
TIMI: Thrombolysis in Myocardial Infarction
TLR: target lesion revascularisation
TMP: Thrombolysis in Myocardial Perfusion
TVR: target vessel revascularisation
Introduction
Several studies have elucidated the role of circulating endothelial progenitor cells (EPC) in the healing process after vascular endothelial injury in patients with ST-elevation myocardial infarction (STEMI)1. The use of EPC cell capture stent (Genous™ stent, OrbusNeich, Fort Lauderdale, FL, USA), a bioengineered R stent with immobilised antibodies on the stent struts against the CD34 antigen on the EPCs, is an attractive option in this thrombogenic setting. The ability of the antibodies to capture and retain circulating EPC cells may accelerate the process of endothelisation and hence the healing process, leading to improved clinical outcomes.
The objective of this study is to evaluate the long-term outcomes of the use of EPC capture stents in primary percutaneous coronary intervention (PCI) during acute STEMI.
Methods
This is a prospective, observational study initiated and performed in our centre. A total of 321 patients who had acute STEMI without cardiogenic shock, and received EPC capture stent implantation during primary PCI were enrolled in this study. All patients received clopidogrel loading doses of 600 mg and 300 mg of aspirin prior to the procedure. Unfractionated heparin of 50-100 IU/kg body weight was given at the start of the procedure depending on the concomitant administration of platelet glycoprotein (GP) IIb/IIIa receptor inhibitors. The activated clotting time (ACT) was kept between 200-250 seconds and above 360 seconds in patients who did and did not receive GP IIb/IIIa inhibitors respectively. The PCI procedure was carried out in the standard fashion with thrombectomy device or balloon predilatation employed according to the discretion of the operators. Patients were thereafter maintained on 100 mg of aspirin indefinitely unless otherwise contraindicated, and 75 mg of clopidogrel daily for a month. Immediate statin therapy in the form of simvastatin 20 mg was initiated soon after the PCI procedure. This was subsequently titrated according to the patient’s low-density lipoprotein (LDL) cholesterol level. Patients who had any contraindication to dual antiplatelet therapy were excluded from the study.
Data collection
Clinical and demographic data were collected and included age, gender, smoking history, family history of premature coronary artery disease and comorbidities such as diabetes mellitus, hypertension and dyslipidaemia. Adjunct therapies were also taken into account and included the use of thrombolytic therapy, glycoprotein IIb/IIIa antagonist agents, and thrombosuction or thrombectomy devices. Lesion characteristics with regard to lesion location, ACC/AHA lesion morphology classification, vessel size, lesion length, Thrombolysis in Myocardial Infarction (TIMI) flow score and Thrombolysis in Myocardial Perfusion (TMP) score were recorded. Off-line quantitative coronary analysis was done by one of the investigators and reviewed by a second investigator. Stent thrombosis was defined according to the Academic Research Consortium (ARC) classification in which definite stent thrombosis is defined as angiographic and pathologic confirmation of acute thrombosis in patients with acute coronary syndromes; and probable stent thrombosis as any unexplained death within 30 days or as target vessel MI without angiographic confirmation of thrombosis or other identified culprit lesion.
Safety profiles with regard to no-reflow, acute, subacute and late stent thrombosis were recorded. Patients were monitored for in-hospital events. The 1-month, 6-month and 12-month follow-up were carried out either by phone enquiry or clinic visits. Major adverse cardiac events (MACE) of death, myocardial infarction and target vessel revascularisation were accounted for and verified by hospital and census records.
Statistical analysis
Data were analysed using the SPSS version 13.0 statistical software. Discrete data was presented as frequencies and percentage while continuous variables were presented as means and standard deviations.
Results
A total of 357 EPC capture stents were implanted in 321 patients in our cohort. The mean age is 55±12 years and 260 (81%) are males. The demographic characteristics of the study population are listed in Table 1.
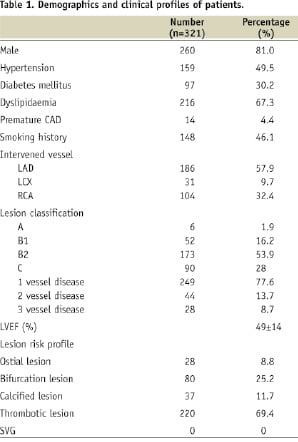
Diabetes mellitus was present in 30%. Platelet GP IIb/IIIa inhibitor was used in 13% of patients and 58% received adjunctive thrombectomy treatment (55% with a thrombus aspiration catheter and 2.5% X-SIZER device) prior to stent implantation (Table 2).
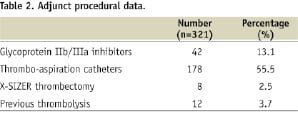
The lesion characteristics of the study population are defined in Table 3.
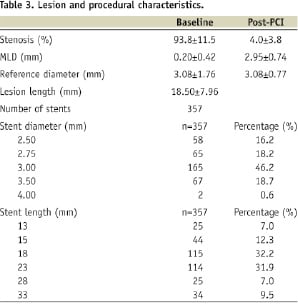
Only the culprit lesion was treated during the primary intervention. The left anterior descending artery is the treated artery in 58% of patients with ACC/AHA type B lesions accounting for 70% of the cases. The mean lesion length was 20.98±5.50 mm and mean reference vessel diameter was 2.99±0.32 mm. The median diameter of stent implanted was 3.0 mm, with 94% of the patients achieving TIMI 3 flow at the end of the procedure (Tables 4 & 5).
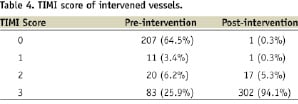

The pre-discharge left ventricular ejection fraction on two-dimensional echocardiogram was 49±14%. Follow-up was achieved in all of the patients in this cohort.
During hospitalisation, one patient had acute closure due to an untreated proximal stent edge dissection which accounted for the one in-hospital myocardial infarction and repeat revascularisation. He survived the recurrent MI. There was one case of in-hospital mortality from an acute stroke.
At one month, there were three patients with stent thromboses. Two patients had subacute stent thromboses, one of which occurred two days after the index procedure, the other at day seven, with resultant myocardial infarction and subsequent target lesion revascularisation. The patient who had subacute stent thrombosis at day seven died during hospitalisation. In addition to these three patients, there was one patient with probable stent thrombosis according to ARC classification. He presented with chest pain and demised in the emergency department at day 13 after index procedure. There were 23 patients (7.2%) who died at one month, 19 from cardiac causes and four from non-cardiac causes (Tables 6 & 7).
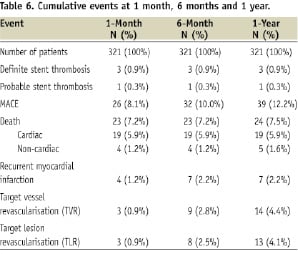
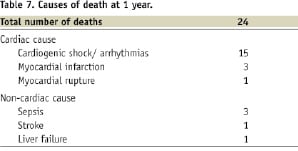
Of the 19 patients who died from cardiac causes in the first month, 15 had cardiogenic shock with progressive heart failure culminating in asystolic or arrhythmic arrest, three had recurrent myocardial infarctions and one died from ventricular septal rupture.
At six months, the MACE rate was 10.0% compared with 8.1% at one month. This was driven mainly by recurrent MIs and TVR, but not cardiac deaths. Six patients had target lesion revascularisation. One patient whose angiogram showed in-stent restenosis was referred for coronary artery bypass surgery (CABG) in view of multivessel coronary artery disease.
At 12-month follow-up, five additional patients underwent TVR (4 PCI and 1 CABG). There was one non-cardiac cause of death due to acute liver failure from hepatitis.
Discussion
This study represents the largest cohort of patients to date on the use of EPC capture stent in patients with STEMI undergoing PPCI. The implantation of this novel stent has proven to be safe and efficacious with low rates of MACE of 8.1% at 30 days, 10.0% at six months and 12.2% at one year. Early definite stent thrombosis occurred in three patients: one acute and two subacute cases. There was no late thrombosis and no late cardiac mortality (>30 days) observed in our cohort. The need for TVR was low at 4.4% at one year.
Primary PCI in acute myocardial infarction is the preferred therapy to fibrinolytic therapy in patients with STEMI2. However, concerns with regard to the thrombogenic milieu predisposing to increased risk of stent thrombosis with the implantation of a metallic implant remain3,4. While drug-eluting stents (DES) have been shown to be effective in reducing rates of angiographic restenosis and target vessel revascularisation, its use in the setting of AMI remains uncertain with serious concerns regarding its safety given the delayed impaired endothelisation process observed in pathological specimens5. Indeed, the presence of thrombus is a predictor of stent thrombosis in patients with received DES4.
Recent research suggests that EPCs mobilised from bone marrow into peripheral blood contribute to endothelial cell regeneration and postnatal neo-revascularisation6. PCI is known to stimulate mobilisation of EPC from the bone marrow7. During AMI, EPC is released in large quantities from the bone marrow acutely, occurring within the first few hours of the event to peak at day seven1. The functional role of these EPCs are unknown but are thought to be able to participate in neovascularisation. Other factors that affect circulating EPC counts include age of patient, cardiovascular risk factors and prior administration of statins8,9.
Endothelial progenitor cell capture Genous™ stent is a stainless steel bioactive stent coated with murine monoclonal anti-human CD34 antibodies. CD34 antigen is targeted because it is a haematopoietic stem/progenitor cell marker which is expressed on the surface of EPCs. The EPC capture stent is designed to attract circulating CD34 EPCs onto its stent surface, which leads to differentiation of these captured cells into a functional endothelial lining over the stent, and thus rapidly stabilising the repair process and promoting healing10,11. Re-establishment of a functional endothelial cell layer restores the cellular vascular integrity and homoeostasis. The recovery of vascular function prevents platelet aggregation and in-stent thrombus formation. Smooth muscle cell migration and proliferation are inhibited, preventing neointimal hyperplasia. Animal models showed complete coverage of the Genous stent by a functional monolayer after seven to 14 days, whereas DES showed eminent delayed vascular healing5,12. The concept of increased circulating EPCs attracted to the surface of the broken atherosclerotic plaque to promote healing forms the basis of this study.
This first-in-man (FIM) study of the EPC capture stent demonstrated the safety and feasibility of this device for the treatment of de novo coronary artery disease13. We reported a 6-month MACE event rate of 5.8% in our first 120 patients and noted that the rate of TVR was low at 2.5%14, which compares favourably with published series on the use of DES or BMS in AMI15,16. Our current study expanded the cohort number with longer term follow-up and continued to demonstrate the safety and efficacy of this stent in this setting. The need for target lesion revascularisation (TLR) was low at 2.5% at six months and 4.1% at one year in our study. This has a lower rate of TLR compared with the study by Miglionico et al, which showed that the use of EPC capture stents in 80 high-risk patients with acute coronary syndrome had a TLR rate of 13% and no incidence of stent thrombosis at one year17.
Our study results of MACE rates of 10.0% and 12.2% at six months and 12 months respectively were indeed encouraging, given that this is a high-risk subset of patients. The MACE was driven by recurrent MIs, mostly due to in-stent restenoses; and TVRs. The definite stent thrombosis rate of 0.9% at one year is comparable with that of the e-HEALING registry. More importantly, there was no incidence of late stent thrombosis despite the use of dual antiplatelet therapy for only a month. Interim analysis of 12-month follow-up data of 3,200 ‘real-world’ patients in the registry showed an overall MACE rate of 8.5% and target lesion revascularisation of 5%, subacute stent thrombosis of 0.4% and late stent thrombosis of 0.3%18. It suggests that the use of EPC capture stent in high risk patients with STEMI can achieve similar high safety and efficacy results.
Patients who were treated with EPC capture stents in our study were prescribed an immediate dose of simvastatin 20 mg upon completion of the procedure. This might be subsequently titrated to reach the appropriate LDL-cholesterol target. It is based on the subset analysis of the small published HEALING II registry study which showed that patients with statins had augmented EPC count with resultant better clinical and angiographic outcomes10. Statins have been shown to promote the survival, migration and differentiation of adult bone marrow derived EPC and enhance EPC recruitment to sites of neovascularisation9. In our patient cohort, 39.6% had further increase of simvastatin dose at discharge to achieve the target LDL-cholesterol level of 2.6 mmol/L.
Our study is however limited by the fact that it is a single centre study which includes only clinical follow-up and lacks angiographic information to evaluate the late loss of this novel stent.
Conclusion
In conclusion, the use of EPC capture stent in the setting of STEMI is feasible and safe with no incidence of late stent thrombosis and an acceptable rate of repeat revascularisation. It sets the stage for potential wider use of this bioactive stent in patients with AMI for enhanced safety and efficacy. A head-to-head randomised comparative study with either a BMS or DES will be imperative in fully evaluating its relative efficacy.
Declaration of Helsinki
The study complies with the Declaration of Helsinki. The ethics committee of National Health Care Group Domain Specific Review Board (DSRB) C has approved the research protocol. Informed consent was obtained from the subjects and/or their guardians for the study.
Definition of terms
Acute stent thrombosis is the occurrence of stent thrombosis within 24 hours after stent implantation.
In-stent is defined as the segment of the vessel covered by the stent.
In-segment is defined as the segment of the vessel covered by the stent and includes 5 mm before and after the stented segment.
Late stent thrombosis is defined as occurrence of stent thrombosis at least 30 days after stent implantation.
Major adverse cardiac event is defined as cardiac death, non-fatal myocardial infarction and clinically-driven target lesion revascularisation.
Minimal luminal diameter is defined to be the smallest diameter determined by quantitative coronary analysis.
Myocardial infarction is defined as elevation of cardiac enzymes three times the upper limit of normal.
Procedural success is defined as achieving residual stenosis of less than 10% with restoration of TIMI 3 flow upon termination of the procedure.
Reference vessel diameter (RVD) is defined as the average diameter of the vessel determined by the average of the diameter of the vessel proximal and distal to the lesion.
Subacute stent thrombosis is defined as the occurrence of stent thrombosis more than 24 hours but less than 30 days after stent implantation.
Target lesion is defined as the index lesion treated.
Target vessel is defined to be the vessel in which the treated lesion is located.

