
Abstract
Aims: The optimal duration of DAPT in ACS patients treated with DES is still unclear. Therefore, the aim of the current study was to investigate a short versus a standard 12-month DAPT regimen in ACS patients undergoing new-generation DES implantation.
Methods and results: REDUCE was a prospective, open-label, multicentre, investigator-initiated study that randomised 1,496 ACS patients after treatment with the COMBO stent to either three (n=751) or 12 months (n=745) of DAPT. The primary study endpoint was a composite of all-cause mortality, myocardial infarction, stent thrombosis, stroke, target vessel revascularisation and bleeding at 12 months. No difference was observed in the demographic and clinical characteristics between the two groups, except for gender (p=0.01). At one-year follow-up, non-inferiority of three- versus 12-month DAPT in the primary endpoint was met (8.2% vs 8.4%, pnon-inferiority<0.001). The similar outcome between the two groups was confirmed at two-year follow-up (11.6% vs 12.1%, p=0.76), with no significant difference in overall mortality (3.1% vs 2.2%, p=0.27), cardiac mortality (1.8% vs 1.1%, p=0.28), stent thrombosis (1.6% vs 0.8%, p=0.16) and major bleeding (3.3% vs 4.0%, p=0.46).
Conclusions: The results show that, among ACS patients treated with the COMBO stent, three months is non-inferior to 12 months of DAPT. However, given the numerically higher rates of mortality and ST in the three-month DAPT group, one-year DAPT should still be recommended in ACS until more information becomes available. A three-month DAPT strategy should be considered only if clinically mandated.
Introduction
Due to the significant improvement in antithrombotic therapies and stent technologies, percutaneous revascularisation is currently the most preferred therapy for the treatment of coronary artery disease (CAD), especially in the setting of acute coronary syndromes (ACS). However, the optimal duration of dual antiplatelet therapy (DAPT) remains under debate. In fact, first-generation drug-eluting stents (DES) were associated with higher rates of late stent thrombosis (ST) and mortality1,2, leading to the recommendation of 12-month DAPT after DES implantation. However, robust evidence to support this antithrombotic strategy in ACS is still lacking, as it was adopted from old ACS trials on the impact of DAPT in populations with low rates of intervention3. Although prolonged DAPT may prevent thrombotic complications, this benefit may be counterbalanced by the subsequent increase in major bleeding complications, especially with newer antiplatelet agents (i.e., ticagrelor or prasugrel)4,5. However, efforts have recently been made in the search for new stent technologies to promote vascular healing and endothelial repair6,7,8,9 that may justify a shorter DAPT duration. In a recent randomised trial including patients with non-ST-elevation myocardial infraction (NSTEMI), the COMBO™ dual-therapy stent (OrbusNeich, Hong Kong, China) was non-inferior in terms of target vessel failure to the XIENCE stent (Abbott Vascular, Santa Clara, CA, USA), with a better stent strut coverage, as shown by OCT (91.3% vs 74.8%) (p<0.001)10. The Randomised Evaluation of short-term DUal antiplatelet therapy in patients with acute coronary syndrome treated with the COMBO dual-therapy stEnt (REDUCE trial) is the first study conducted in ACS-only patients treated with a new-generation DES to test the hypothesis that treatment with DAPT for three months is non-inferior compared to standard 12-month DAPT with regard to a combined safety and efficacy endpoint. We report the final two-year results of the REDUCE trial.
Methods
STUDY DESIGN AND RANDOMISATION
The REDUCE trial (NCT02118870) was an investigator-initiated, prospective, open-label, multicentre, randomised study with two groups, as previously described11. Briefly, ACS patients who were successfully treated with the COMBO stent were subsequently randomised, after obtaining informed consent, during index hospitalisation (before discharge), in a 1:1 ratio, to either three-month or 12-month DAPT. Patients requiring staged PCI received their secondary PCI during index hospitalisation prior to randomisation.
Treatment assignments were performed centrally through a dedicated website as part of the electronic case report form according to computer-generated random permuted blocks with stratification by site. The intervention (DAPT administration) was not blinded. The central ethics committee (Isala Hospital, Zwolle, the Netherlands) initially approved the study. The ethics committees and competent authorities of each participating centre (Supplementary Table 1) approved the study. The study complies with the Declaration of Helsinki.
TREATMENT AND FOLLOW-UP PROCEDURES
Eligible patients (Supplementary Table 2) were treated with aspirin (ASA) and a P2Y12 inhibitor. Prasugrel and ticagrelor were preferred over clopidogrel. The final choice of P2Y12 inhibitor was at the discretion of the treating physician. Patients received DAPT according to their randomisation and continued on ASA monotherapy afterwards. If contraindications for ASA emerged, monotherapy with P2Y12 inhibition was allowed. In case additional coronary intervention was required, only patients in whom these procedures were performed with a COMBO stent, and before discharge, were eligible to be randomised. Physical follow-up visits to the outpatient clinic were performed at three and 12 months, whereas a telephone contact was planned at six months and 24 months.
STUDY ENDPOINTS
The primary endpoint was the composite occurrence of all-cause death, myocardial infarction (MI; based on the third universal definition12), stent thrombosis (ST; definite/probable, Academic Research Consortium [ARC] definition13), stroke, target vessel revascularisation (TVR) and bleeding (Bleeding Academic Research Consortium [BARC] 2/3/514) within the 12-month follow-up. The independent clinical events committee, while blinded to the randomisation of the patients, adjudicated all serious adverse events and determined whether any revascularisation event was related to the index procedure target vessel. A detailed list of primary and all secondary endpoints is provided in Supplementary Table 3.
STATISTICAL ANALYSIS
Continuous data are expressed as median (25th-75th percentile) and categorical data as percentages. The analysis of variance was appropriately used for continuous variables. χ² testing or the Fisher’s exact test was used for categorical variables.
The sample size calculation was based on a non-inferiority design, with a one-sided log-rank test for comparison of independent survival curves at the 2.5% significance level, a power of 80%, and a margin for non-inferiority of 5%. Based on a recent study including ACS patients treated with DES15, we assumed an event rate of the primary endpoint of 12% in both DAPT groups with a counterbalance of thrombotic and bleeding complications. On the basis of this assumption and an extension for dropouts, 750 patients per group were required to demonstrate non-inferiority.
The primary endpoint was evaluated after completion of the one-year follow-up. Statistical analyses were performed by an independent contract research organisation (Diagram BV, Zwolle, the Netherlands). Data acquisition and analyses were performed independently. Primary and secondary analyses were based on the intention-to-treat population. Additionally, a pre-specified landmark analysis of the primary endpoint without TVR was performed from three to 12 months, since treatment during the first three months was equal in both groups. Analyses based on the per protocol analysis populations were considered secondary and confirmatory. With the sole exception of the primary endpoint, all other analyses were tested for superiority. Statistical analyses were performed using SAS 9.4 (SAS Institute, Cary, NC, USA).
Results
From June 2014 to May 2016, 1,500 eligible ACS patients undergoing successful COMBO stent implantation were included in the study, as described in the protocol. Four patients withdrew their informed consent soon after randomisation, leaving 1,496 patients randomised to three (n=751) or 12 months (n=745) of DAPT. The study flow chart is shown in Supplementary Figure 1. Baseline characteristics are shown in Table 1, Table 2, and Supplementary Table 4. No differences were observed between the groups, except for gender (17.4% vs 22.7%, p=0.01). STEMI was observed in almost 50% in both groups. Median DAPT duration in the two groups was 91 (89-96) vs 365 (363-369) days; p<0.001, respectively. Almost 60% of patients were discharged on new adenosine-diphosphate (ADP) antagonists (prasugrel or ticagrelor) (Supplementary Table 5).
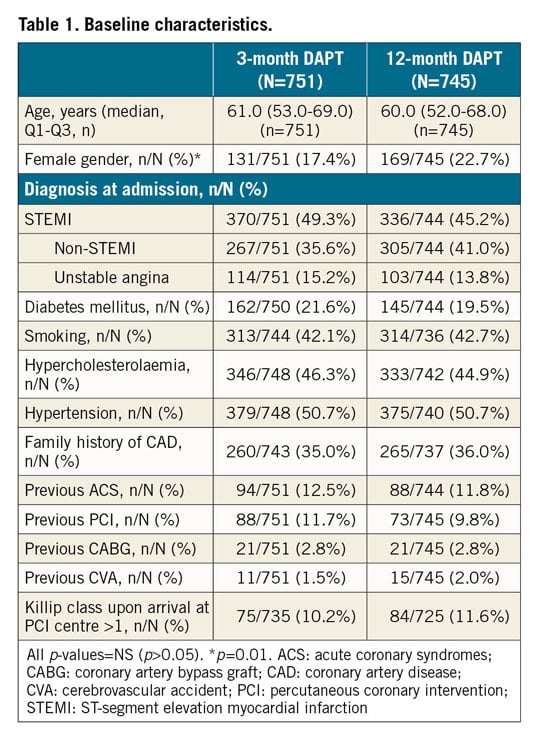
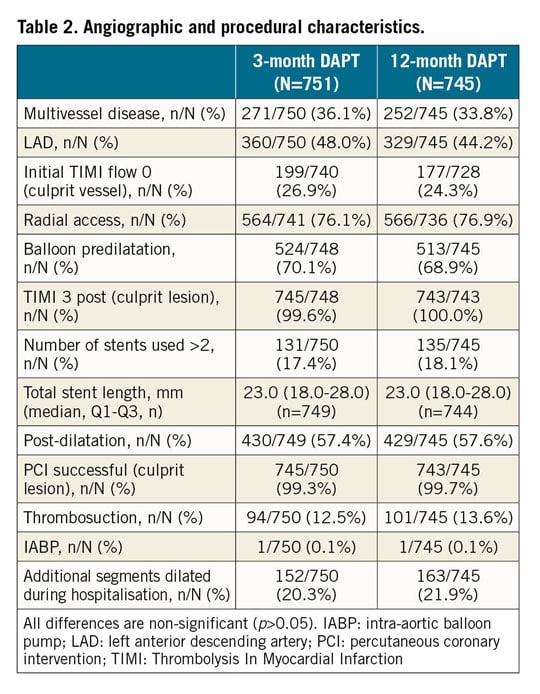
PRIMARY STUDY ENDPOINT
As detailed in Supplementary Figure 1, a total of 1,462 patients (729 in the three-month and 733 in the 12-month DAPT groups) were analysed. No difference was observed in the primary endpoint (8.2% vs 8.4%, risk difference –0.0022, upper limit 95% confidence interval [CI]: 0.027, p<0.001; HR 0.97, 95% CI: 0.68-1.39). The result was confirmed after adjustment for gender (adjusted HR 0.96, 95% CI: 0.67-1.36, p=0.80). Kaplan-Meier curves are shown in Figure 1.
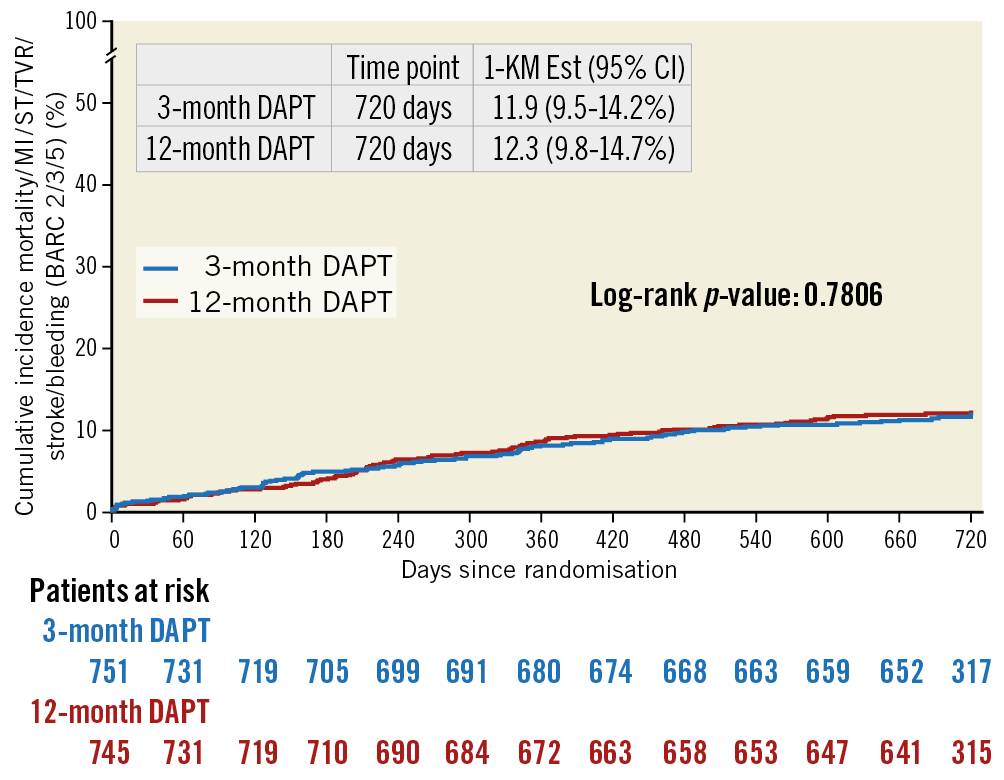
Figure 1. Kaplan-Meier curves for the primary study endpoint.
No difference between three- and 12-month DAPT was observed in the occurrence of the primary endpoint in the per protocol (8.1% vs 6.8%, p<0.001; HR 1.19, 95% CI: 0.78-1.83) and actual treatment analyses (8.0% vs 7.3%, p<0.001; HR 1.10, 95% CI: 0.72-1.66).
SECONDARY STUDY ENDPOINTS
As detailed in Supplementary Figure 1, a total of 1,460 patients (733 in the three-month and 727 in the 12-month DAPT groups) were analysed. The composite occurrence of all-cause death, MI, ST, stroke, TVR and bleedings was observed in 173 patients (11.8%) at two-year follow-up, without any significant difference between three and 12 months of DAPT (11.6% vs 12.1%, HR 0.96, 95% CI: 0.71-1.29, p=0.76). The result was confirmed after adjustment for gender (adjusted HR 0.95, 95% CI: 0.70-1.28, p=0.81). Kaplan-Meier curves are shown in Figure 1.
At two-year follow-up, no difference between three and 12 months of DAPT was observed in the occurrence of the composite of all-cause death, MI, ST, stroke, TVR or bleeding in the per protocol (11.2% vs 10.8%, p=0.82, HR 1.05, 95% CI: 0.74-1.49) and actual treatment analyses (11.1% vs 11.2%, p=0.97; HR 1.0, 95% CI: 0.71-1.41).
As shown in Table 3 and Supplementary Table 6, no statistically significant difference was observed between three- and 12-month DAPT in any secondary endpoint at one- and two-year follow-up. A total of 39 deaths (2.7%) were observed at two-year follow-up, with 21 of them (54%) classified as cardiovascular (Supplementary Table 7). A non-significant difference in cardiac mortality was observed between the groups (1.8% vs 1.1%, p=0.28). An overall low rate of definite/probable ST was observed in our study (18 events, 1.2%), without any significant difference between the groups (1.6% vs 0.8%, p=0.16). Kaplan-Meier curves are shown in Figure 2. A non-significantly higher rate of major bleeding was observed with 12 months as compared to three months of DAPT (4% vs 3.3%, p=0.46) (Supplementary Figure 2). No difference was observed in other secondary endpoints (Supplementary Figure 3-Supplementary Figure 6).
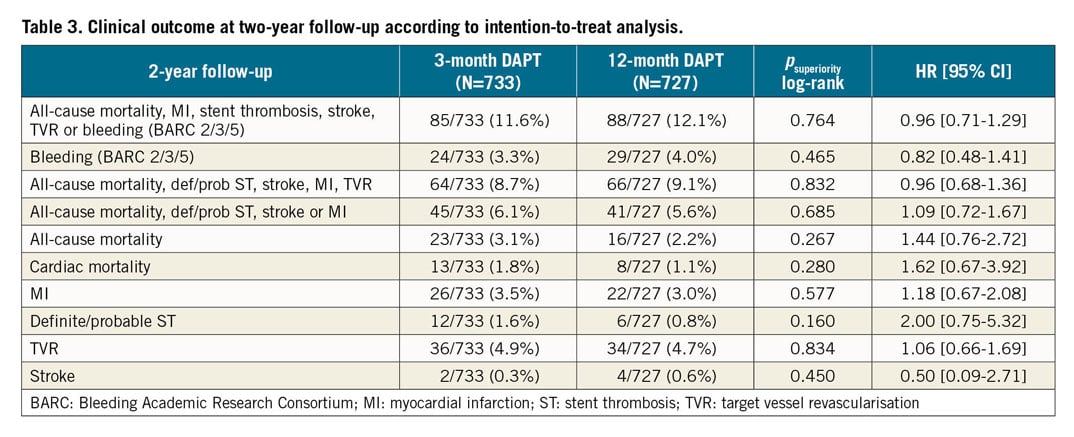
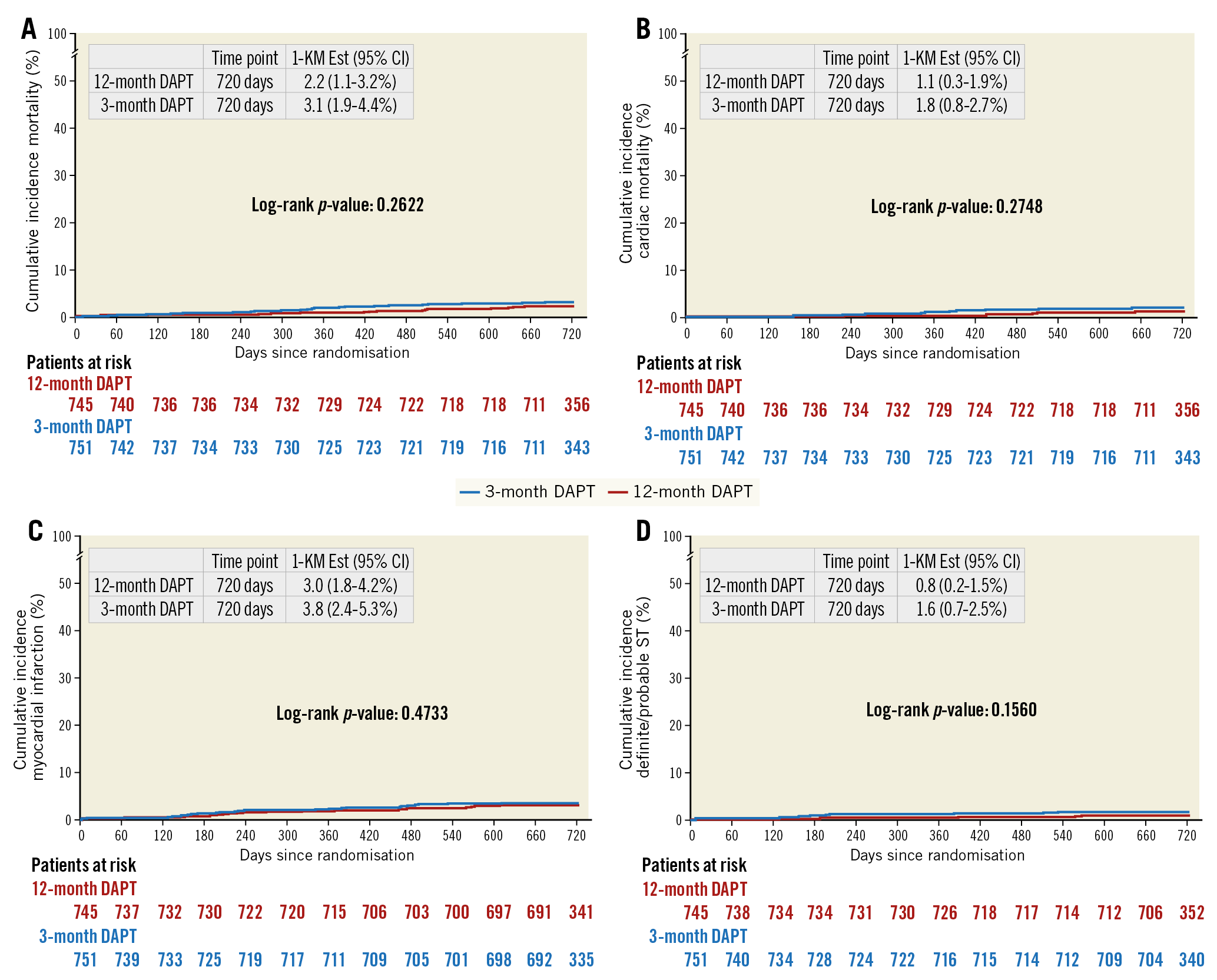
Figure 2. Kaplan-Meier curves. A) Mortality. B) Cardiac mortality. C) Myocardial infarction. D) Definite/probable stent thrombosis.
As shown in Supplementary Figure 7, in a pre-specified landmark analysis no statistically significant difference in the occurrence of all-cause death, MI, definite or probable ST, stroke, or bleeding (BARC 2, 3, 5) was observed between the groups including only patients who were free from events at three-month follow-up (p=0.76).
SUBGROUP ANALYSES
The difference in primary outcome between three- and 12-month DAPT was explored (for superiority) in several high-risk subsets of patients, defined according to age, gender, diabetes, chronic kidney disease and clinical presentation (STEMI vs NSTEMI/unstable angina [UA]). Results were quite consistent across the subgroups, including gender, without any significant statistical interaction at one- (Supplementary Figure 8) and two-year follow-up (Figure 3).
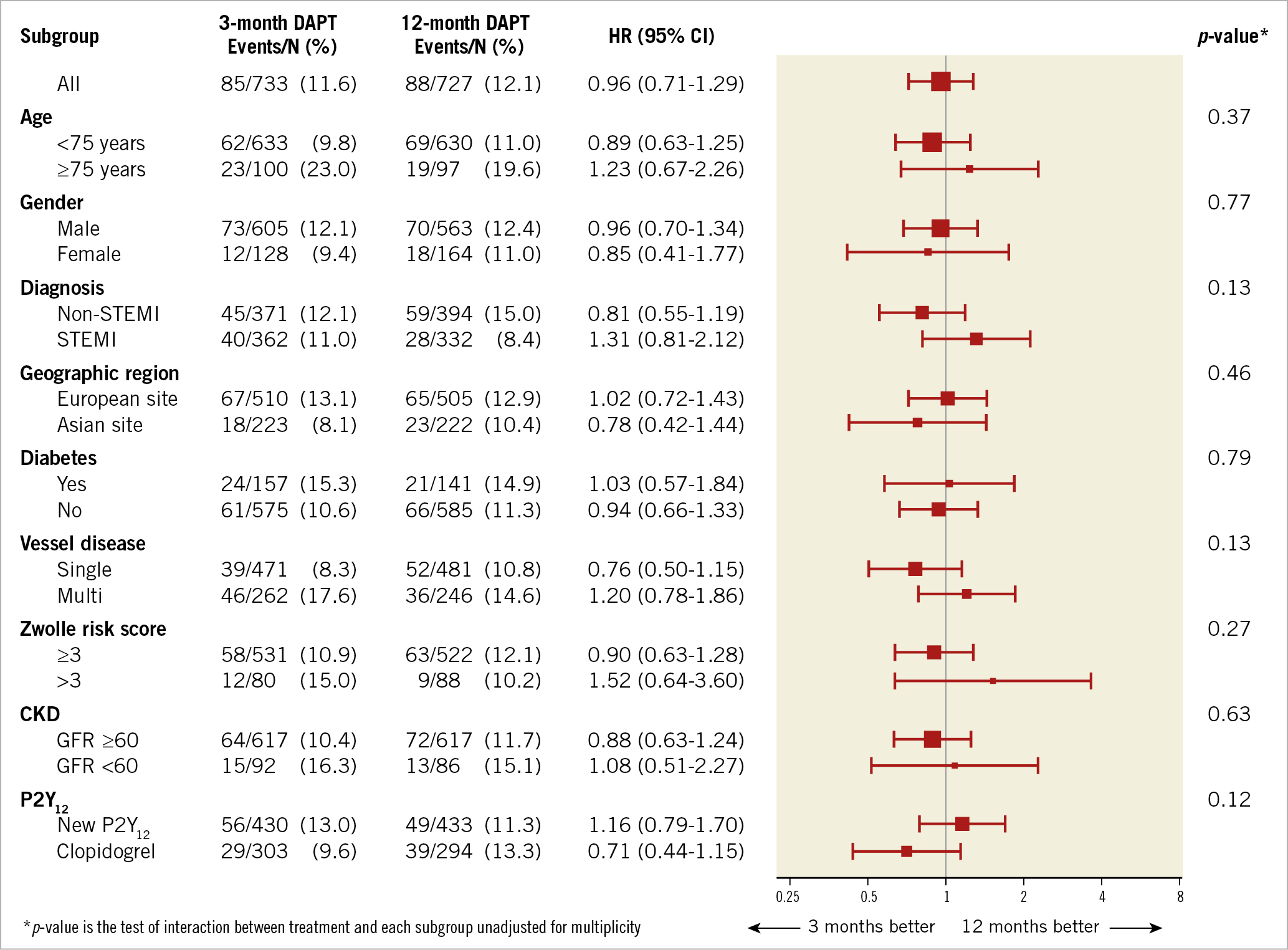
Figure 3. Forest plot showing the composite occurrence of all-cause death, MI, ST, stroke, TVR and bleeding (BARC 2/3/5) at two-year follow-up across several major subgroups of patients. CKD: chronic kidney disease
Discussion
The REDUCE trial is the first study restricted to ACS patients comparing a short (three-month) versus a standard 12-month DAPT strategy after successful stent implantation. In our study, among ACS patients treated with the COMBO stent, no difference was observed between three and 12 months of DAPT at one- and two-year follow-up.
OPTIMAL DAPT DURATION IN ACS
The optimal duration of DAPT after DES implantation in ACS is still a matter of debate, because thrombotic risk needs to be balanced with bleeding risk.
While protecting from thrombotic complications, a significant increase in bleedings may be expected in case of prolonged DAPT that may counterbalance the net benefit. This may be of relevance in consideration of the higher risk profile of current patients undergoing PCI, at higher risk of bleedings, which may impact on survival.
Based on the CURE trial3, scientific societies have been in favour of DAPT after an ACS. However, this trial was conducted 20 years ago, whereas new-generation DES technologies have been shown to minimise the risk of ST.
So far, only a few dedicated trials have investigated this issue in ACS, especially in the era of new ADP antagonists and new-generation DES. In the multicentre DAPT STEMI trial16 a total of 870 STEMI patients treated with primary angioplasty and the Resolute Onyx™ stent (Medtronic, Minneapolis, MN, USA) who were taking DAPT and were event-free at six months were randomised 1:1 to single antiplatelet therapy or to DAPT for an additional six months. New ADP antagonists were used similarly in both groups (58%). All patients who were randomised were then followed for another 18 months (i.e., 24 months after the primary PCI). The primary endpoint (a composite of all-cause mortality, any MI, any revascularisation, stroke, and Thrombolysis In Myocardial Infarction [TIMI] major bleeding at 18 months after randomisation) occurred in 4.8% of patients receiving single antiplatelet therapy versus 6.6% of patients receiving DAPT (pnon-inferiority=0.004). In the multicentre SMART-DATE trial17, a total of 2,712 ACS patients treated with PCI and DES with permanent (XIENCE or Resolute Onyx) or bioresorbable (Orsiro; Biotronik, Bülach, Switzerland) polymer were randomly assigned to six-month (n=1,357) and 12-month or longer DAPT (n=1,355). Clopidogrel was used in 79.7% of patients in the six-month DAPT and in 81.8% of patients in the 12-month DAPT groups. The primary endpoint (a composite of all-cause death, MI, or stroke at 18 months) occurred in 4.7% with six-month DAPT and in 4.2% with 12-month DAPT (pnon-inferiority=0.03). Although all-cause mortality did not differ significantly between six-month DAPT and 12-month DAPT (2.6% vs 2.9%, p=0.90) and neither did stroke (0.8% vs 0.9%, p=0.84) or ST (1.1% vs 0.7%, p=0.32), MI occurred more frequently in the six-month DAPT group than in the 12-month DAPT group (1.8% vs 0.8%, p=0.02). No significant difference was observed in the rate of BARC type 2/3/5 bleeding between the two groups (2.7% vs 3.9%, p=0.09).
In a recent comprehensive meta-analysis restricted to ACS which included 17,941 patients18, a shorter DAPT strategy was associated with a reduction in bleedings, whereas no difference in cardiovascular mortality, MI or ST was observed with shorter versus standard 12-month DAPT.
REDUCE TRIAL
REDUCE is the first trial comparing, in a total of 1,496 ACS patients, a very short DAPT strategy (three months) versus a standard 12-month DAPT strategy. The use of a new-generation DES, the large inclusion of STEMI patients (almost 50%), and the predominant use of new ADP antagonists in almost 60% of the population make this study contemporary. Differently from DAPT-STEMI but similarly to SMART-DATE, patients were randomised during initial hospitalisation.
Our study showed that three-month DAPT was non-inferior to 12-month DAPT with regard to the primary endpoint (a composite of mortality, MI, ST, stroke, TVR or bleeding [BARC 2/3/5]). A similar outcome between the two groups was observed at two-year follow-up (11.6% vs 12.1%, respectively), and also confirmed in the per-protocol analysis, actual treatment analysis, and for major subgroups such as age, diabetic status, gender, type of ACS (STEMI versus NSTEMI/ACS) and kidney function.
No significant differences were observed in secondary endpoints (mortality, MI, ST, TVR and bleedings). A numerically higher occurrence of (cardiac) mortality and definite/probable ST was observed in the three-month DAPT group. However, these findings must be interpreted with caution. In fact, this study was not powered for these very low event rates. In addition, about half of the deaths were non-cardiovascular, and some were even observed when patients were still on DAPT. Nevertheless, these outcomes do not justify a liberal use of the shorter DAPT therapy. In fact, short DAPT should be applied only when clinically indicated, for example due to high bleeding risk.
The overall low rates of definite/probable ST at one-year follow-up (0.8%), despite the short DAPT duration in half of the population, may be explained by the use of a new-generation DES with faster re-endothelialisation, whereas in TRITON-TIMI 38 and PLATO most of the patients received either BMS (about 50%) or first-generation DES. In fact, our data are consistent with other STEMI trials with new-generation DES19.
Even though we included BARC 2 bleedings, domination of the primary endpoint by minor bleeding was not observed. Similarly, in the DAPT trial20 there was only a difference of 1.6% in BARC 2 bleeding incidence between 12 and 30 months (3.1% in the continued DAPT group versus 1.5% in the placebo group). Furthermore, the recent PEGASUS trial21 has shown that prolonged three-year ticagrelor in patients with previous MI reduced the rate of thrombotic complications, that was, however, counterbalanced by a higher risk of bleedings. In fact, a similar mortality was observed between ticagrelor and placebo. These data on prolonged DAPT are in line with the large DAPT trial20 even trending towards higher mortality with prolonged DAPT22. In fact, current guidelines provide a class 2B recommendation for prolonged DAPT after ACS23,24.
Limitations
The lower than expected event rates observed in our study may be due to the randomisation strategy after successful stenting (freedom from in-hospital events) which may have led to selection bias of lower-risk ACS patients. Although the non-inferiority margin of 5% may appear relatively large, especially in comparison with an actual primary endpoint event rate of 8.3%, our study should be placed in the context of other non-inferiority stent studies, with comparable relative margins16,17. Furthermore, our sample size estimation was based on a postulated event rate of 12%, which was higher than the actual rate of 8.3% at one-year follow-up, but consistent with the rate observed at two-year follow-up showing a similar outcome between the groups (11.6% vs 12.1%).
We observed a significant difference in gender between the two groups. However, our results were confirmed after adjustment for gender and were consistent in both male and female gender, without significant interaction.
The study was performed unblinded, without placebo control. Furthermore, the use of P2Y12 inhibitors was heterogeneous, reflecting real-world practice.
While most of the baseline characteristics of included patients were similar to those observed in other trials, the younger age (mean of 60 years), in addition to the use of new ADP antagonists in less than 60% of the population, the exclusion of patients at high risk of bleeding, such as those needing oral anticoagulation, may have contributed to minimising the benefits in bleeding complications expected with a shorter DAPT duration. In fact, shorter DAPT was associated with a numerically lower occurrence of bleeding complications, without reaching statistical significance. However, in addition to its prognostic impact and the costs related to bleeding complications, it must be recognised that a short-term DAPT strategy (especially in the era of new ADP antagonists) certainly provides advantages in terms of cost reduction (when applied on a large scale).
Based on the data collected, we could not exactly identify patients at high risk of bleeding, with the exception of elderly patients who carry higher risk primarily due to their comorbidities.
Finally, the current results are pertinent to the COMBO stent, and therefore cannot be extrapolated to other DES.
Conclusions
The results of the REDUCE trial show that, among ACS patients treated with the COMBO stent, three months is non-inferior to 12 months of DAPT. However, given the numerically higher rates of mortality and ST in the three-month DAPT group, one-year DAPT should still be recommended in ACS until more information becomes available. A three-month DAPT strategy should be considered only if clinically mandated.
|
Impact on daily practice Until more information becomes available, one-year DAPT should still be recommended in ACS patients. In case of intolerance to one-year DAPT, such as high bleeding risk, a shorter duration of DAPT, even for three months, should be safe, even for ACS patients. |
Funding
The study was supported by a research grant from OrbusNeich Medical Inc., Fort Lauderdale, USA.
Appendix. Study collaborators
Lucia Barbieri, MD; Queen Mary Hospital, University of Hong Kong, Hong Kong, China. Stephen W. Lee, MD; Queen Mary Hospital, University of Hong Kong, Hong Kong, China. Jacques Lalmand, MD; Centre Hospitalier Universitaire, Charleroi, Belgium. René J. van der Schaaf, MD, PhD; Onze Lieve Vrouwe Gasthuis, Amsterdam, the Netherlands. Tian H. Koh, MD, PhD; National Heart Center, Singapore, Singapore. Philippe Timmermans Sr, MD; Jessa Ziekenhuis, Hasselt, Belgium. Dagmara Dilling-Boer, MD; Jessa Ziekenhuis, Hasselt, Belgium. Leo F. Veenstra, MD; Maastricht University Medical Center, Maastricht, the Netherlands; Zuyderland Atrium Medical Center, Heerlen, the Netherlands. Arnoud W. van ‘t Hof, MD, PhD; Maastricht University Medical Center, Maastricht, the Netherlands; Zuyderland Atrium Medical Center, Heerlen, the Netherlands. Vincent Roolvink, MD, PhD; Isala Hospital, Zwolle, the Netherlands. Evelien Kolkman, MSc; Diagram BV, Zwolle, the Netherlands. Marc A. Brouwer, MD, PhD; Radboud University Medical Center, Nijmegen, the Netherlands.
Conflict of interest statement
A. van ‘t Hof reports grants from Medtronic, grants and personal fees from AstraZeneca, grants and personal fees from Abbott, outside the submitted work. J. Lalmand reports grants and personal fees from OrbusNeich, during the conduct of the study. The other authors and the other study collaborators have no conflicts of interest to declare.

