Abstract
Aims: To evaluate outcomes of the endothelial progenitor cell (EPC) capture stent in patients on chronic anti-vitamin K (AVK) regimen, requiring percutaneous coronary intervention (PCI).
Methods and results: Between February 2007 and February 2008, 78 consecutive patients under chronic AVK treatment undergoing PCI were enrolled in the registry and received an EPC capture stent. The incidence of comorbid conditions was analysed by the Charlson index. Dual antiplatelet therapy (DAT, aspirin and clopidogrel) was prescribed for one month only together with the AVK treatment, after PCI. Major adverse clinical events (MACE) rate, included death, acute myocardial infarction (MI) or target lesion revascularisation (TLR), incidence of stent thrombosis and rate of haemorrhagic events were collected. A Charlson index >3 was present in 89% of patients. At 14±8 months the cumulative rate of MACE was 22%: 10 deaths (six cardiac deaths), and six TLR. No MI or definitive/probable stent thromboses occurred during follow-up. Four major haemorrhagic episodes occurred during follow-up, all of them after the first month.
Conclusions: Patients on AVK treatment represent a highly comorbid population with a high event rate after PCI. The strategy of PCI with an EPC capture stent and short duration of DAT may be used in patients who need a short-term DAT.
Introduction
In the last years, the increasing use of drug-eluting stents (DES) has markedly reduced in-stent restenosis compared to bare metal stents (BMS).1,2 However, the antiproliferative drugs coated into DES impede the natural healing response by delaying the formation of a functional endothelial layer covering stent’s struts with the risk of stent thrombosis.3 To reduce this risk, an optimal antiplatelet regimen after stent implantation is mandatory, and currently, it consists of a combination of aspirin and ADP-receptor antagonists, such as ticlopidin or clopidogrel, for at least six to 12 months after DES implantation.4-6
A prolonged dual antiplatelet therapy after DES implantation may be difficult to reach in patients in whom long-term anti-vitamin K (AVK) treatment is required. This group of patients generally includes a rather old population with comorbidities and a high risk of bleeding and cardiovascular events. In fact, the risk of bleeding in patients already on AVK therapy is increased with the addition of dual antiplatelet therapy (DAT) but, on the other hand, a temporary discontinuation of anticoagulation may be associated with a higher risk of thromboembolism.7 Conversely, the use of BMS in this population may expose them to a higher risk of recurrent ischaemic events.8 Recently, a stent has been designed (Genous™ Bio-engineered R stent; OrbusNeich, Hong Kong) which incorporates endothelial progenitor cells (EPC) antibodies with covalent ligand on the surface. These antibodies may be able to capture circulating EPC and accelerate the healing process. As a consequence, this feature may have an impact in shortening the duration of DAT.9 Data from the multicentre registry and randomised trials have already shown that although having less clinical efficacy when compared to DES, EPC capture stents appear to show a low rate of stent thrombosis, despite the use of DAT for only one month.10-12
The aim of this study was to evaluate the outcomes of a medical strategy of EPC capture stent implantation followed by one month of DAT in patients under chronic AVK treatment, in whom short-term DAT may be useful.
Methods
Study population
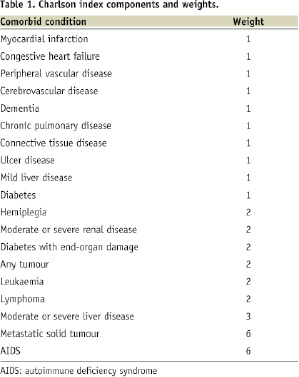
Between February 2007 and February 2008 all consecutive patients, who were on chronic anticoagulant therapy and who needed percutaneous coronary intervention (PCI), were included in the strategy of EPC capture stent implantation followed by 1-month DAT together with their AVK treatment. There were no specific exclusion criteria. Statins were also prescribed to all patients for at least one month. Those patients who discontinued AVK treatment after index PCI were excluded from the analysis. Clinical and angiographic characteristics of all patients were recorded. In order to estimate comorbidity of the patients included, the Charlson score was calculated. Overall, a value ≥ 3 defines a high level of comorbidity. Briefly the Charlson score is the sum of 19 predefined comorbidities, that were assigned weight of 1, 2, 3 or 6 (Table 1).13
All patients signed a written informed consent prior to PCI procedure and agreed to be clinically followed up. At the time of the study an IRB approval was not formally necessary for observational registries that use a CE-mark approved device. Clinical follow-up was performed by a clinical visit or telephone interview. Angiographic follow-up was not mandated unless it was clinically indicated by symptoms or by a positive non-invasive test of ischaemia.
Procedure
Stent implantation was performed according to the experience of the operator following standard guidelines. Either direct stent implantation or balloon predilatation was allowed. IIb/IIIa administration was left to the discretion of the operator. All patients were treated with a therapeutic dose of aspirin at the moment of PCI (100 mg daily from at least five days before or 500 mg administered intravenously immediately before the procedure). Clopidogrel was administered in a loading 300 mg dose directly before or immediately after the procedure. As a routine strategy in our centre, the radial approach was preferred in patients under AVK treatment. In these patients, AVK therapy was not discontinued as long as the radial artery was patent with good collateral filling of the palmar arch from the ulnar artery (tested by the Allen manoeuvre or by pletismography), and the international normalised ratio (INR) was lower than 4.0 at the time of the procedure. No additional anticoagulation was administered during PCI in these patients. In the event that the radial artery could not be used for the above-mentioned reasons, AVK treatment was discontinued and a weight-adjusted dose of low molecular weight heparin (LMWH) was substituted. Whenever INR was normal, the procedure was performed via the femoral artery. In such patients, PCI was performed under LMWH. AVK treatment was reinitiated within 24 hours after the procedure while LMWH was finally discontinued as soon as the INR level was above 2.0.
Definitions
The outcomes of the patients were assessed by measuring the rates of major adverse cardiac events (MACE) as the combination of cardiac death, myocardial infarction and target lesion revascularisation and of its individual components. Death was classified as cardiac and non-cardiac. Any death was considered cardiac unless a non-cardiac cause could be adjudicated.
Periprocedural myocardial infarction was defined as a rise in troponin T level above the upper reference limit. Acute myocardial infarction was defined as appearance of unequivocal ECG changes together with an increase in creatine-kinase levels to at least twice the upper normal limit or increase of troponin levels.
Any revascularisation performed on the treated segment was defined as target lesion revascularisation.
Stent thrombosis was defined and categorised according to Academic Research Consortium, into early (within 30 days), late (more than 30 days to one year after stent implantation) and very late (more than one year after stent implantation) and into definitive, probable and possible.14
Cerebrovascular events, included stroke (ischaemic or haemorrhagic), cerebrovascular haemorrhage, transient ischaemic attacks, and reversible ischaemic neurological deficits adjudicated by a neurologist and confirmed by computed tomography scanning.
Bleeding complications were divided into minor and major. Major bleeding was defined as the occurrence of intracranial or retroperitoneal bleeding, haemorrhage at the vascular access site requiring intervention, a reduction in haemoglobin levels of at least 5 gr/dl, reoperation for bleeding or transfusion of a blood product (at least 2U), or bleeding causing substantial hypotension requiring the use of inotropic agents. All other bleeding events were considered minor (e.g., epistaxis).15
Statistical analysis
Continuous variables are presented as mean±SD and categorical variables as percentages. Continuous variables were compared using independent sample t-test or U-Mann-Whitney test where appropriate. Categorical variables were compared with χ2-square or Fisher’s statistics test where appropriate. Cumulative survival of MACE was illustrated by the Kaplan-Meier method. We explored possible independent predictors of MACE using Cox regression modelling. The final model was obtained using a forward stepwise method with a threshold for exit set at p higher than 0.10. Only variables with p<0.2 in the univariate analyses were entered into Cox regression model. Proportional hazards assumption was tested exploring the time-dependence of covariates all at once. Statistical significance was accepted for a two-sided value of p<0.05. Analysis was performed with SPSS version 13 (SPSS Inc., Chicago, IL, USA).
Results
Baseline characteristics
During the study period, 78 consecutive patients (98 lesions) were managed by the proposed strategy of EPC capture stent implantation followed by 1-month DAT. All patients were kept on the AVK treatment during follow-up. Baseline clinical characteristics are summarised in Table 2.
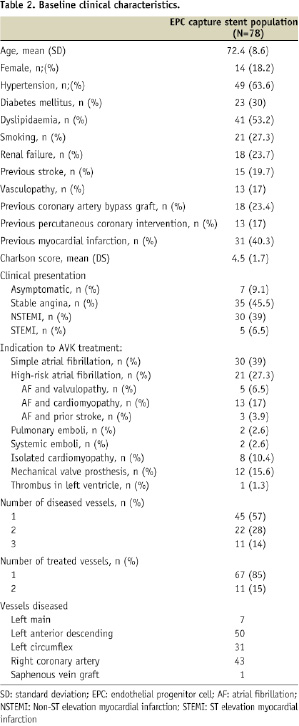
Briefly, the mean age of patients was 72-years-old; 30% of patients had diabetes and 89% of patients had a high comorbidity index, as measured by the Charlson score ≥3. All patients received the prescribed regimen of DAT (aspirin 100 mg daily and clopidogrel 75 mg daily) for one month together with the AVK treatment. Compliance to the treatment was 100%. AVK treatment was kept in the recommended range according to its clinical indication. At one month clopidogrel was discontinued while aspirin was continued lifelong and AVK treatment according to its indication.
Angiographic and procedural data
Angiographic and procedural data are summarised in Table 3.
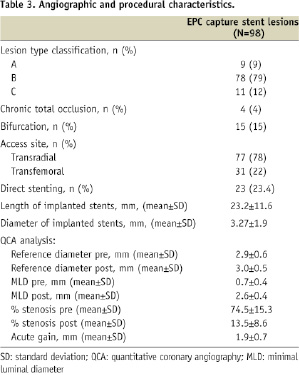
Ninety-eight lesions were treated with 122 EPC capture stent implantations (1.2 stent per lesion). Type C lesions accounted for 12%. Most of the procedures were performed by radial approach (78%) and 23.4% of stents were directly implanted. At the moment of PCI, the mean INR value in our population was 1.6±0.5.
In-hospital clinical outcomes
In-hospital outcomes are summarised in the Table 4.
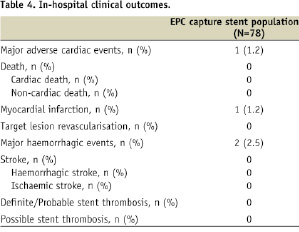
During hospital stay, no patient died. One patient suffered from a periprocedural non-Q myocardial infarction, due to an occlusion of the side branch. No cases of definitive or probable stent thrombosis were recorded. Two patients suffered from major haemorrhagic events during hospitalisation (two femoral pseudoaneurysms, requiring surgical intervention or red blood cells transfusion).
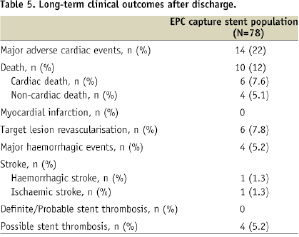
Long-term clinical outcomes
Complete follow-up was available for all patients (100%). Mean clinical follow-up was 14±8 months. Long-term outcomes are summarised in Table 5.
Kaplan Meier curve of event-free survival is shown in Figure 1A.
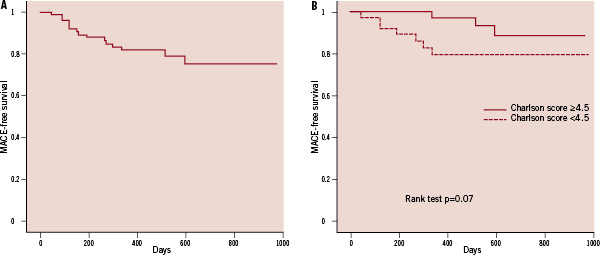
Figure 1. Panel A: MACE-free survival of all population. Panel B: MACE-free survival stratified according to Charlson score. Patients with Charlson score above median show higher MACE rate.
Not a single MACE was recorded after discharge up to first month, when the patients were on triple therapy.
Overall, a total of 10 patients (12.8%) died during clinical follow-up beyond one month. Of them, a cardiac cause was responsible of six deaths (7.6%). Sudden death accounted for four patients; end-stage cardiac failure for two patients. Target lesion revascularisation (TLR) rate was 7.8% (6.1% per lesion analysis). Four out of six TLR were due to proliferative in-stent restenosis (ISR), while the remaining two involved occlusive ISR. Late loss in these patients was 1.8±0.5, while acute gain was 1.7±0.5. All revascularisations due to ISR were performed by PCI. During follow-up, no patients suffered from myocardial infarction or definitive/probable stent thrombosis. Rate of possible stent thrombosis was 5.2%.
Four major haemorrhagic episodes were recorded: one patient died due to haemorrhagic stroke with INR higher than 9; two patients required red blood cells transfusion; one had a digestive haemorrhage. All these haemorrhagic events occurred after the first month, when the patients were only on aspirin and the AVK regimen.
No independent predictors of MACE or of its single components were identified. However, patients with a Charlson score equal or above the median value of all population showed a higher MACE rate during follow-up (Figure 1B).
Discussion
This analysis highlights that patients on chronic AVK therapy may represent a high-risk population for cardiovascular events. In general, patients on chronic anticoagulation regimen represent a highly comorbid population, as demonstrated by the high value of the Charlson score (average of 4.5), regardless of the angiographic and procedural data. Halg et al have already demonstrated that patients on chronic AVK present a worse clinical profile compared to patients receiving PCI but who are not on AVK therapy.16 Usually they are older, more often they have had previous coronary bypass surgery and have worse renal function than patients without AVK therapy. The high incidence of MACE after PCI in this category of patients is also well-known, as shown by Rogacka et al.8 Their MACE rate was similar to that observed in our population (23.6% vs. 22%), where the worst outcome was in patients with a higher Charlson comorbidity index. Ruiz-Nodar and colleagues also evidenced an even higher MACE rate (36.6%) in patients under AVK treatment receiving stent implantation.17
This high risk of developing MACE after PCI may justify the use of DES in these patients. However it is important to weigh the potential bleeding risk against the risk and consequences of restenosis: these patients have not only a high risk of ischaemic events but also a high risk of haemorrhagic complications, especially when adding dual antiplatelet therapy to their chronic AVK therapy. To date, the antiplatelet management of a patient on chronic AVK therapy receiving PCI is a field without clear evidence-based guidelines. In this regard, in many observational studies, variable antithrombotic combinations have been reported.8,17-19 In our study, we explored the long-term outcome of a strategy using a stent designed to capture circulating CD34 receptor-positive EPCs and a standardised DAT regimen for only one month. The EPC capture stent promotes vascular healing and undergoes more rapid re-endothelialisation that may allow the shorter duration of DAT therapy, and potentially a less risk of haemorrhagic events.20 The use of EPC capture stents has been demonstrated to be safe and feasible for the treatment of de novo coronary artery disease, as well as in high-risk patients.20,21 The incidence of stent thrombosis for the EPC capture stent ranges from 0.0% to 6.0%: in particular the GENIUS-STEMI study reported a rate of 6.0% of stent thrombosis in ST elevation myocardial infarction patients treated with this stent.22 On the other hand, in the Registry from Ruiz-Nodar et al17 the stent thrombosis rate in patients with atrial fibrillation requiring anticoagulation with the use of drug eluting stents was 2.8% vs. 0% with the use of bare metal stents. In a recent study, a comparison of the EPC capture stent with the Taxus Liberte stent (Boston Scientific, Natick, MA, USA), the EPC stent resulted in a higher incidence of repeated revascularisation compared to the other stent, but without a statistically significant increase in cardiac death or myocardial infarction during one year follow-up.10 In particular, the rate of TLR was higher than that found in our registry (12.3% vs. 7.8%), although our patients had a worse clinical profile. However, none of the patients in our registry developed myocardial infarction during the long-term follow-up.
On the other hand, in patients on chronic AVK regimen, one should consider the haemorrhagic complications related to DAT. The collateral risk of bleeding resides in the need of discontinuing anticoagulation and/or antiplatelet therapy which exposes these patients to ischaemic events. Halg showed that in patients on combined antithrombotic treatment with AVK regimen and antiplatelet agents, the total bleeding rate was considerably higher than in those without (OR=4.6, 95% CI 2.1-10.1), resulting mainly from late bleedings (OR=9.3, 95%CI 3.8-22.8), whereas early bleeding did not differ significantly between the two groups (OR=1.5, 95%CI 0.35-6.8).16 In our registry we found a 2.5% rate of early in-hospital major haemorrhagic events, related to the site of arterial access for the procedure. Femoral approach has already been associated with higher haemorrhagic complications in AVK patients.23 However, at long-term follow-up, we observed a 5.2% rate of haemorrhagic events after the first month of DAT on top of the AVK regimen. This rate is lower than that described other series where it ranged from 9.2% to 18%.16,19,24 Therefore, bleeding complications have to be included in the equation when deciding the type of stent to be implanted and as a rule, the safest stent in patients requiring AVK is one requiring less duration of DAT. The operator must weigh the potential bleeding risk against the risk and consequences of restenosis. In many instances, the balance between the risk and benefits of prolonged triple therapy might lead the operator to choose a BMS and commit the patient to a short course of DAT in addition to warfarin. Although regimens as short as two to four weeks have been reported to offer adequate protection from early stent thrombosis after a BMS, some specific clinical conditions (i.e., acute coronary syndromes) may lead to a prolonged DAT.6,25,26 Another alternative is to place a DES for treatment of very high-risk lesions and to accept a small-to-moderate risk of bleeding. The currently available data regarding triple therapy after DES were gathered in patients treated with first-generation paclitaxel- and sirolimus-eluting stents. Probably, second-generation DES, with thinner struts or bioresorbable polymers, could be less prone to thrombosis, present faster endothelial coverage and might potentially require shorter duration of DAT.27
In this high-risk population, in which there are no evidence-based guidelines and many different type of stents and antithrombotic regimens are being used in real-world practice, our observational registry shows that the use of the EPC capture stent combined with one month DAT together with an AVK regimen might be a useful strategy.17,19 The efficacy of the EPC-capture stent in patients receiving AVK therapy remains to be elucidated in larger randomised trials. Specifically, the future combination of this EPC capture technology with antiproliferative drugs may warrant further randomised controlled trials.
Study limitations
This is a non-randomised clinical registry, without a control group. Thus, we cannot draw firm conclusions regarding safety or efficacy of the EPC technology in patients receiving chronic AVK therapy. However, this is the first time long-term outcomes of EPC stents are described in this high-risk population. Data on circulating EPCs from each patient were not collected. Due to the period of inclusion of the historical cohort, we could not use the ESC/AHA/ACC consensus definition of myocardial infarction.28 Thus, comparison of myocardial infarction rates between groups was based on the classical WHO definition.29 This definition, however, has been historically used in most of the studies on AVK patients. Finally, due to the small sample size, we cannot rule out that the absence of stent thrombosis in our population might also be due to chance.
Acknowledgement
Dr. Salvatore Brugaletta is a recipient of an EAPCI grant in interventional cardiology.