Abstract
Aims: Percutaneous coronary stenting is synonymous with dual antiplatelet therapy, ranging from four weeks to lifelong. However, even short-term (four weeks) therapy with aspirin and thienopyridines is occasionally contraindicated. No study has ever appraised very short-term dual antiplatelet therapy after stenting. We thus aimed to exploit the pro-healing features of the Genous™ Bio-engineered R stent™ (Genous) (OrbusNeich Medical Technologies, Hong Kong, People’s Republic of China) and evaluate the safety of a 10-day dual antiplatelet regimen after its implantation in up to 50 patients.
Methods and results: Forty-nine consecutive patients with de novo lesions located in vessels able to receive a 2.5 mm Genous stent were included. After stenting, they received lifelong aspirin plus clopidogrel for 10 days. The primary endpoint of the study was sudden cardiac death, myocardial infarction or angiographic evidence of stent thrombosis ascribable to the study stent. Almost 70% of patients effectively discontinued clopidogrel nine to 11 days after stenting. At three-month clinical follow-up, no patient had died or reached the primary endpoint (95%; confidence interval 0-7.3%). Repeat revascularisation occurred instead in three (6.1%[2.1-16.5%]), with target lesion revascularisation in two (4.1%[1.1-13.7%]).
Conclusions: Even very short-term dual antiplatelet therapy seems safe after coronary stenting with Genous in de novo coronary artery lesions located in secondary branch vessels. This preliminary exploratory study gives some support to planning a large trial to test the hypothesis of short dual antiplatelet therapy following Genous stent implantation.
Introduction
While stenting as a practice has been widely accepted, the ideal stent remains elusive. Drug-eluting stents (DES) exploiting antiproliferative, immunosuppressive, and anti-inflammatory drugs have demonstrated that intimal hyperplasia and restenosis can be safely retarded. However, based on the potential local toxicity of these drug-polymer combinations, many questions still remain in regard to their long-term effect on vessel healing1. Thus, bare metal stents (BMS) remain important for many patients at low or moderate risk of restenosis2. Indeed, to minimise the risk of acute, subacute (still the most common type of stent thrombosis with both BMS and DES), and late thrombotic stent events (encompassing sudden cardiac death, myocardial infarction, and stent thrombosis), dual antiplatelet therapy and adequate stent expansion remain paramount3. Typical duration of dual antiplatelet therapy ranges from four weeks (for BMS implanted in stable patients) to 12 months or more (for DES and/or unstable patients) in the modern stent era4. Yet, even four weeks of combined therapy with aspirin and thienopyridine may not be acceptable for patients at high risk of bleeding (e.g., those with profound thrombocytopenia) or those requiring non-cardiac surgery early after stenting5. To date, no study has ever appraised the safety of a very short-term dual antiplatelet regimen in patients undergoing stenting.
The Genous™ bio-engineered R stent™ (Genous, OrbusNeich Medical Technologies, Hong Kong, People’s Republic of China) is based on a polysaccharide matrix coating with murine, monoclonal anti-human CD34+ antibodies covalently bonded to the surface of a 316L stainless steel stent with modular structure6. Given its capability to selectively bind endothelial progenitor cells, Genous may lead to earlier endothelialisation and a lower risk of stent thrombosis, in keeping with its pro-healing design. This device has already been tested in formal studies, including the HEALING I (16 patients)7, and the HEALING II trials8. Notably, in both studies no stent thrombosis occurred despite the protocol recommendation for only one month of dual antiplatelet therapy.
Our hypothesis was that even a very short-term dual antiplatelet regimen could prove safe and effective in patients undergoing coronary stenting with Genous. Thus, we designed a prospective multicentre observational study to appraise the risk of stent thrombotic events in patients receiving a 10-day regimen of aspirin and clopidogrel after Genous stent implantation.
Methods
Design
The Genous trial was designed as a prospective observational study in up to 50 patients.
Patients
Patients were considered eligible if fulfilling all the following inclusion criteria: stable or unstable angina pectoris or documented silent ischaemia, able to understand and sign a witnessed informed consent, age ≥18, no participation in another investigational trial, willingness to comply with the specified follow-up evaluation, target lesion diameter stenosis >50% (by visual estimate), need for stent implantation in a de novo lesion in a vessel able to receive a 2.5 mm Genous (multiple stents were allowed if implanted in thesame vessel and all were Genous), target vessel supplying a viable territory, no expected interventions within the following three months. In particular, the decision to focus only on patients requiring stent implantation in a vessel able to receive a 2.5 mm stent was taken to guarantee safety in case of stent occlusion.
Any of the following criteria would lead to exclusion: known allergy to aspirin, clopidogrel, contrast media or stainless steel; acute medical illness or chronic medical condition with an anticipated survival of less than one year or coexisting condition that could affect patient’s compliance with the protocol; female gender with child-bearing potential; serum creatinine level of more than 2.0 mg/dl (177 µmol/l); acute or subacute (≤72 hours) myocardial infarction; concomitant medical condition requiring systemic anticoagulation; gastrointestinal bleeding or active ulcer within the last month; haemorrhagic diathesis; thrombocytosis (total platelet count greater than 500,000 mm3); immediate need to intervene on a major epicardial vessel; need to treat more than one branch vessel in the index procedure; need to assume thienopyridine therapy for long term; left ventricular ejection fraction below 0.40; patients who do not satisfy the criteria of successful stenting (residual peri-stent dissection, >30% residual in-stent stenosis or >50% persistent stenosis in the target vessel with Thrombolysis in Myocardial Infarction [TIMI] flow <3 post-stenting); occluded target lesion at baseline; restenotic target lesion; previously implanted stent in the target vessel, less than two years before; lesion length >25 mm by visual estimation. Specifically, patients who had a BMS implanted less than a month or a DES implanted less than a year prior to enrolment into this study and who needed to continue clopidogrel for an extended time period were excluded.
Procedures
Percutaneous coronary intervention was performed according to state of the art techniques and approaches, leaving access site, choice of predilatation versus direct stenting, maximum balloon dilation pressure, and ancillary devices at the physician’s discretion. Nonetheless, explicit recommendations were given for stent implantation based on adequate balloon inflations, at least at nominal pressure and lasting 10 or more seconds, to ensure adequate stent expansion. In the case where the patient required treatment in another vessel, the second vessel had to be treated no earlier than three months following the index treatment. After this period other lesions could be treated with any stent and the patient could be restarted on any combination antiplatelet therapy, as by protocol the study was considered completed when the patient had reached three months of clinical follow-up.
Medical treatment and follow-up
The protocol was planned to run in two phases with progressive reduction of the thienopyridine regimen. All patients received five days of pre-treatment with aspirin at a minimum dose of 100 mg and clopidogrel 75 mg (a 300-600 mg loading dose of clopidogrel could be administered at the time of the procedure if the patients were not pre-treated). In phase 1, patients received dual antiplatelet therapy for 10 days post-stenting, whereas patients in phase 2 were supposed to receive dual antiplatelet therapy for five days post stenting. Phase 2 has not yet begun enrolling and thus only results of phase 1 will be reported onwards. The decision on the use of glycoprotein IIb/IIIa receptor inhibitors, bivalirudin or other antithrombotic agents during or after the procedure was left to the operator.
Study endpoints
The primary endpoint of the study was the absence of any occlusion of the Genous stent up to three months, defined by the occurrence after successful stenting of any of the following events: sudden cardiac death not attributable to other causes, myocardial infarction in the area of the study stented vessel, or clinically-driven angiography showing evidence of study stent occlusion. Additional endpoints included all-cause death and repeat revascularisation distinguished in target vessel percutaneous coronary intervention, target vessel non-target lesion percutaneous coronary intervention, and target lesion percutaneous coronary intervention. Follow-up clinical visits were performed at one and three months.
Ethical aspects
All included patients signed a written informed consent form. The study was formally approved and monitored by the ethics committees of participating centres. A dedicated critical event committee (CEC) monitored the study. Specifically, investigators notified any adverse event to the CEC, in case of death, myocardial infarction, or urgent revascularisation within 24 hours from the occurrence. The CEC routinely evaluated each event and defined if it was attributable to stent thrombosis or to any other condition, with the agreement that the study would be stopped following the occurrence of a study stent thrombosis after successful stenting.
Statistical analysis and sample size
Continuous variables are reported as mean±standard deviation. Categorical variables are reported as n (%). Statistical inference was based on the computation of 95% confidence intervals according to the Wilson method with Confidence Interval Analysis (University of Southampton, Southampton, UK). Given the novelty of the approach and the lack of previous data no formal sample size computation was performed.
Results
A total of 49 patients with baseline characteristics reported in Table 1 were included from six centres. These baseline characteristics are reported in Table 1. Specifically, age was 64.2±10.2 years, there were 41 (83.4%) males and nine (18.7%) diabetics. Prior percutaneous coronary intervention had been performed in 21 (42.9%), whereas coronary artery bypass grafting in three (6.1%). Most patients were admitted for stable angina (39 [79.6%]).
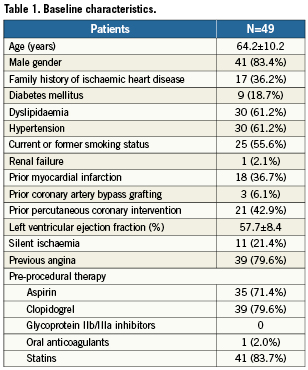
Procedural details are reported in Table 2. In particular, left anterior descending artery branches were the most commonly treated (30 [61.2%]), and a total of 55 stents were implanted (53 [96.3%]) with a 2.5 mm diameter and two lesions required the implantation of 3.0 mm Genous. In two instances, additional stents needed to be implanted to treat periprocedural dissections. There were no residual dissections, no transient vessel occlusions, or occlusion of the branch within the segment, nor any occlusion of any other vessel. Forty-three patients had TIMI 3 flow established post-procedure, while five patients had TIMI 2 flow and only one patient had TIMI 1 flow at the completion of the index procedure.
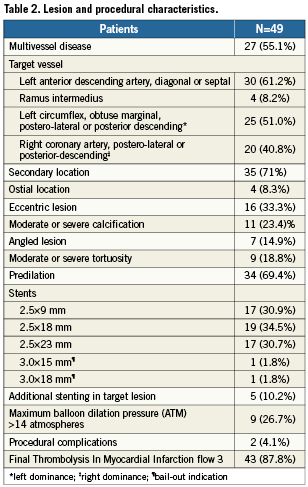
Data on medical therapy from discharge to study completion are available in Table 3. Specifically, all patients were on dual antiplatelet therapy for at least five days prior to the index procedure with the Genous stent and all remained on dual antiplatelet therapy upon discharge, with 34 (69.4%) discontinuing clopidogrel at 10 days, 10 (20.4%) discontinuing clopidogrel at 30 days, and one (1.9%) discontinuing it after 30 days. Reasons for not continuing clopidogrel beyond 10 days were: admission to emergency room for isolated episode of chest pain (1 [1.9%]), occurrence of severe adverse event (1 [1.9%]), staged percutaneous coronary intervention (2 [3.8%]), patient’s decision (1 [1.9%]), referring cardiologist’s decision (2 [3.8%]), and unspecified causes (4 [7.6%]). Additionally, four patients stopped the use of dual antiplatelet therapy, but subsequently restarted it for medical reasons unrelated to the Genous stent implantation, including preparation for another non-target lesion scheduled PCI, occurrence of a non-sent related adverse event, and upon decision by the referring cardiologist, all before the three-month end of the study.
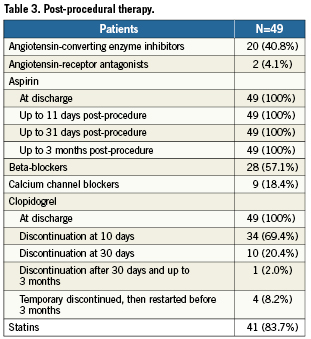
Clinical events are reported in Table 4. In particular, there were no deaths, myocardial infarction or stent thromboses due to the study stent. However, two patients required repeat revascularisation procedures within the three-month follow-up period. One patient, at three-month follow-up after the index procedure, who complained of persistent anginal pain, underwent repeat target lesion percutaneous revascularisation for a 50% diffuse intra-stent restenosis of the Genous stent, while the presence of thrombus was excluded by angiography. This patient was a 50 year old male ex-smoker with a history of hypercholesterolaemia and who, two years previously, had experienced a non-target vessel Q-wave MI, which at that time required percutaneous intervention. Another patient, a 60 year old male former smoker with hypertension, hypercholesterolaemia, and a family history of ischaemic heart disease, had stent thrombosis in a non-Genous stent. This subject, who had previously undergone several percutaneous coronary interventions, including stenting in the mid-left anterior descending artery with a BMS, had subsequently undergone implantation of a 2.5 mm Genous stent in the second diagonal branch. Twenty-six days after the index procedure, he was admitted for Q-wave myocardial infarction, with coronary angiography disclosing thrombotic occlusion of the stent implanted in the left anterior descending artery, whereas the nearby Genous appeared patent and free from stent thrombosis.
Discussion
This study, reporting for the first time ever on a very short-term (10 days) dual antiplatelet regimen following coronary stenting with the Genous stent, suggests that a 10-day dual antiplatelet regimen appears safe and effective in preventing stent thrombotic events in target lesions not located in large diameter or proximal segments of the coronary system, provided that a highly biocompatible stent (i.e., the endothelial-progenitor-cell capturing Genous) is employed.
Current research context
Since the introduction of coronary stents, these endovascular metallic prostheses have been fraught with a significant risk of stent thrombotic occlusion. From the dramatic risk of thrombosis in the late 80s and early 90s, which was as high as 18%9, improvements were achieved by means of high pressure balloon inflation and dual antiplatelet therapy with both aspirin and ticlopidine3, leading to rates of stent thrombosis as low as 0.6%10. Despite pioneering attempts proposing single-agent antiplatelet therapy with aspirin after coronary stenting, this approach was not adopted because of safety concerns11. Thus, dual antiplatelet therapy has been recommended universally for four weeks after BMS implantation, with non-cardiac surgery recommended at least six weeks after BMS implantation, to minimise the risk of stent thrombosis5.
The issue of stent thrombosis has become even more important in the DES era, with the reported risk of this complication escalating in patients prematurely discontinuing dual antiplatelet therapy (hazard ratios as high as 161 in those discontinuing versus not discontinuing antiplatelet therapy12). Thus, dual antiplatelet therapy is now recommended for 12 months in most patients treated with DES, with non-cardiac surgery recommended at least 12 months after BMS implantation, to minimise the risk of stent thrombosis5.
Despite this consensus on duration of dual antiplatelet therapy, several patients may not comply with this protracted regimen, either because of bleeding risk or because of the need for early invasive non-cardiac procedures. Thus, there is a need for more biocompatible stents amenable to short-term antiplatelet therapy. Indeed, the Genous bio-engineered R stent exploits a coating with monoclonal anti-human CD34+ antibodies to selectively bind endothelial progenitor cells, thus promoting early healing and endothelialisation of stent struts6. Its safety and efficacy when followed by a four-week dual antiplatelet regimen has already been proven in prospective observational studies, including the HEALING I and the HEALING II trials7,8,13. In addition, there is preliminary evidence from studies including patients undergoing non-cardiac surgery that even shorter term antithrombotic regimens can be safe and effective after Genous implantation14. However, so far no study has reported on very short-term (10 days) dual antiplatelet therapy following Genous stent implantation.
Contributions of the present study
The current GENOUS trial, reporting on 49 patients successfully treated with coronary Genous stent implantation in secondary coronary vessels and discharged with a 10-day dual antiplatelet regimen, suggests that even such a very short-term aspirin and clopidogrel therapy can be safe and effective in preventing stent thrombosis. Specifically, we did not observe any death, myocardial infarction or stent thromboses involving the Genous stent. Indeed, a patient who had previously received a BMS in the left anterior descending artery and who had subsequently undergone implantation of a Genous stent in a diagonal branch experienced stent thrombosis during follow-up, but this event occurred almost one month after Genous implantation, and thrombosis was limited to the left anterior descending artery and did not involve the Genous stent. This patient unfortunately had an event associated with a previously treated lesion after the index procedure with the Genous stent, but the occurrence of this event suggests anecdotally that the Genous stent is even more biocompatible than the previously implanted BMS.
Target lesion revascularisation occurred in only one patient during study follow-up, despite the inclusion of small vessels and the use of small diameter stents. Thus, this finding provides indirect evidence in support of the anti-restenotic efficacy of Genous, which is in keeping with results from the HEALING I and HEALING II trials7,8,13, especially in subjects with adequate statin therapy, which is well-known for favouring mobilisation and homing of endothelial progenitor cells.
Avenues for further research
Completion of the phase 1 of the GENOUS trial might lead to enrolment in phase 2, where dual antiplatelet therapy would be prescribed for an even shorter duration: five days. The present results, when combined with the upcoming findings of the GENOUS trial phase 2, could indeed provide a novel approach to the treatment of coronary artery disease in patients at high risk of bleeding or those requiring non-cardiac surgery early after coronary stenting. In addition, the future availability of second-generation (based on a cobalt-chromium platform) and third-generation Genous devices (based on a cobalt-chromium platform and combining both sirolimus elution and endothelial progenitor cell capturing features) will likely further improve the safety and efficacy of this stent technology.
The appeal of such a strategy of combining drug elution and endothelial progenitor cell capturing is self-evident, especially in patients with coronary artery disease and significant comorbidities, invasive cancer, requiring surgery, and/or at high bleeding risk, who require both prevention of restenosis after stenting and freedom from stent thrombosis. The Genous stent seems to be ideal for treatment of patients with prior bleeding or in patients awaiting surgery. It has the potential to greatly change who we can safely intervene upon.
Limitations
The limitations of this pilot study include the relatively small sample size, the high proportion of male subjects, the lack of mid-term clinical and angiographic follow-up, and the lack of randomisation to an appropriate control device. However, the innovative and paradigm-shifting scope of this work mandated a set of careful selection criteria, and small sample size with thorough safety monitoring of all adverse events. In addition, reliance on an external event committee and statisticians for, respectively outcome adjudication and data analysis, further enhance the internal validity of this work. Nonetheless, additional studies with larger size, longer follow-up, and controlled design are warranted to confirm or disprove the present findings. The lack of longer-term clinical and systematic angiographic follow-up might have reduced the precision and accuracy of restenosis estimates in the current study and will need to be seriously considered in further studies of dual antiplatelet therapy duration with the use of the Genous stent. Finally, prolongation of dual antiplatelet therapy beyond 10 days in 11 patients might have limited the systematic appraisal of the safety of such a short dual antiplatelet therapy regimen, so a larger sample size should be considered in future studies to allow for stratification of clinical results as a function of dual antiplatelet therapy duration.
Conclusions
The prospective GENOUS study suggests that even very short-term dual antiplatelet therapy seems safe after coronary stenting with endothelial progenitor cell capturing devices in de novo coronary artery lesions located in secondary branch vessels. This preliminary exploratory study gives support to planning a large trial to test the hypothesis of short dual antiplatelet therapy following Genous stent implantation.
Acknowledgements
We thank Steve Rowland, OrbusNeich Medical Inc., Hong Kong, People’s Republic of China, for his editorial assistance.
Conflict of interest statement
The authors have no conflict of interest to declare
Funding
This study was supported by an unrestricted grant from OrbusNeich, Hong Kong, People’s Republic of China.
Appendix
Critical event committee (CEC): Giuseppe Biondi-Zoccai, Modena, Italy; Marco Ferlini, Pavia, Italy. Data analysis: Xavier Jouven, Paris, France. Data monitoring: Mediolanum Cardio Research, Milan, Italy.

