Abstract
Aims: The MGuard stent (bare metal stent wrapped externally with a polymer mesh sleeve) is designed to prevent distal embolisation by reducing thrombus and plaque fragments released during and post percutaneous coronary intervention (PCI). The aim of this study was to confirm the clinical feasibility, safety and performance of the MGuard stent during primary PCI for ST-segment elevation myocardial infarction (STEMI).
Methods and results: The present study was a multicentre, prospective, single arm study in which 60 patients with STEMI <12 hours were enrolled. Predilatation was performed in 61.7% of the cases and thrombus aspiration in 18.3%. In one (1.7%) patient the stent could not cross the lesion. Final TIMI grade 3 flow was observed in 90.0% of patients, with myocardial blush grade 3 in 73.3% of patients and complete (>70%) ST-segment resolution 60 minutes after PCI in 61.4% of patients. In 5.0% of cases distal embolisation occurred. The total major adverse cardiac events rate during the 6-month follow-up was 1.7%.
Conclusions: Based on this experience, the MGuard stent implantation in STEMI patients is safe and highly effective. A larger randomised trial is warranted to confirm the clinical endpoints.
Background
Primary percutaneous coronary intervention (PCI) is the recommended way of reperfusion in ST-segment elevation myocardial infarction (STEMI) when logistically feasible.1 This method is highly effective in the restoration of infarct-related artery patency. However, presence of normal coronary flow (Thrombolysis in Myocardial Infarction [TIMI] grade 3 flow) in the infarct-related artery is not equal to the restoration of myocardial perfusion through cardiac microcirculation.2 After conventional primary PCI with stent implantation, even with glycoprotein (GP) IIb/IIIa inhibitor, in two thirds of patients impaired, myocardial perfusion expressed by angiographic (myocardial blush grade [MBG]) and electrocardiographic parameters (ST-segment resolution) is observed.2,3 These patients are at higher risk of larger irreversible myocardial injury, higher incidence of adverse remodelling of the left ventricle leading to consequent heart failure, as well as at higher risk of death during short- and long-term follow-up.3,4
Impaired myocardial reperfusion may be caused by distal embolisation of thrombus or plaque debris.4,5 Importantly, the risk of distal embolisation during primary PCI may be reduced by the use of thrombectomy and distal protection devices, especially in the setting of high-thrombus burden.6,7 Another possible option is to use the specially designed MGuard stent (MGuard™ Coronary Stent System; Inspire-MD Ltd., Tel-Aviv, Israel).8-11 This stent is covered by a micron level mesh. The mesh is intended to prevent distal embolisation by blocking the atherothrombi extrusion through the stent struts during its implantation To date, data concerning the MGuard stent implantation in the STEMI setting are still limited.10,11
The purpose of the present study was to confirm the clinical feasibility, safety and performance of the MGuard stent during primary PCI for STEMI.
Methods
Study population
The MGuard™ coronary stent system in ST elevation myocardial infarction – a European post-market clinical study (MAGICAL) study was a multicentre, prospective, single arm study. Between July 2008 and May 2009, a total of 60 patients meeting the following clinical inclusion criteria were included into the study: age >18 years; presence of clinical symptoms consistent with acute myocardial infarction (e.g., chest pain, arm pain, etc.); unresponsive to nitroglycerine >30 minutes duration and ≤12 hours before signing informed consent; presence of ≥2 mm of ST-segment elevation in two or more contiguous electrocardiographic leads; primary PCI was indicated for revascularisation based on coronary anatomy; vessel diameter of the target lesion was ≥2.5 mm and ≤4.0 mm in diameter by visual estimation at the proposed site of stent deployment; the target lesion was located within the native coronary artery and was >50% stenosed; treatment of only one native coronary artery lesion with only one MGuard stent was needed in a previously unstented coronary tree; distal vascular bed after wiring lesion was adequately visualised and allowed to assess lesion length, and vessel size; and, the infarct-related artery was without excessive tortuosity, diffuse disease or severe lesion/vessel calcification. Women with known pregnancy were excluded from the study. Also, patients with contraindications to antiplatelet and/or antithrombotic treatment (i.e., active bleeding; major surgery planed within six weeks of the procedure; aspirin, ticlopidine and clopidogrel, or heparin allergy or sensitivity; past history or present lab evidence of current neutropenia [<1000 neutrophils/mm3] or thrombocytopenia [<100,000 platelets/mm3]; history of stroke or transient ischaemic neurological attack within the past 60 days; history of bleeding diatheses, peptic ulceration or other illness, which limits the use of antiplatelet or antithrombotic therapy), as well as stent or mesh material allergy or sensitivity (polyethylene theraphthalate and stainless steel), allergy to contrast media, allergy to anaesthetics; past history or present lab evidence of current hepatic dysfunction (liver function tests >3x upper limits of lab normal), or a history of serum creatinine >2.5 mg/dl; with known history of chronic renal disease or haemodialysis; undergoing cardiopulmonary resuscitation; in cardiogenic shock (systolic blood pressure <80 mmHg for >30 minutes, or requiring IV vasopressors or emergency intraortic balloon pump for hypotension); with left ventricular ejection fraction <30% or heart failure were not included into the study. Based on angiographic features patients with left main stenosis >60%; target stenosis <50%; with multivessel coronary artery disease requiring emergent or urgent (within 30 days) coronary artery bypass surgery after PCI; with stent thrombosis; with the target lesions located within saphenous vein graft; with the target lesion located at a coronary bifurcation with large (>1.5 mm) side-branch or with bifurcation in which exact status cannot be adequately assessed before stenting procedure were also excluded. The use of atherectomy and/or thrombectomy catheters or/and embolic protection devices was not permitted before stent implantation. After enrolment of 30 patients, the protocol of the study was changed and the use of simple aspiration catheters was allowed at the discretion of the operator.
MGuard stent
MGuard™ stent is a bare metal stent covered with an ultra thin, micron level, flexible mesh sleeve by circular knitting. The sleeve is designed to expand seamlessly during stent deployment, without affecting the structural integrity of the stent, and to prevent thrombi and plaque debris detachment during and post PCI. The net captures and locks thrombus and plaque materials against the arterial wall. Also, the sleeve may diffuse stent pressure on the vessel wall, thereby it may reduce injury and lessen the likelihood of restenosis. Unlike a “covered” stent, the MGuard stent allows normal perfusion into the side branches originating at the stent implantation site. Stents with diameter of 3.0, 3.5, and 4.0 mm and length of 15, 19, 24, and 29 mm were available for the study purposes.
Treatment strategy
In all patients, a loading dose of aspirin (325 mg PO or 500 mg IV) and clopidogrel (600 mg PO) was given. The choice of antithrombin (heparin or bivalirudin) and GP IIb/IIIa inhibitor was at the discretion of the operator. Left ventriculography and coronary angiography was performed using standard techniques. Patients fulfilling all angiographic inclusion criteria and without exclusion criteria were enrolled into the study. Additional heparin boluses were given until the activated clotting time was ≥300 seconds (200-300 seconds if a GP IIb/IIIa inhibitor was used). A ≥6 Fr guiding catheters, and conventional wires (> 0.36 mm) were used. Direct implantation of the MGuard stent followed by post-dilatation was allowed only if there was adequate visualisation of the distal vessel following wiring the vessel. If the distal coronary vessel was not visualised with wiring, predilatation with a ≤2.0 mm balloon using low pressures was recommended. As mentioned above, the use of simple aspiration catheters was permitted only in the last 30 patients. Final angiography was obtained in two views with emphasis on assessment of TIMI flow and any distal occlusions, which may have occurred. Double antiplatelet treatment was recommended for 12 months (aspirin 75 mg PO daily, clopidogrel 75 mg PO daily). Adjunctive drugs were used in compliance with the current guidelines for STEMI management.
Study endpoints and clinical assessment
The primary endpoints of the study were: the frequency of TIMI grade 3 flow after PCI and the incidence of complete ST-segment resolution after PCI (defined as ≥70% ST-segment resolution 60-90 minutes after the last angiogram). The major secondary endpoints consisted of the composite endpoint of major adverse cardiac events (all cause death, target vessel reinfarction, or ischaemic target lesion revascularisation or stroke) at discharge, 30 days and six months post procedure [safety endpoint], and the proportion of patients achieving normal myocardial blush [efficacy endpoint]. Additional procedural (i.e., procedure success, angiographic success, device failure) and angiographic information (i.e., angiographic thrombus score and burden, incidence of slow and no reflow and visible distal embolisation, side branch occlusion, quantitative coronary angiography vessel parameters) were assessed. Clinical outcome was evaluated through monitoring of cardiovascular events: all-cause and cause-specific death, reinfarction of target vessel, repeat ischaemic target lesion and vessel revascularisation, stroke, new onset of severe heart failure, new onset of severe hypotension, bleeding events, and overall and cause specific re-hospitalisation during in-hospital, 30-day and 6-month follow-up period. TIMI definition of bleeding was used to assess severity of bleeding events. Data quality and completeness were monitored.
Angiographic analysis
Angiograms were acquired using a standard protocol and were reviewed by an independent angiographic core laboratory (Cardiovascular Research Foundation, New York, NY, USA) using standard definitions for TIMI flow and MBG assessment. All quantitative measures of reference and minimal luminal diameter were made using standard methodology (Medis, Leiden, The Netherlands).12-14 Myocardial blush was graded in the distribution of the infarct artery based on the maximal densitometric degree of contrast opacification: MBG 0/1=no or minimal myocardial contrast opacification; MBG 2=moderate contrast opacification, but less than in either an ipsilateral or contralateral non-infarct-related artery; and MBG 3=normal myocardial blush or contrast opacification, comparable with the other coronary arteries. When myocardial blush persisted (“staining”), MBG 0 was assigned.
Electrocardiographic analysis
All electrocardiograms were reviewed by an independent core laboratory (Cardiovascular Research Foundation, New York, NY, USA) to assess (1) Q waves (Q wave duration >0.03 seconds for most leads and >0.04 seconds for leads V1, aVL, II and aVF in at least two contiguous leads [posterior infarcts were defined by an initial R wave > 0.04 seconds in V1 and V2 with and R wave > S wave]), (2) ST-segment deviation defined by upward convexity in the leads representing the area of infarction (for acute posterior wall injury was defined as having horizontal or down sloping ST-segment with upright T-waves in V1-V3), and (3) new ischaemic changes. Complete ST-segment resolution was defined as >70% ST-segment resolution from the maximum deviation measured 60 to 90 minutes after the last angiogram.
Statistical analysis
Data were analysed according to the established standards of descriptive statistics. Results were presented as percentages of patients or mean±standard deviation where applicable. Differences between pre-specified patients subgroups (with and without: aspiration catheter use, predilatation, post dilatation, TIMI grade 0 or 1 flow at baseline, and right coronary artery as infarct-related artery) were analysed using chi-square test and the Fisher’s exact test as appropriate, and results were presented as odds ratios with 95% confidence intervals. All tests were two-sided and a P value of <0.05 was considered statistically significant. All statistical analysis was performed using SPSS 15.0 (SPSS Inc., Chicago, IL, USA).
Results
A total of 60 patients were enrolled into the study. Baseline characteristics, treatment before event and clinical status on admission to primary PCI centre are shown in Table 1. All patients were pretreated with clopidogrel loading dose of 600 mg, and 59 (98.3%) patients received aspirin loading dose of 325 mg before angiography. In most patients (65.0%) right coronary artery was identified as infarct-related artery, and patent infarct-related artery (TIMI grade 2 or 3 flow) was found in 22 (36.6%) patients (Table 2). In all patients a weight-adjusted dose of unfractionated heparin was given. Additionally, in 24 (40.0%) patients GP IIb/IIIa inhibitor (abciximab) was used during primary PCI. The mean time-delay from chest pain onset to stent implantation was 269±155 minutes.
Invasive treatment and angiography core lab assessment details are presented in Table 2. In one (1.7%) patient there was no possibility to cross the lesion with study stent and it was implanted within proximal stenosis. Additional non-study, bare-metal stent was implanted at the site of infarct-related artery occlusion. In one (1.7%) patient, while retrieving the non-expanded stent (3.5x15 mm MGuard) back into the guiding catheter (6 Fr), the stent shifted along the balloon catheter, but did not fall off the shaft of the balloon catheter, and was successfully removed. Ultimately, a 3.0x15 mm MGuard stent was successfully implanted at the site of occlusion. In two (3.3%) patients, distal edge dissection requiring additional bare-metal stent implantation occurred. Final TIMI grade 3 flow was observed in 54 (90.0%) patients with MBG 3 in 44 (73.3%) patients (MBG 0 – 0%, MBG 1 – 3.3%, MBG 2 – 23.4%). In three (5.0%) of the cases, distal embolisation was identified in the coronary angiography after primary PCI. Transient slow-flow was observed in two (3.3%) patients, and coronary spasm in two (3.3%) cases. Due to technical issues ST-segment resolution 60-90 minutes after PCI was assessed in 57 patients. Complete (>70%) ST-segment resolution 60-90 minutes after procedure was observed in 35 of 57 (61.4%) cases (ST-segment resolution <30% – 3.5%, ST-segment resolution 30-70% – 35.1%, mean ST-segment resolution 76.9±24.4%). Frequency of combined endpoints is presented in Figure 1.
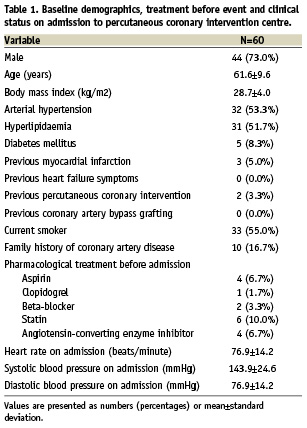
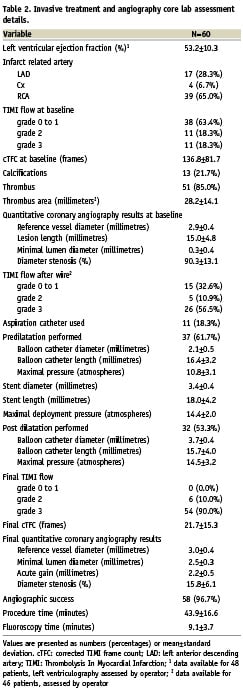
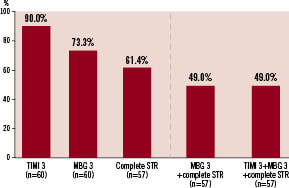
Figure 1. Primary and combined endpoints of the MAGICAL study. Frequency of final Thrombolysis In Myocardial Infarction (TIMI) grade 3 and Myocardial Blush Grade (MBG) 3, and complete ST-segment resolution (STR) >70% 60 to 90 minutes after procedure.
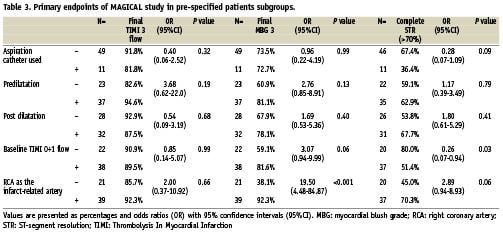
Subgroups analysis for primary outcomes are presented in Table 3. Thrombus aspiration was associated with a trend toward less frequent optimal myocardial reperfusion. However, it was used more frequently in patients with occluded infarct-related artery at baseline (TIMI grade 0 to 1 flow in patients with or without aspiration catheter used: 90.9% vs. 57.1%; P=0.043).
Mean hospital stay was 5.2±1.7 days. There was one (1.7%) TIMI major bleeding and no ischaemic complications were observed during index hospital stay. Aspirin and clopidogrel were prescribed at discharge to all patients. During the 30-day follow-up period, one (1.7%) patient was hospitalised due to the exacerbation of angina symptoms (significant myocardial ischemia was excluded based on non-invasive diagnostics). Additionally, during the 6-month follow-up another (1.7%) patient was hospitalised due to atypical chest pain (myocardial ischaemia was excluded based on non-invasive diagnostics). In one (1.7%) patient, non-ST-elevation myocardial infarction was diagnosed, and successfully treated with bare metal stent implantation within the vessel other than treated during index procedure. The study stent was patent and without significant restenosis. In one (1.7%) patient, PCI within infarct-related artery was performed, but the stent was implanted in the segment other than the one treated during the index procedure (non-target lesion revascularisation). The study stent was patent and without significant restenosis. One (1.7%) patient was hospitalised due to gastrointestinal bleeding requiring blood transfusion (classified as TIMI major bleeding). In the same patient ischaemic stroke of the left hemisphere occurred (stroke rate 1.7%). Three (5.0%) patients were readmitted for planed control angiography and non-infarct-related artery revascularisation. One (1.7%) patient was re-hospitalised two times due to new onset of atrial fibrillation. Finally, there was no death, target vessel reinfarction, or ischaemic target lesion revascularisation during the 6-month follow-up. By protocol definition, total major adverse cardiac events rate at 6-months was 1.7%. Total TIMI major bleeding rate for the 6-month follow-up period was 3.3%.
Discussion
In this prospective series of 60 STEMI patients using the MGuard stent, we achieved a high rate of complete epicardial and myocardial reperfusion, and 1.7% major adverse cardiac events rate at 6-months.
The aim of STEMI treatment is the achievement of early and sustained reperfusion not only on epicardial but primarily on the microcirculatory level, as the optimal myocardial perfusion is associated with improved patients outcomes.2-4,15,16 Impaired myocardial reperfusion can be caused not only by distal embolisation of thrombotic and atherosclerotic particles, but also by microvascular disruption, endothelial dysfunction, and myocardial oedema.15,16 Several adjunctive mechanical devices, such as thrombectomy catheters, manual aspiration catheters, and distal protection devices have been introduced to reduce the risk of distal embolisation and to improve myocardial reperfusion during primary PCI. Large, randomised studies have failed to show any clinical benefit of distal protection devices during primary PCI in STEMI.7,17-19 Similarly, two randomised studies did not confirm improvement in myocardial reperfusion parameters, and have shown even increased infarct size20 and increased 30-day mortality21 associated with the routine use of thrombectomy devices. On the other hand, in several randomised trials, decreased angiographic complications rates and improved myocardial reperfusion were reported in patients treated with simple aspiration catheters.22-25 Importantly, in the largest TAPAS (Thrombus Aspiration during Percutaneous Coronary Intervention in Acute Myocardial Infarction study) trial this treatment strategy was associated with improved long-term clinical outcome.22,26 These findings were also confirmed by two recent meta-analyses.6,27 Use of aspiration catheters may lead to the decrease of thrombus load at the site of infarct-related artery occlusion and facilitate the use of the direct stenting technique. Importantly, direct stenting was shown to be associated with the reduction of microvascular injury and improved ST-segment resolution after primary PCI in comparison to standard balloon predilatation and stenting.28 However, even in patients with successful thrombus aspiration and direct stenting, the risk of unsuccessful reperfusion still persists.
Another possibility to reduce the angiographic complications rates and to improve myocardial reperfusion is to use the mesh covered stent (MGuard stent). This stent is intended to prevent distal embolisation by blocking of the atherothrombi extrusion through the stent struts during its implantation. Protection during the stent implantation is crucial, as the risk of distal embolisation during stent implantation is high. In the TAPAS study, in patients treated with conventional PCI (without thrombus aspiration) and standard coronary stent implantation, 61.5% of all cases of distal embolisation occurred during stent implantation. In patients with thrombus aspiration, the risk of embolisation related to stent positioning and implantation was lower (43.3%), but still high.29 In our study distal embolisation was observed only in 5.0% of patients. In comparison, it was identified in the angiogram in 16.0% of patients in the study by Napodano et al5, and in 15.2% of patients in the study by Henriques et al4. Similar rates of distal embolisation were reported in the more recent TAPAS study (6.7% for thrombus aspiration group and 6.0% for conventional primary PCI group).29 On the other hand, observed rates of distal embolisation were lower than rates shown in the meta-analysis assessing impact of manual aspiration catheters (8.0% for thrombus aspiration group and 20.0% for conventional primary PCI group)6, as well as in meta-analysis of the studies with early use of abciximab before primary PCI (11.1%).30
Also, achieved results of final TIMI flow, MBG and ST-segment resolution after primary PCI were favourable for MGuard stent use. In our study final TIMI grade 3 flow was observed in 90.0% of patients. This rate was comparable, and even better than achieved in the TAPAS study (86.0% for aspiration arm and 83.0% for no aspiration arm)22 and meta-analysis by De Luca et al (87.0% for aspiration arm and 81.0% for no aspiration arm).6 Final MBG 3 was observed in 73.3% of patients and similarly, this rate was higher than in above mentioned studies (TAPAS study: 46.0% for aspiration arm and 32.0% for no aspiration arm; De Luca et al: 52.0% for aspiration arm and 32.0% for no aspiration arm).6,22 Even higher rate (90.0%) of MBG 3 was reported by Piscione et al in the first, large cohort of patients with STEMI treated with the MGuard stent. However, in that study the angiographic parameters represent not independent core laboratory assessments, but rather physicians assessments.
Frequency of complete ST-segment resolution after primary PCI (61.4%) was similar to the observed in patients treated with aspiration catheters (57.0%), and higher than achieved in patients treated with conventional primary PCI (44.0%) in the TAPAS study.22 Piscione et al reported a 90.0% rate of complete ST-segment resolution after primary PCI with MGuard stent use.11 Observed differences between the studies favouring the MGuard stent should be interpreted with caution as there is the possibility of selection bias associated with enrolment of patients fulfilling angiographic criteria. For example patients with calcified, very complex lesions and excessive vessel tortuosity were not included. These patients are at higher risk of angiographic complications.5 As the use of atherectomy and/or thrombectomy catheters or/and embolic protection devices was not permitted before stent implantation (except last 30 patients in whom use of simple aspiration catheters was allowed at the discretion of operator) there was also a chance of exclusion of the highest risk patients with very large thrombus load due to planned use of such devices. On the contrary, in the TAPAS study, randomisation to aspiration and to no aspiration was done before angiography.22 Also, meta-analysis by De Luca et al consists of studies with different inclusion and exclusion criteria, including patients with occluded infarct-related artery and/or large thrombus burden and potentially with the highest risk of angiographic complications.6 On the other hand, in the MAGICAL trial, the study stent was implanted more frequently within right coronary artery. Importantly, primary PCI within the right coronary artery is associated with a higher risk of distal embolisation and impaired myocardial reperfusion.5 However, this was not confirmed in subgroup analysis of our study. Additionally, efficacy of the MGuard stent in prevention of myocardial injury during PCI was also confirmed in the first-in-man trial to include patients with stenosis within saphenous coronary vein grafts or native coronary vessels.8
Small-size balloon predilatation at low pressure was recommended before the MGuard stent implantation. In many situations, this inflation may establish the flow within the infarct-related artery, and facilitate visualisation of the distal vessel allowing for proper stent size selection. Creation of a small channel may also reduce the risk of thrombus dislodgement during stent passage. Alternatively, manual aspiration catheters may be used to reduce thrombus burden and to achieve distal flow before stent implantation. In our study thrombus aspiration was associated with a trend toward less frequent optimal myocardial reperfusion. However, the study was underpowered to assess such relationships. The use of aspiration catheters was non-randomised and there was the risk of selection bias associated with operators preferences. Aspiration catheters and the MGuard stent could be used rather as complementary, than competitive devices during primary PCI in STEMI. Also, large-balloon and/or high-pressure predilatations should be avoided during primary PCI.
The MGuard stent use is not recommended in highly calcified and/or highly tortuous vessels. Also, passage through a previously implanted stent may be associated with increased risk of the mesh entanglement, leading to stent dislodgment. Importantly, patients with the target lesion located at a coronary bifurcation with large (>1.5 mm) side branch were not included, as the side branches can potentially be compromised by the presence of the stent struts or the polymer mesh. Data concerning possibility of the use of such stent within the bifurcation lesion, including flow preservation, and the accessibility to the branch are very limited.8
There was no death, as well as infarct-related artery-related events during 6-month follow-up. Similar, favourable clinical outcome of STEMI patients treated with the MGuard stent was reported by Piscione et al.11 Extent of restenosis within the study stent cannot be assessed, as the control angiography was not routinely performed. On the other hand, there was no need of clinically driven coronary angiography (except for the two above-mentioned patients). In the first-in-man study (most of the patients with vein graft stenosis) the observed rate of target vessel revascularisation for the MGuard stent was 11.1%, with a mean late loss of 0.372±0.23 mm and a mean percent diameter stenosis of 30.6%.8
Limitations of the study
The main limitation of the present study is the small sample size and the non-randomised study design. As discussed above, study population was preselected based on inclusion and exclusion criteria. Study was also underpowered to properly assess the clinical events. On the other hand, this is the second11, large cohort of consecutive STEMI patients treated with the MGuard stent implantation during primary PCI, but the first with independent, core lab assessment of angiographic and electrocardiographic parameters, as well as with 6-month follow-up results.
Conclusions
Based on this experience, the MGuard stent implantation in STEMI patients is safe and highly effective. A larger randomised trial is warranted to confirm the clinical endpoints.
Appendix
List of participating centres: 1. Department of Interventional Cardiology, University Hospital, Krakow, Poland (PI: Dariusz Dudek, coordinating center, n=32); Department of Interventional Cardiology, District Hospital, Nowy Sacz, Poland (PI: Jacek Dragan, n=20); Department of Interventional Cardiology, District Hospital, Nowy Targ, Poland (PI: Jacek Legutko, n=7); Department of Interventional Cardiology, District Hospital, Krosno, Poland (PI: Janusz Szczupak, n=1).

