Abstract
Background: Current guidelines recommend treating atrial fibrillation (AF) patients who undergo percutaneous coronary intervention (PCI) with triple antithrombotic therapy (TAT) for up to one month in patients at high thrombotic risk. It is unclear how to select these high-risk patients.
Aims: The aim of this study was to identify patients at high thrombotic risk who might benefit from TAT over double antithrombotic therapy (DAT).
Methods: This study was a post hoc subanalysis of the RE-DUAL PCI trial. A Cox proportional hazards model was built by stepwise selection of plausible predictor variables for a composite ischaemic endpoint, defined as cardiovascular death, myocardial infarction (MI), stent thrombosis (ST) or ischaemic stroke. The effect of TAT versus DAT was calculated for those patients with the highest proportion of predicted thrombotic risk. A simplified risk score was constructed based on beta-coefficients.
Results: For 209 patients (7.7%) the composite ischaemic endpoint occurred during the first year. The simplified risk score contained six variables. In patients with a score ≥5 (n=154, 5.7%), a significant reduction in the composite of MI and ST was observed with TAT versus DAT (6.3% vs 21.0%, p=0.041), without a penalty in terms of bleeding. In patients at low thrombotic risk, a significant increase in bleeding was observed without a reduction of ischaemic events.
Conclusions: Our findings support the use of DAT in the majority of patients. A small subgroup of patients might benefit from TAT and we propose a novel clinical risk score to select these patients.
Introduction
Antithrombotic regimens in patients with atrial fibrillation (AF) undergoing percutaneous coronary intervention (PCI) represent one of the most challenging topics for cardiologists in daily practice. In AF patients with a CHA2DS2-VASc score ≥1 in males and ≥2 in females, oral anticoagulation (OAC) is warranted to reduce the risk of systemic thromboembolic events, including stroke, whereas patients undergoing PCI have an indication for dual antiplatelet therapy, consisting of aspirin and a P2Y12 inhibitor, in order to reduce the risk of stent thrombosis (ST) or other recurrent atherothrombotic events123. Approximately one in five patients with AF undergo PCI at some point, illustrating the relevant overlap in clinical practice4. From the PCI perspective, one in twelve patients undergoing coronary stenting has concomitant AF and an indication for OAC3. The combination of dual antiplatelet therapy and OAC is referred to as triple antithrombotic therapy (TAT). A drawback of TAT is that it confers at least a two-times higher risk of bleeding as compared to double antithrombotic therapy (DAT), i.e., with the omission of aspirin56.
Current international guidelines and consensus documents recommend TAT for one week and up to one month in patients at high thrombotic risk278. To date, it is unclear how to select patients at high thrombotic risk.
Five randomised controlled trials compared TAT with the combination of (N)OAC and an antiplatelet agent. The WOEST study was the first to investigate a regime of omitting aspirin in anticoagulated patients undergoing PCI9. The WOEST study showed that treatment with a vitamin K antagonist (VKA) and the P2Y12 inhibitor clopidogrel (DAT) was associated with a reduction of bleeding without an increase in ischaemic events, compared to patients treated with TAT. Four more recent studies, which used the same approach of TAT versus DAT with non-vitamin K oral anticoagulants (NOAC), did not show any differences in ischaemic outcomes, whereas all but the ENTRUST-AF PCI study showed a reduction of bleeding complications in patients treated with DAT10111213.
Although no differences in ischaemic outcomes were observed in the individual trials, it must be noted that all studies were largely underpowered for thromboembolic endpoints and the trials included mostly low-risk patients, with a small proportion of patients with acute coronary syndrome (ACS)1415. Some meta-analyses suggested a significant but small increase of myocardial infarction (MI) and ST in patients treated with DAT1416. A subanalysis of the AUGUSTUS trial pointed to a trade-off in ischaemic versus bleeding risks. A significant reduction in ischaemic events was observed when TAT was used in the first month after PCI, but at the equal cost of bleeding17. After 30 days, TAT continued to increase bleeding without significantly reducing ischaemic events. The authors propose a patient-centric decision-making strategy for the use of TAT. The recent meta-analysis by Gargiulo et al14 supports this concept of a personalised strategy. The authors found evidence for a subgroup of patients who had a net benefit from TAT versus DAT in favour of reducing ischaemic events; however, they could not provide tools for the identification of this subgroup of patients, nor do international guidelines provide specific guidance for patient selection.
In this study, we sought to find subgroups of patients at high thrombotic risk, and to develop a risk score to identify the high-risk patients who might benefit from TAT.
Methods
Patient cohorts
This study was a post hoc subanalysis of the RE-DUAL PCI trial. The study protocol for this trial has been previously published18. In short, in the RE-DUAL PCI trial, the DAT group was treated with dabigatran, 110 mg b.i.d. or 150 mg b.i.d., in combination with a P2Y12 inhibitor (actual treatment: clopidogrel in 87% and ticagrelor in 12% of patients). The TAT group consisted of VKA, aspirin and a P2Y12 inhibitor (actual treatment: clopidogrel in 90% and ticagrelor in 8% of patients). TAT was given for three months in patients undergoing PCI with drug-eluting stents (DES) and for one month in patients receiving a bare metal stent (BMS). The study received the proper ethical oversight; the study protocol and any amendments were approved by the ethics committee at each participating centre.
Ischaemic and bleeding endpoints
The composite ischaemic endpoint was defined as cardiovascular death, MI, ST (definite or probable according to Bleeding Academic Research Consortium [BARC] criteria) or ischaemic stroke. The bleeding endpoint was defined as the first BARC 2, 3 or 5 bleeding within 365 days. Also, separate components of these endpoints were explored. For the purpose of a sensitivity analysis, follow-up was truncated after the first event (either ischaemic or bleeding event).
Follow-up
The mean follow-up after PCI was 14 months. For this analysis follow-up was truncated at 365 days to obtain risk estimates for the first year of DAT versus TAT. Groups were compared according to the intention-to-treat principle.
Statistical analysis
Baseline characteristics were compared between patients with and without ischaemic events during the one-year follow-up by t-tests or chi-square tests, or their non-parametric equivalents, as appropriate.
Predictors
Based on clinical plausibility and availability in both trial datasets, variables were considered as candidate predictors. The variables included age, sex, body mass index, hypertension, hypercholesterolaemia, diabetes mellitus including subgroups of insulin dependent diabetes, smoking, alcohol use, medical history (bleeding, MI, PCI, coronary bypass artery grafting, stroke, venous thrombo-embolisms or systemic embolism, renal failure, malignancy, peripheral artery disease [with intervention, or ankle-brachial index <90], heart failure), MI at presentation, left ventricular ejection fraction (LVEF), laboratory tests at presentation (haemoglobin, haematocrit, platelet count, leukocyte count, creatinine, estimated glomerular filtration rate by Chronic Kidney Disease Epidemiology Collaboration [CKD-EPI]), and angiographic and procedural characteristics (number of diseased vessels [>50% diameter stenosis], left main disease, thrombus-containing lesion, number of stented vessels, stented vessel/graft, bifurcation PCI, lesion length >30 mm and in-stent restenosis stenting). We did not include modifiable factors like medication use, periprocedural heparin use, factors of which reporting might be unreliable like New York Heart Association classification, and factors with uncertain causal relation like index electrocardiogram (ECG) rhythm and type of atrial fibrillation.
Model development
Since we wanted to characterise the patients’ thrombotic risk based on their single clinical features, irrespective of receiving DAT or TAT, individual candidate variables for the model were selected from a univariate Cox regression, stratified for randomisation arm and BMS placement (the latter directly influenced aspirin treatment duration). Variables showing a p-value <0.30 for the ischaemic events model were considered as candidate variables. A Cox model for the composite ischaemic endpoint was constructed with forced entry of the stratification variables. Stepwise selection from the candidate variables using a 0.05 significance level was performed. Missing values in the dataset for stepwise model selection were imputed by simple means (Supplementary Table 1). Based on the beta coefficients of the Cox model, a point score was constructed using the methods as proposed in the Framingham Study risk scores19. Continuous variables were at that point categorised, based on clinically relevant categories. After dichotomisation, the least prevalent category would be retained in the model.
Validation
With the Cox model, the expected hazard of the ischaemic endpoint, given the covariates, was predicted at 365 days. Also, the point score was calculated for each patient. Predictive accuracy of the model and point score were evaluated by the area under the receiver operating curve (C-statistic). The cohort was divided into quintiles, deciles and demi-deciles based on the expected hazards, or grouped by point score to assess calibration. Also, goodness-of-fit was assessed by the Hosmer-Lemeshow test.
Observed risks within the different risk categories and risk scores of ischaemic events, bleeding, and mortality were compared between the patients that received TAT or DAT, to evaluate if a benefit of TAT over DAT could be found in patients at higher thrombotic risk.
To compare the performance of the new risk score in relation to existing risk scores, an assessment of the C-statistic of the CHA2DS2-VASc for the ischaemic endpoint in the RE-DUAL PCI cohort was performed.
External validation was performed in the WOEST 2 registry (ClinicalTrials.gov: NCT02635230). The (as yet unpublished) WOEST 2 registry is a cohort of 1,059 patients treated with OAC undergoing PCI. Baseline characteristics of this cohort are summarised in Supplementary Table 2. Differences in outcomes between TAT and DAT cohorts were not evaluated, as an inherent indication bias exists between the groups due to the observational design of this cohort.
Results
A total of 2,725 patients were included in this analysis. In 209 patients (7.7%) the composite ischaemic endpoint occurred during the first year. Baseline characteristics of patients with and without ischaemic events are depicted in Table 1. Approximately half of the cohort (50.5%) underwent PCI for the indication of ACS. Patients with an ischaemic event during follow-up were more likely to have a medical history of MI, heart failure, diabetes, renal failure, peripheral artery disease or prior stroke and were more likely to have presented with ACS. LVEF was lower in patients with an ischaemic event during follow-up and multivessel disease was more prevalent. Furthermore, there were significant differences in haemoglobin levels, white blood cell counts, platelet counts and creatinin clearance. BARC 2, 3 or 5 bleedings were documented in 520 patients (19.1%).
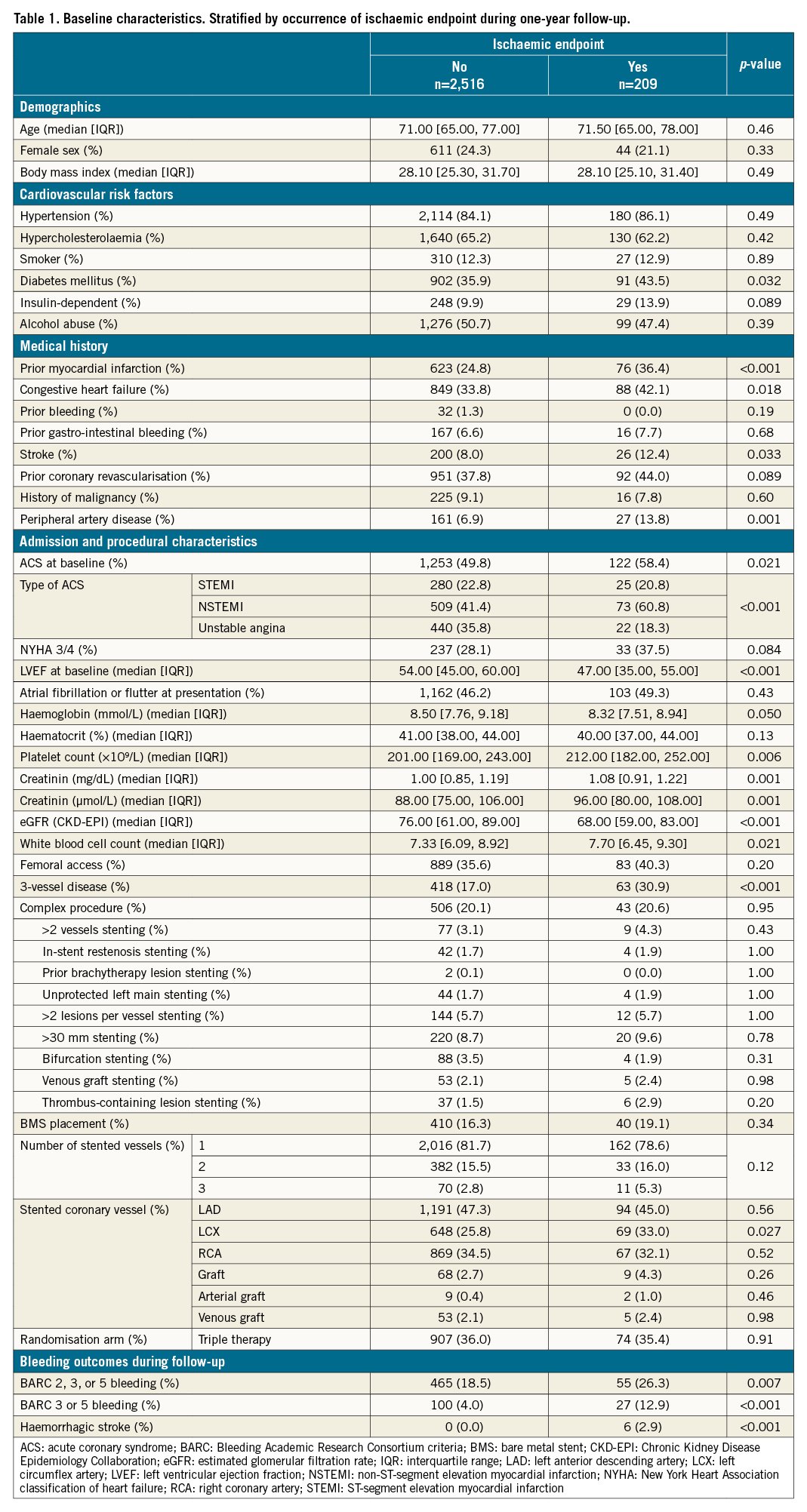
Table 2 shows results of the stratified univariate Cox regression. Strong predictors for ischaemic events were: decreased LVEF, multivessel disease or multivessel stenting, MI, diabetes mellitus, peripheral artery disease or stroke and a history of heart failure or renal failure.
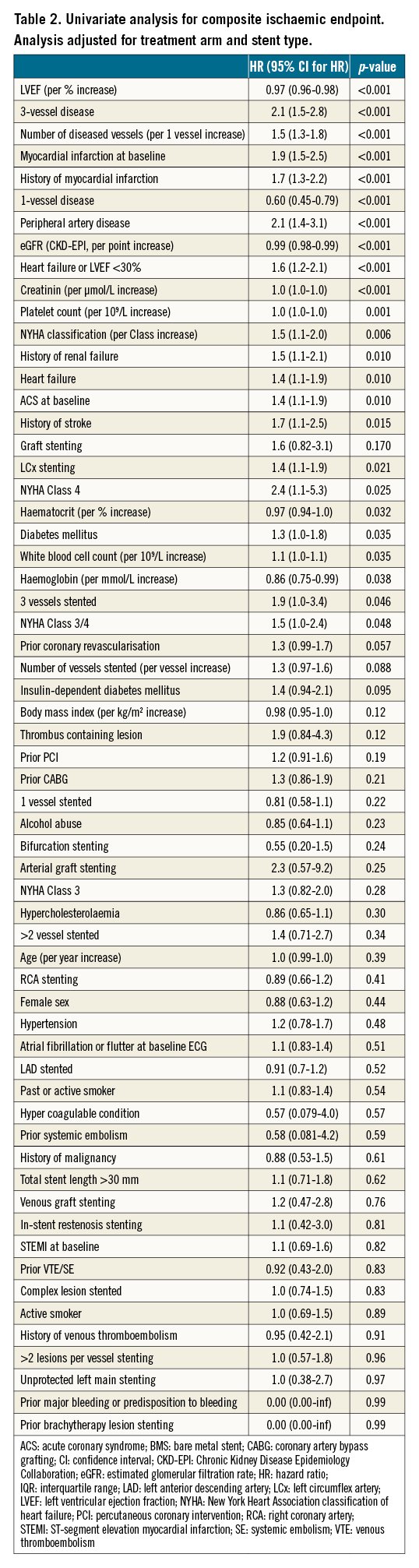
After the stepwise selection, the multivariable Cox regression model predicting ischaemic events contained the LVEF, number of diseased vessels, MI as an indication for index-PCI, platelet count, peripheral artery disease and creatinine clearance (Table 3). The discriminatory capacity of the ischaemic model was fair (C-statistic 0.68, 95% CI: 0.64-0.72) (Supplementary Figure 1).
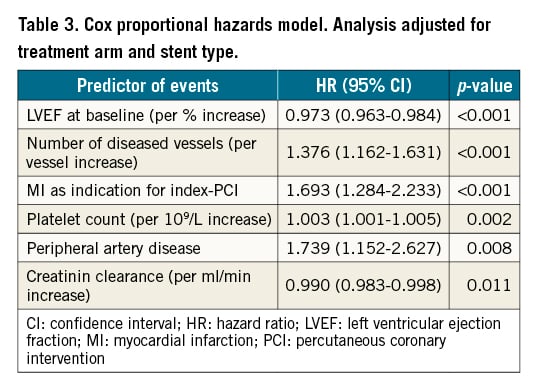
Observed ischaemic and bleeding outcomes and mortality rates by percentiles and quintiles for predicted hazards are depicted in Figure 1 and Supplementary Table 3. Goodness-of-fit as assessed by the Hosmer-Lemeshow test was found to be good (p=1.00). Incidence of ischaemic events ranged from 3.9% for the lowest quintile of thrombotic risk to 15.8% for the highest risk quintile. In comparing high-risk patients to low-intermediate risk patients, significantly more thromboembolic events were found in the high-risk patients (15.8% vs 5.6%; p<0.001) (Supplementary Table 3).
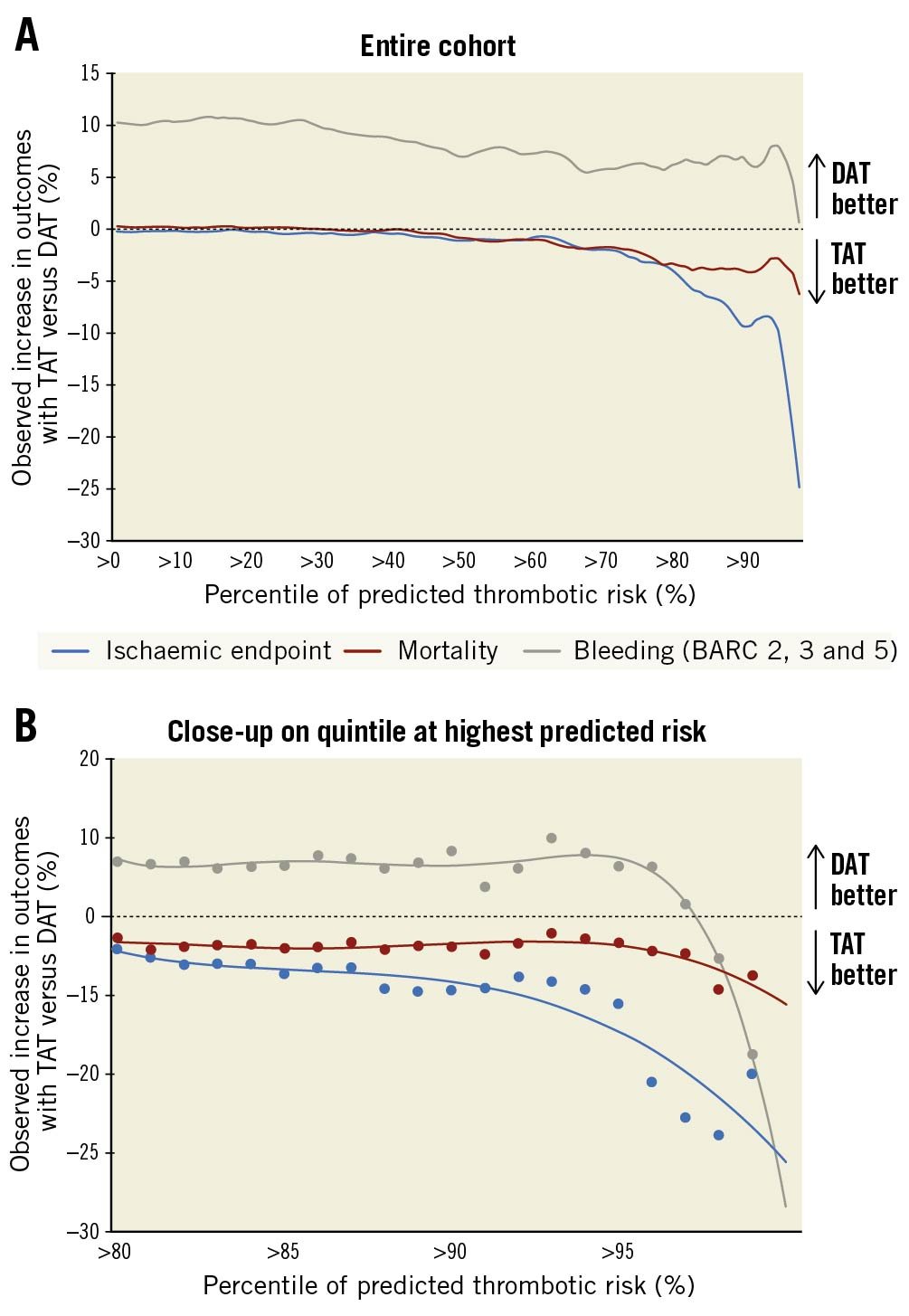
Figure 1. Treatment effect of TAT versus DAT on ischaemic endpoint, mortality, and bleeding per percentile of predicted thrombotic risk. Farther to the right = higher thrombotic risk and smaller fraction of patients. A) In the entire cohort. B) Close-up on quintile at highest predicted risk. BARC: Bleeding Academic Research Consortium criteria; DAT: double antithrombotic therapy; TAT: triple antithrombotic therapy
A numerical reduction of the ischaemic endpoint was observed in patients at the highest thrombotic risk treated with TAT as compared to DAT (Figure 1A). This effect was most pronounced in patients in the >95th percentile of thrombotic risk (Figure 1B).
The simplified risk score based on the Cox regression model contained six variables (Central illustration) and retained fair accuracy comparable to the original Cox regression model (C-statistic 0.66, 95% CI: 0.62-0.70) (Supplementary Figure 2). The total risk score ranged from one point to eight points. Higher scores were associated with a higher risk of ischaemic events (ptrend<0.001). The calibration curve demonstrated excellent calibration for the composite ischaemic endpoint (Supplementary Figure 3).
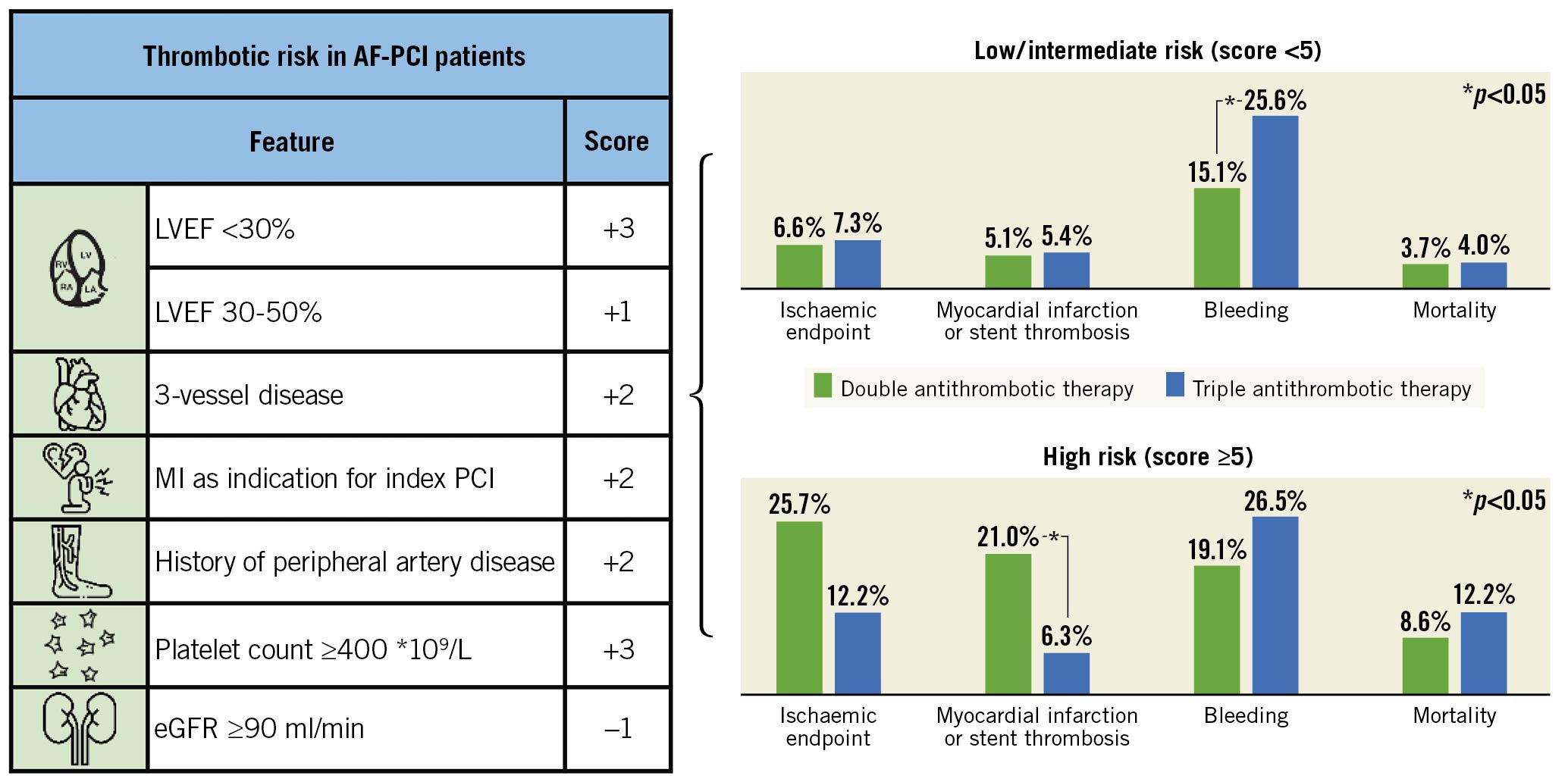
Central illustration. Score for thrombotic risk in AF-PCI patients. The score contains six clinical variables to predict ischaemic events after PCI in patients with AF (LVEF, three-vessel disease, MI as indication for index PCI, history of peripheral artery disease, platelet count ≥400 *109/L and eGFR ≥90 ml/min). In patients with low and intermediate risk (score <5) the use of TAT was significantly associated with higher bleeding rates than DAT (25.6% vs 15.1%, p<0.001) without benefits regarding ischaemic endpoints. In patients with high risk (score ≥5) the use of TAT was associated with significantly less myocardial infarction and stent thrombosis (6.3% vs 21.0%, p=0.041). Thus, the score identifies a minority of high-risk patients that might benefit from TAT after PCI. AF: atrial fibrillation; DAT: double antithrombotic therapy; eGFR: estimated glomerular filtration rate; LVEF: left ventricular ejection fraction; MI: myocardial infarction; PCI: percutaneous coronary intervention; TAT: triple antithrombotic therapy
When using a >5 point cut-off, a trend towards a reduction of the composite ischaemic endpoint in patients treated with TAT as compared to DAT was observed (p=0.092) (Figure 2A). A significant reduction of MI or ST was also observed (6.3% vs 21.0%, p=0.041) (Figure 2B). When considering other outcomes, bleeding events outnumbered ischaemic events across all risk categories, with a significant increase in patients using TAT as compared with DAT in patients with low thrombotic risk (Figure 2C, Supplementary Figure 4).
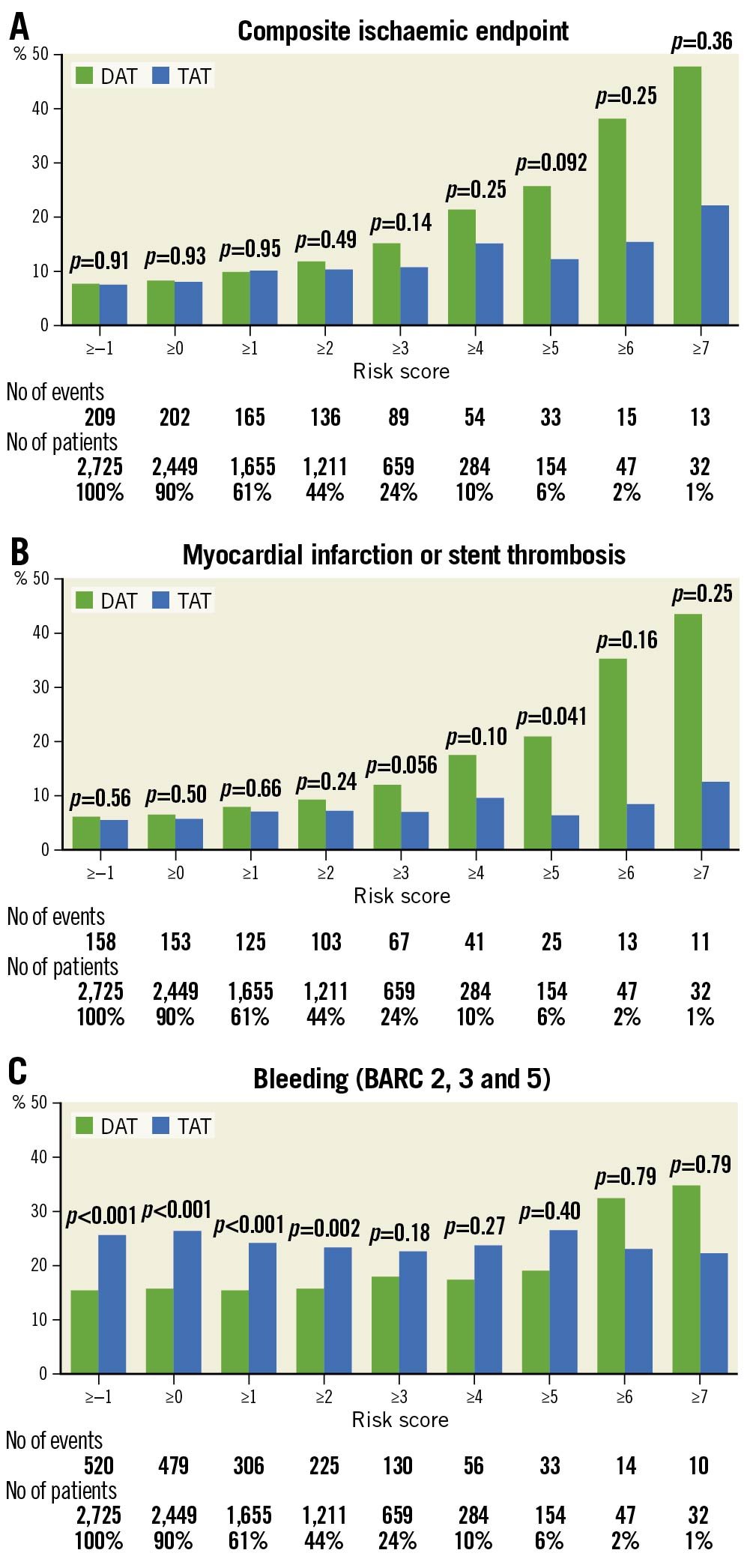
Figure 2. Observed outcomes with DAT and TAT for different risk score cut-offs. A) Composite ischaemic endpoint. B) Myocardial infarction or stent thrombosis. C) Bleeding. BARC: bleeding academic research consortium criteria; DAT: double antithrombotic therapy; TAT: triple antithrombotic therapy
External validation
The risk score was externally validated in the WOEST 2 registry. Discriminative capacity was similar to the internal validation (C-statistic 0.63, 95% CI: 0.56-0.70) (Supplementary Figure 5).
The score had an excellent overall ability to identify high-risk patients, with higher scores corresponding to a higher risk of ischaemic endpoints (ptrend<0.001) (Figure 3). A score ≥5 identified 11.8% of all patients at high thrombotic risk. Thrombotic risk in these high-risk patients was significantly higher as compared to the remainder of the cohort (16.3% vs 6.7%, p=0.001).
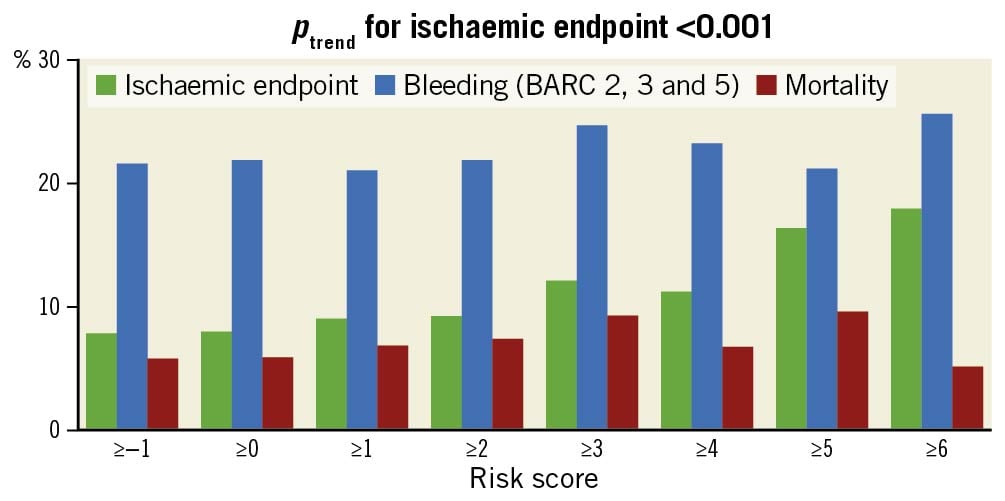
Figure 3. Outcomes for different risk score cut-offs in the external validation cohort (WOEST 2 registry). BARC: bleeding academic research consortium
Duration of triple therapy
To study the timing of ischaemic events, a Kaplan-Meier curve was constructed (Supplementary Figure 6) with separate outcomes for high-risk patients (risk score ≥5) and patients not at high risk (risk score <5). Interestingly, the curves of high-risk patients continued to diverge beyond the first month, up to 90 days after PCI. Although this study cannot provide a definitive answer to the question of optimal treatment duration when TAT is given, this observation suggests that a substantial proportion of events continue to take place after the first month in patients treated with DAT but not in those treated with TAT. Therefore, when TAT is prescribed in patients at high thrombotic risk and is well tolerated by the patient in the first month, continuing TAT up to three months might be considered.
Performance of the CHA2DS2-VASc score
When applying the CHA2DS2-VASc in the RE-DUAL PCI cohort, it showed only modest accuracy for the composite ischaemic endpoint (C-statistic 0.58, 95% CI: 0.54-0.62) (Supplementary Figure 7).
Discussion
This study sought to identify AF patients undergoing PCI at high risk for recurrent ischaemic events who might benefit from TAT. The model was able to identify patients with high thrombotic risk. A simplified risk score found a significant reduction in MI/ST with TAT in patients with a risk score >5. However, this subgroup of “high-risk” patients comprised only ~5% of all patients. In the majority of patients, no benefit with TAT was observed. Importantly, the lower incidence of ischaemic events with TAT as compared to DAT was outnumbered by an increase in bleeding events in the overall population, but not in patients at the highest thrombotic risk.
The observation of a possible reduction in ischaemic events in high-risk patients is in line with some meta-analyses and subgroup analyses which pointed to a possible benefit of TAT, especially in high-risk patients. Two meta-analyses of randomised controlled trials signalled a reduction in terms of ST (and a trend for MI) associated with TAT1416. Although incidence rates were very low, ST was significantly reduced. Other meta-analyses did not support this finding52021.
Our study is the first study to investigate the effect of TAT in patients at high thrombotic risk, represented by a combination of high-risk characteristics. Several subgroup analyses of the randomised controlled trials based on single clinical variables (e.g., diabetes, age ≥80 years, ACS patients) could not demonstrate a reduction in ischaemic events associated with TAT222324. This illustrates the complex and multifactorial aspect of high thrombotic risk, which was adequately addressed in the current study by combining multiple patients' characteristics.
Further randomised controlled studies will be needed to adequately address the question of TAT versus DAT, particularly in high-risk patients. Of note, the MASTER DAPT trial enrolled 4,434 patients with high bleeding risk, of whom 1,666 used OAC. The subgroup using OAC was randomised to a duration of one month of TAT versus at least three months of TAT before switching to DAT. The shorter duration of TAT had a lower risk of bleeding without an additional ischaemic risk25. Similar results were observed in a post hoc analysis of the AUGUSTUS trial17. This demonstrates that, if TAT is prescribed, it should be limited to one month.
Clinical implications
Using this risk score containing seven clinical, angiographical and procedural parameters, a significant reduction in MI/ST associated with TAT was seen in a small subgroup of patients at high thrombotic risk undergoing PCI. Our findings are an important “proof of concept”, which is in line with the general beliefs of many cardiologists with regard to high-risk patients.
On the other hand, for the majority of the population, no benefit of TAT was found - and it could even harm patients at low thrombotic risk, in whom only an increase in bleeding was observed. Therefore, our findings support the utilisation of DAT rather than TAT in the majority of AF patients undergoing PCI, while reserving TAT for a small proportion of patients – as was adapted in the most recent ESC guideline on non-ST-segment elevation myocardial infarction (NSTEMI)7. If TAT is prescribed, it should be limited to 30 days, as its antithrombotic effect shows no clear value beyond 30 days post-PCI1725.
Limitations
The TAT arm in this study comprised vitamin K antagonists whereas in contemporary clinical practice NOACs are standard care for their favourable safety profile. In the RE-DUAL PCI trial, DAT including a NOAC was compared to TAT using a VKA, which might have exaggerated the bleeding risk in the latter group.
We did adjust the analyses for the duration of TAT, which was directly related to the use of BMS (one month) or DES (three months). However, we did not evaluate the effect of different dabigatran dosages (110 mg b.i.d. or 150 mg b.i.d.). Generally, as per international guidelines, the lowest approved dose should be applied in this population with multiple antithrombotic agents.
Although an overall increase of bleeding associated with TAT was observed, numerically fewer bleeding events were observed in patients with a risk score of ≥5. This is an interesting observation that could be due to greater degrees of thrombin activation or might be a play of chance due to small patient numbers. However, a similar asymmetrical treatment effect was observed in the PRECISE-DAPT bleeding score, in whom patients at high bleeding risk no longer had a benefit in terms of reduction in ischaemic events.
The performance of the score was good in the WOEST 2 registry which served as an external validation cohort. Although the C-statistic of 0.63 was modest (and typical of risk scores based on clinical factors), this applies to the overall fit of the risk score and does not necessarily correspond to the ability of the score to identify the patients at the highest risk. Finally, differences between DAT and TAT could not be tested, due to the indication bias inherent in the design of an observational registry. Before adapting this novel risk score into daily clinical practice, further external validation in randomised controlled trials such as the AUGUSTUS, PIONEER AF-PCI, or ENTRUST PCI is needed.
Conclusions
A clinical risk score was developed to estimate thrombotic risk in AF patients undergoing PCI. The model identified a small subgroup of high-risk patients comprising ~5% of all patients, in whom a significant reduction in MI/ST was observed with TAT. For patients not at high thrombotic risk, no benefit was observed and even harm was found.
Our findings support the use of DAT in the majority of AF patients undergoing PCI, while reserving TAT for a small and selected subgroup of high-risk patients. We propose a risk score which might aid in identifying these patients.
Impact on daily practice
The majority of AF patients undergoing PCI do not benefit from TAT. However, we identified a small subset (~5%) of patients at high thrombotic risk in whom a significant reduction in myocardial infarction and stent thrombosis was observed with TAT. A risk score is proposed which might aid in identifying these patients.
Funding
The RE-DUAL PCI trial was funded by Boehringer Ingelheim.
Conflict of interest statement
B. Zwart reports having received consultancy/speakers’ fees from Bayer, AstraZeneca and Sanofi. A.J.W.M. de Veer reports advisory/consulting/speakers’ fees from Bayer and Boehringer Ingelheim. G.Y.H. Lip reports consultancy fees for Bayer/Janssen, Boehringer Ingelheim, Bristol-Myers Squibb/Pfizer, Daiichi-Sankyo and Verseon; speakers’ fees from Boehringer Ingelheim, Bristol-Myers Squibb/Pfizer and Daiichi-Sankyo although no fees are directly received personally. D.L. Bhatt serves on the advisory boards of Cardax, CellProthera, Cereno Scientific, Elsevier Practice Update Cardiology, Level Ex, Medscape Cardiology, MyoKardia, PhaseBio, PLx Pharma, and Regado Biosciences; serves on the Board of Directors of the Boston VA Research Institute, the Society of Cardiovascular Patient Care, and Tobesoft; is the Chair of the American Heart Association Quality Oversight Committee; serves on the Data Monitoring Committees of the Baim Institute for Clinical Research, the Cleveland Clinic, Contego Medical, Duke Clinical Research Institute, the Mayo Clinic, Mount Sinai School of Medicine, and the Population Health Research Institute; has received honoraria from the American College of Cardiology, the Baim Institute for Clinical Research, Belvoir Publications, the Canadian Medical and Surgical Knowledge Translation Research Group, Duke Clinical Research Institute, HMP Global, Journal of the American College of Cardiology, K2P, Level Ex, Medtelligence/ReachMD, MJH Life Sciences, Population Health Research Institute, Slack Publications, the Society of Cardiovascular Patient Care, and WebMD; has other affiliations with Clinical Cardiology, the NCDR-ACTION Registry Steering Committee, and the VA CART Research and Publications Committee; has received research funding from Abbott, Afimmune, Amarin, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Cardax, Chiesi, CSL Behring, Eisai, Ethicon, Ferring Pharmaceuticals, Forest Laboratories, Fractyl, Idorsia, Ironwood, Ischemix, Lexicon, Lilly, Medtronic, MyoKardia, Pfizer, PhaseBio, PLx Pharma, Regeneron, Roche, Sanofi, Synaptic, and The Medicines Company; has received royalties from Elsevier; is a Site Co-Investigator for Biotronik, Boston Scientific, CSI, St. Jude Medical, and Svelte; is a Trustee of the American College of Cardiology; and has done unfunded research for FlowCo, Merck, Novo Nordisk, and Takeda. C.P. Cannon reports research grants and personal fees from Amgen, Applied Therapeutics, Ascendia, Better Therapeutics, Boehringer-Ingelheim, Bristol-Myers Squibb, Corvidia, Daiichi Sankyo, Eli Lilly, HLS Therapeutics, Innovent, Kowa, Lexicon, Janssen, Merck, Pfizer, Rhoshan, and Sanofi; consulting fees from Aegerion/Amryt, Alnylam, Amarin, Amgen, and grants from NovoNordisk, outside the submitted work. J.M. ten Berg reports having received research grants and personal fees from, Accumetrics, AstraZeneca, Boehringer-Ingelheim, Bayer, Bristol-Myers Squibb, CeleCor, Daiichi Sankyo, Eli Lilly, Ferrer, The Medicines Company, Pfizer and ZonMw. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

