Abstract
BACKGROUND: The Atrial Fibrillation and Ischemic Events with Rivaroxaban in Patients with Stable Coronary Artery Disease (AFIRE) trial demonstrated non-inferior efficacy endpoints for rivaroxaban monotherapy versus combination therapy (rivaroxaban plus a single antiplatelet) and superior safety endpoints in patients with atrial fibrillation and stable coronary artery disease.
AIMS: This post hoc analysis investigated whether the AFIRE trial results reflected the presence or absence of prior revascularisation.
METHODS: Among 2,215 patients, 1,697 (76.6%) had previously undergone revascularisation, and the remaining 518 (23.4%) had not undergone prior revascularisation. The primary efficacy endpoint was a composite of stroke, systemic embolism, myocardial infarction, unstable angina requiring revascularisation, or death from any cause, while the primary safety endpoint was major bleeding.
RESULTS: In 1,697 patients with prior revascularisation, the efficacy and safety endpoints were superior for monotherapy versus combination therapy (efficacy: hazard ratio [HR] 0.62, 95% confidence interval [CI]: 0.45-0.85; p=0.003; safety: HR 0.62, 95% CI: 0.39-0.98; p=0.042). Among 518 without prior revascularisation, there were no significant differences in endpoints (efficacy: HR 1.19, 95% CI: 0.67-2.12; p=0.554; safety: HR 0.47, 95% CI: 0.18-1.26; p=0.134). There was borderline interaction of the efficacy endpoints (p=0.055) between two treatments. The safety benefit of monotherapy on any bleeding was significant in patients without prior revascularisation (HR 0.59, 95% CI: 0.38-0.93; p=0.022).
CONCLUSIONS: In high-risk thrombosis patients with a history of prior revascularisation, rivaroxaban monotherapy versus combination therapy demonstrated favourable safety and efficacy outcomes.
Oral anticoagulation is considered essential for patients with atrial fibrillation (AF), which is a risk factor for thromboembolic events1234, whereas antiplatelet agents are considered the cornerstone of treatment for patients with stable coronary artery disease (CAD)567. Therefore, patients with AF and CAD often require combination antithrombotic therapy, which increases their risk of fatal and non-fatal bleeding and death. The Atrial Fibrillation and Ischemic Events with Rivaroxaban in Patients with Stable Coronary Artery Disease (AFIRE) trial demonstrated remarkable evidence that rivaroxaban monotherapy was non-inferior to combination therapy with rivaroxaban plus a single antiplatelet agent for composite major adverse cardiovascular and cerebral events. Additionally, rivaroxaban monotherapy was superior for major bleeding in patients with AF and stable CAD at 1 year after revascularisation (prior percutaneous coronary intervention [PCI] or coronary artery bypass grafting [CABG]) and in those with angiographically confirmed CAD not requiring revascularisation8.
However, it is unclear whether the results of the AFIRE trial would remain consistent regardless of whether a patient has had a prior revascularisation procedure (PCI or CABG) or not.
Methods
STUDY POPULATION AND TRIAL DESIGN
The AFIRE trial was a multicentre, randomised, open-label, parallel-group trial9. Full details, including the trial design and results of the primary analysis, have been described previously8. In brief, men and women who were ≥20 years of age and had received a diagnosis of AF with a CHADS2 score ≥1 and stable CAD were enrolled. The eligible patients fulfilled one of the following criteria: (i) angioplasty with or without stenting at least 1 year before enrolment; (ii) evidence of ≥50% coronary stenosis, as detected by coronary computed tomography or coronary angiography; or (iii) a history of undergoing CABG at least 1 year before enrolment. The main exclusion criteria were a history of stent thrombosis, coexistent active tumours, and poorly controlled hypertension. The trial was designed and conducted by an executive steering committee9. The study was conducted in accordance with the principles of the Declaration of Helsinki and was approved by the institutional review board of the National Cerebral and Cardiovascular Center, Japan, as well as the institutional review boards of all participating institutions. An independent data and safety monitoring committee reviewed all data, and all participating patients provided written informed consent. Under the guidance of the authors, Mebix, a contract research organisation, assisted in the selection, supervision, and monitoring of the participating centres; the collection, storage, and analysis of the trial data; the interpretation of trial results; and preparation of the manuscript.
A total of 2,240 patients were enrolled; 2,236 were randomly assigned in a 1:1 ratio to receive monotherapy with rivaroxaban (10 mg once daily for patients with a creatinine clearance of 15-49 ml/min or 15 mg once daily for patients with a creatinine clearance of ≥50 ml/min) or combination therapy with rivaroxaban at the previously stated doses plus an antiplatelet agent (aspirin or a P2Y12 inhibitor) according to the treating physician’s discretion.
In the present post hoc subanalysis of 2,215 patients (1,697 who had undergone prior revascularisation, 518 who had not undergone prior revascularisation) in the modified intention-to-treat population in the AFIRE trial, we analysed the outcomes according to the presence or absence of prior revascularisation (Figure 1).
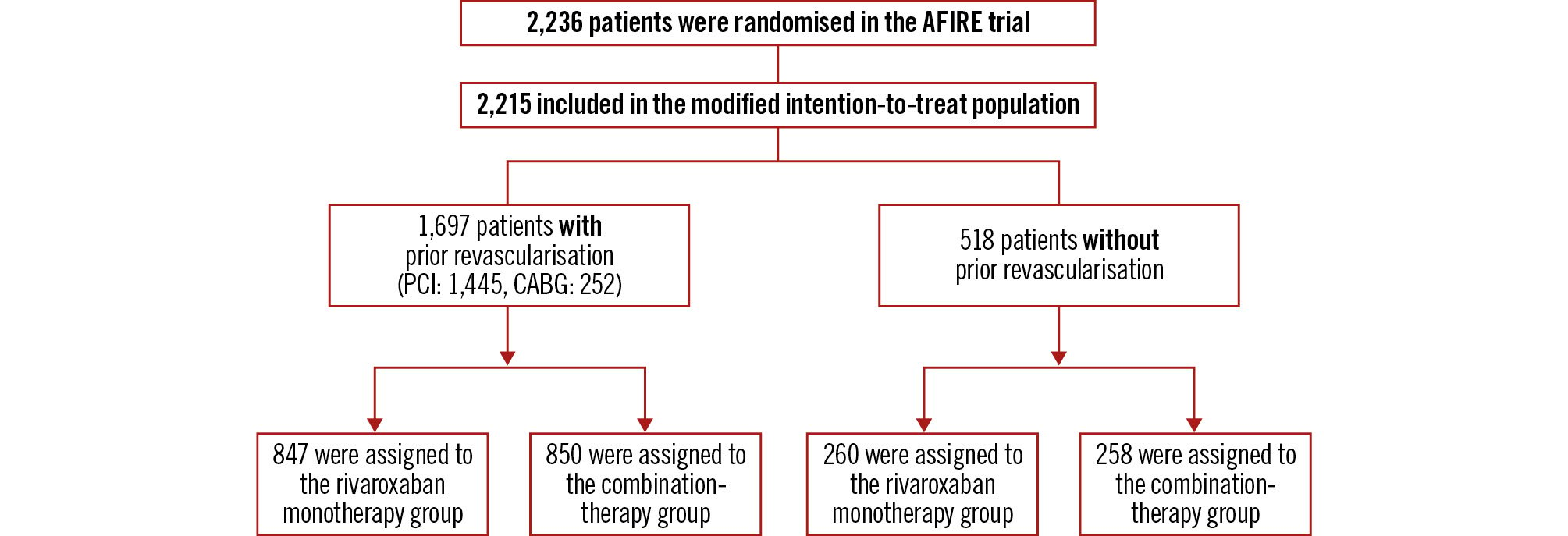
Figure 1. Patient flow of the subanalysis population. AFIRE: Atrial Fibrillation and Ischemic Events with Rivaroxaban in Patients with Stable Coronary Artery Disease; CABG: coronary artery bypass grafting; PCI: percutaneous coronary intervention
OUTCOMES
The primary efficacy endpoint was a composite of stroke, systemic embolism, myocardial infarction, unstable angina requiring revascularisation, or death from any cause. The primary safety endpoint was major bleeding, as defined by the International Society on Thrombosis and Haemostasis criteria. The individual components of the efficacy and safety endpoints were analysed as secondary endpoints. Net adverse clinical events (NACE) were defined as a composite of death from any cause, myocardial infarction, stroke, or major bleeding.
STATISTICAL ANALYSIS
Continuous variables are presented as a mean±standard deviation or median (interquartile range) as appropriate, while categorical variables are presented as count (percentage). Cumulative event rates were estimated using the Kaplan-Meier method, with incidence rates in each treatment arm shown as percentages per patient-year. A Cox proportional hazards model was used to compare the outcomes between monotherapy with rivaroxaban and combination therapy with rivaroxaban and an antiplatelet agent, with the results expressed as hazard ratios (HR) and 95% confidence intervals (CI). The latter were not adjusted for multiple comparisons; therefore, the inferences drawn from these intervals may not be reproducible. Randomisation in the AFIRE trial was adjusted using a minimisation method for age, sex, history of PCI, cardiac insufficiency, heart failure, hypertension, diabetes mellitus, and stroke. All statistical analyses were performed using SAS software version 9.4 for Windows (SAS Institute). All reported p-values were 2-tailed, and those <0.05 were considered statistically significant.
Results
BASELINE CHARACTERISTICS
Among the 2,215 patients in the modified intention-to-treat population, 1,697 had a history of revascularisation (1,445 with PCI, 252 with CABG). The remaining 518 patients had no history of revascularisation. Table 1 shows the patient baseline characteristics by treatment groups (rivaroxaban monotherapy and combination therapy) and the presence or absence of a history of revascularisation. There were no significant intergroup differences in the baseline characteristics of patients with a history of previous revascularisation. The mean CHADS2, CHA2DS2-VASc, and HAS-BLED scores were 2.5, 4.1, and 2.2, respectively. The prevalence of previous myocardial infarction was approximately 40%. In the combination therapy group, 68.8% of patients were treated with aspirin. There were no intergroup differences in the baseline characteristics of the patients without a history of prior revascularisation. The mean CHADS2, CHA2DS2-VASc, and HAS-BLED scores were 2.4, 3.7, and 1.7, respectively. The prevalence of previous myocardial infarction was approximately 10%. In the combination therapy group, 81.8% of patients were treated with aspirin. Compared to patients without a history of prior revascularisation, the CHA2DS2-VASc and the HAS-BLED scores were significantly higher in patients with prior revascularisation (Supplementary Table 1).
Table 1. Patient clinical characteristics by study group.
| Patients with prior revascularisation (n=1,697) | Patients without prior revascularisation (n=518) | |||||
|---|---|---|---|---|---|---|
| Rivaroxaban monotherapy (n=847) | Combination therapy (n=850) | p-value | Rivaroxaban monotherapy (n=260) | Combination therapy (n=258) | p-value | |
| Age, years | 74.3±8.3 | 74.5±8.1 | 0.8421 | 74.2±8.3 | 73.9±8.5 | 0.8467 |
| Male | 683 (80.6) | 693 (81.5) | 0.6645 | 192 (73.8) | 183 (70.9) | 0.4920 |
| BMI, kg/m2 | 24.57±3.71 | 24.55±3.74 | 0.9107 | 24.12±3.44 | 24.35±3.60 | 0.3750 |
| AF type | ||||||
| Paroxysmal | 472 (55.7) | 460 (54.1) | 0.6535 | 124 (47.7) | 120 (46.5) | 0.9184 |
| Persistent | 116 (13.7) | 129 (15.2) | 48 (18.5) | 46 (17.8) | ||
| Permanent | 259 (30.6) | 261 (30.7) | 88 (33.8) | 92 (35.7) | ||
| Previous PCI | 781 (92.2) | 783 (92.1) | 1.0000 | - | - | - |
| Previous CABG | 125 (14.8) | 127 (14.9) | 0.9456 | - | - | - |
| Hypertension | 728 (86.0) | 722 (84.9) | 0.5821 | 219 (84.2) | 222 (86.0) | 0.6217 |
| Diabetes mellitus | 363 (42.9) | 375 (44.1) | 0.6244 | 98 (37.7) | 91 (35.3) | 0.5848 |
| Dyslipidaemia | 634 (74.9) | 615 (72.4) | 0.249 | 147 (56.5) | 142 (55.0) | 0.7907 |
| Angina | 573 (67.7) | 604 (71.1) | 0.1403 | 114 (43.8) | 119 (46.1) | 0.6588 |
| Heart failure | 286 (33.8) | 305 (35.9) | 0.3864 | 103 (39.6) | 94 (36.4) | 0.4701 |
| Previous stroke | 115 (13.6) | 132 (15.5) | 0.2709 | 33 (12.7) | 43 (16.7) | 0.2159 |
| Previous myocardial infarction | 358 (42.3) | 369 (43.4) | 0.6589 | 26 (10.0) | 24 (9.3) | 0.8819 |
| Previous peripheral arterial disease | 53 (6.3) | 63 (7.4) | 0.3868 | 14 (5.4) | 9 (3.5) | 0.3942 |
| CrCL | 62.9±26.7 | 61.6±24.6 | 0.5439 | 62.5±22.1 | 62.2±21.9 | 0.9794 |
| <30 ml/min | 44 (5.2) | 52 (6.1) | 0.5991 | 10 (3.8) | 8 (3.1) | 0.7115 |
| ≥30 and <50 ml/min | 231 (27.3) | 229 (26.9) | 69 (26.5) | 64 (24.8) | ||
| ≥50 ml/min | 535 (63.2) | 511 (60.1) | 164 (63.1) | 175 (67.8) | ||
| CHADS2 score | 2.5±1.1 | 2.5±1.2 | - | 2.4±1.2 | 2.4±1.2 | - |
| 0-2 | 487 (57.5) | 474 (55.8) | 0.4715 | 150 (57.7) | 147 (57.0) | 0.8692 |
| ≥3 | 360 (42.5) | 376 (44.2) | 110 (42.3) | 111 (43.0) | ||
| CHA2DS2-VASc score | 4.1±1.4 | 4.1±1.5 | - | 3.8±1.4 | 3.7±1.6 | - |
| 0-3 | 314 (37.1) | 316 (37.2) | 0.9645 | 115 (44.2) | 120 (46.5) | 0.6021 |
| ≥4 | 533 (62.9) | 534 (62.8) | 145 (55.8) | 138 (53.5) | ||
| HAS-BLED score | 2.2±0.8 | 2.2±0.7 | - | 1.7±0.8 | 1.8±0.8 | - |
| 0-2 | 574 (67.8) | 575 (67.6) | 0.9570 | 212 (81.5) | 201 (77.9) | 0.3043 |
| 3-5 | 246 (29.0) | 245 (28.8) | 37 (14.2) | 45 (17.4) | ||
| Treatment at baseline | ||||||
| Dose of rivaroxaban | ||||||
| 10 mg | 397 (46.9) | 397 (46.7) | 0.9805 | 100 (38.5) | 116 (45.0) | 0.1527 |
| 15 mg | 441 (52.1) | 445 (52.4) | 158 (60.8) | 140 (54.3) | ||
| Use of antiplatelet agent | ||||||
| Aspirin | 7 (0.8) | 585 (68.8) | - | 2 (0.8) | 211 (81.8) | - |
| P2Y12 inhibitor | 5 (0.6) | 265 (31.2) | 0 (0) | 42 (16.3) | ||
| Data are expressed as n (%) or mean±standard deviation. AF: atrial fibrillation; BMI: body mass index; CABG: coronary artery bypass grafting; CrCL: creatinine clearance; PCI: percutaneous coronary intervention | ||||||
PRIMARY EFFICACY AND SAFETY ENDPOINTS
Figure 2A and Figure 2B show the primary efficacy endpoints of the monotherapy (rivaroxaban only) and combination therapy (rivaroxaban plus a single antiplatelet agent) groups. Among the 1,697 patients with a history of prior revascularisation, rivaroxaban monotherapy was superior to combination therapy. Primary efficacy endpoint events were observed in 63 of 847 patients and 100 of 850 patients receiving rivaroxaban monotherapy and combination therapy, respectively, corresponding to incidence rates of 3.83% and 6.18% per patient-year, respectively (HR 0.62, 95% CI: 0.45-0.85; p=0.003), whereas no significant differences were noted in the primary efficacy endpoints among the 518 patients without a history of prior revascularisation. Primary efficacy endpoint events occurred in 26 of 260 patients and 21 of 258 patients receiving rivaroxaban monotherapy and combination therapy, respectively, corresponding to incidence rates of 5.14% and 4.34% per patient-year, respectively (HR 1.19, 95% CI: 0.67-2.12; p=0.554). There was borderline interaction for the primary efficacy outcome between prior revascularisation and antithrombotic therapy (p=0.055) depending on the randomised treatment allocations.
As for the primary safety endpoint (Figure 3A, Figure 3B), rivaroxaban monotherapy was superior to combination therapy in the 1,684 patients with a history of prior revascularisation. Primary safety endpoint events were observed in 29 of 841 patients and 46 of 843 patients receiving rivaroxaban monotherapy and combination therapy, respectively, corresponding to incidence rates of 1.76% and 2.85% per patient-year, respectively (HR 0.62, 95% CI: 0.39-0.98; p=0.042), while no significant intergroup difference was noted among the 518 patients without a history of prior revascularisation. Primary safety endpoint events were observed in 6 of 258 patients and 12 of 256 patients receiving rivaroxaban monotherapy and combination therapy, respectively, corresponding to incidence rates of 1.17% and 2.48% per patient-year, respectively (HR 0.47, 95% CI: 0.18-1.26; p=0.134). There was no interaction in the primary safety outcomes between prior revascularisation and antithrombotic therapy (p=0.633).
In relation to the treatment effect based on the type of revascularisation, we observed a significant difference in the primary efficacy endpoint among a subgroup of 1,445 patients with prior PCI. In this subset, patients receiving rivaroxaban monotherapy exhibited lower event rates compared to those on combination therapy (event rates of 3.84% and 6.25% per patient-year, respectively; HR 0.62, 95% CI: 0.44-0.87; p=0.0054) (Supplementary Figure 1). Conversely, among the 252 patients with prior CABG, there was no significant intergroup variation in the primary efficacy endpoint when comparing patients receiving rivaroxaban monotherapy and combination therapy (event rates of 3.76% and 5.76% per patient-year, respectively; HR 0.66, 95% CI: 0.29-1.53; p=0.337) (Supplementary Figure 2). Additionally, there was no interaction in the primary efficacy endpoint between the type of revascularisation and the antithrombotic therapy (p=0.158).
Furthermore, our analysis revealed no significant differences concerning the primary safety endpoint. This was observed only in patients with either prior PCI (event rates of 1.70% and 2.77% per patient-year for rivaroxaban monotherapy and combination therapy, respectively; HR 0.62, 95% CI: 0.37-1.03; p=0.064) or prior CABG (event rates of 2.08% and 3.27% per patient-year for rivaroxaban monotherapy and combination therapy, respectively; HR 0.65, 95% CI: 0.21-1.98; p=0.445). In addition, there was no interaction in the primary safety endpoint between the type of revascularisation and the antithrombotic therapy (p=0.891) (Central illustration).
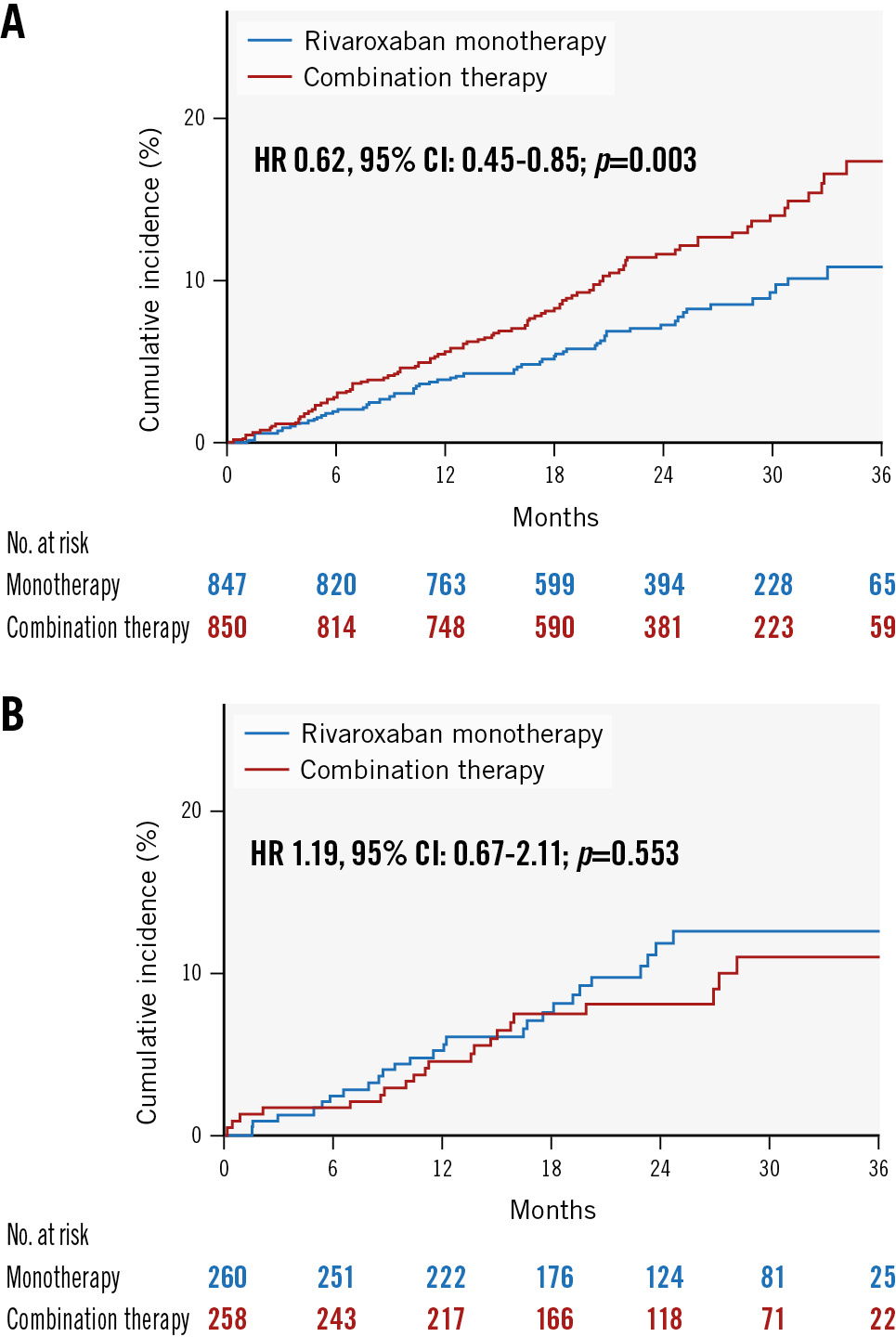
Figure 2. Kaplan-Meier curves for efficacy endpoints among patients with/without revascularisation. A) Efficacy endpoints (composite of stroke, systemic embolism, myocardial infarction, unstable angina requiring revascularisation, and death from any cause) in rivaroxaban monotherapy versus combination therapy groups among patients with a history of prior revascularisation. B) Efficacy endpoints in rivaroxaban monotherapy versus combination therapy groups among patients without a history of prior revascularisation. CI: confidence interval; HR: hazard ratio; No.: number
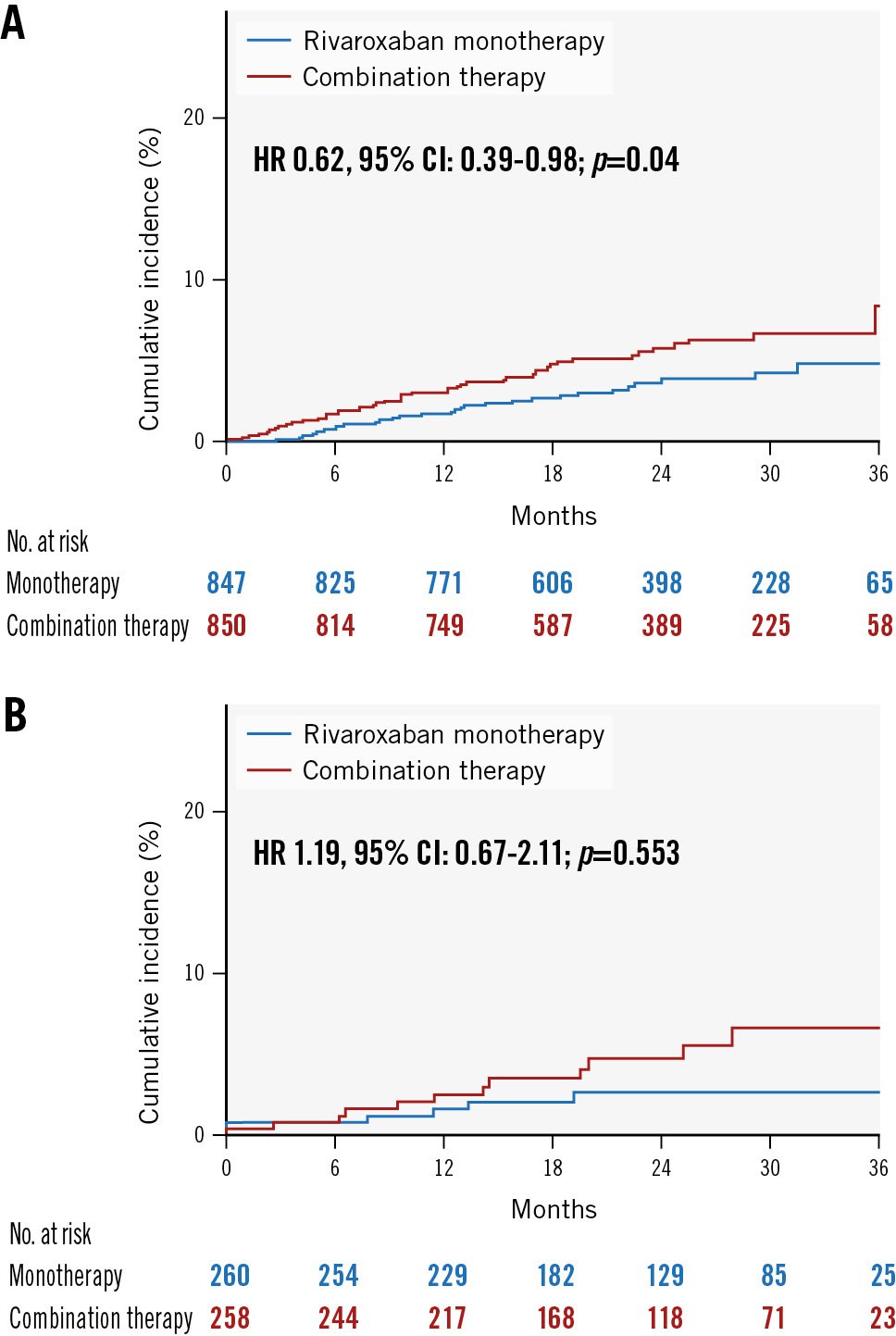
Figure 3. Kaplan-Meier curves for safety endpoints among patients with/without revascularisation. A) Safety endpoints for major bleeding of rivaroxaban monotherapy versus combination therapy groups of patients with a history of prior revascularisation. B) Safety endpoints of rivaroxaban monotherapy and combination therapy groups of patients without a history of prior revascularisation. CI: confidence interval; HR: hazard ratio; No.: number
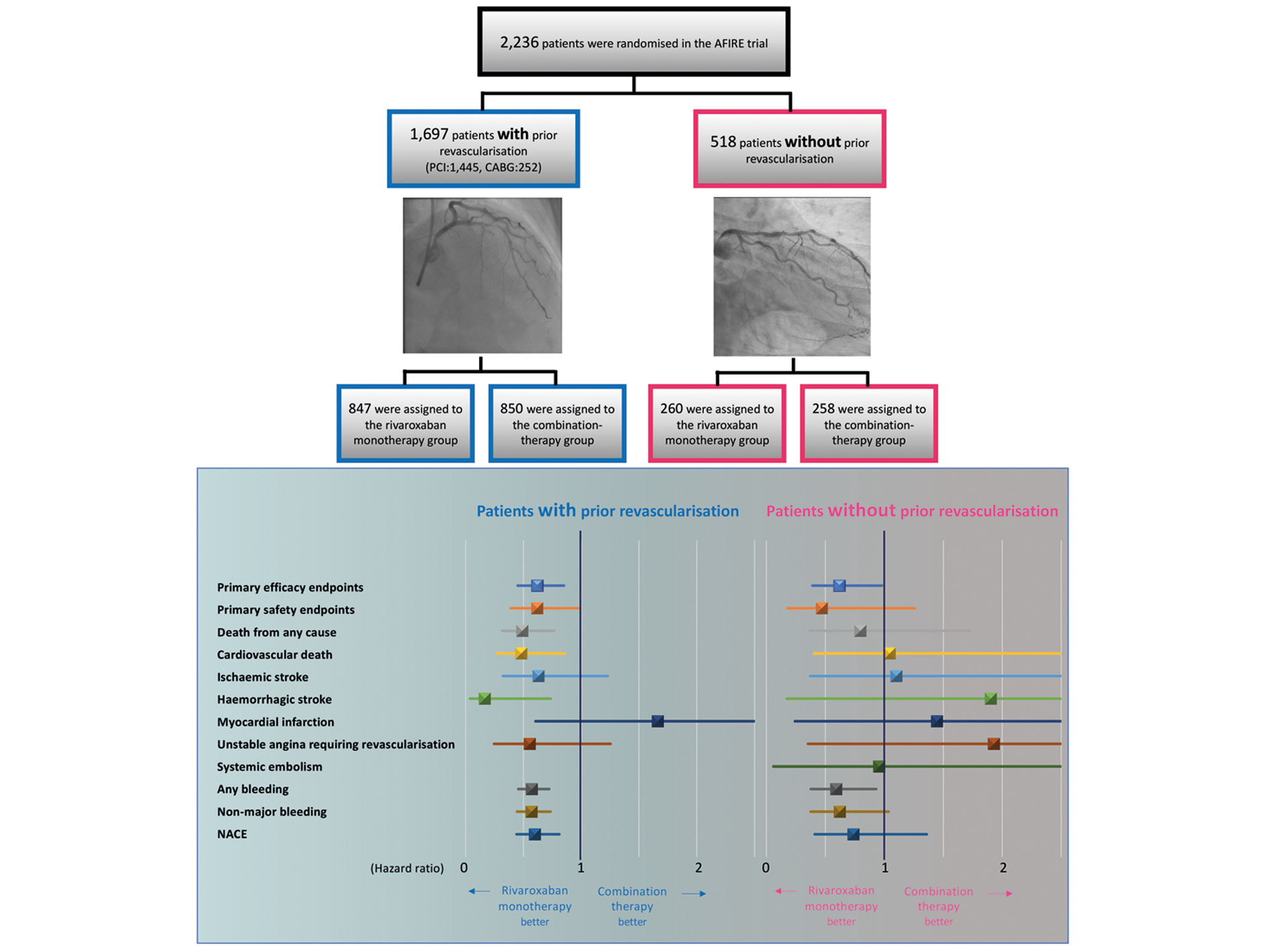
Central illustration. Relative risk of efficacy and safety endpoints, secondary efficacy and safety endpoints, and net adverse clinical events in patients with and without prior revascularisation. In the subgroup of patients who had undergone prior revascularisation, rivaroxaban monotherapy demonstrated superiority over combination therapy with respect to the primary efficacy and safety endpoints. Conversely, for patients without a history of prior revascularisation, no statistically significant disparities were observed in the primary efficacy and safety endpoints. AFIRE: Atrial Fibrillation and Ischemic Events with Rivaroxaban in Patients with Stable Coronary Artery Disease; CABG: coronary artery bypass grafting; NACE: net adverse clinical events; PCI: percutaneous coronary intervention
SUBGROUP ANALYSES AMONG PATIENTS WITH PRIOR REVASCULARISATION
As shown in Figure 4, the effects of rivaroxaban monotherapy versus combination therapy on the primary efficacy endpoint among patients with a history of prior revascularisation were consistent across subgroups, including sex, age, stroke and bleeding risk scores, renal function, and AF type. With respect to the primary safety endpoint, there was similar consistency in the effect of rivaroxaban monotherapy in patients with a history of prior revascularisation (Figure 5). However, there was a statistically significant interaction for the primary safety endpoint between patients with versus without previous myocardial infarction (HR 0.31 vs 0.89; p for interaction=0.046).
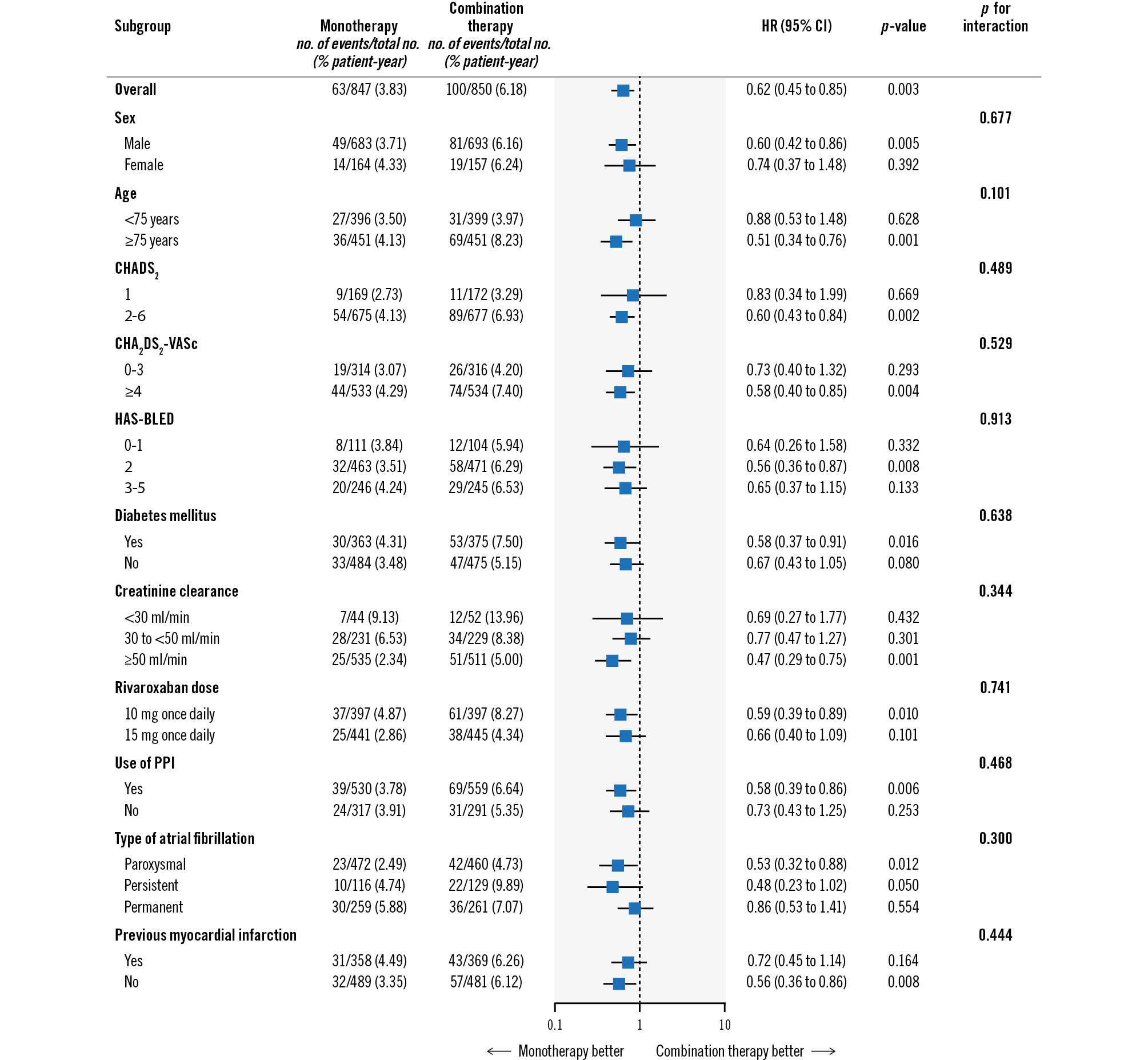
Figure 4. Primary efficacy endpoint according to subgroup based on revascularisation history. Hazard ratios for the primary efficacy endpoint (composite of stroke, systemic embolism, myocardial infarction, unstable angina requiring revascularisation, or death from any cause) in the two trial groups among patients with a history of prior revascularisation. CI: confidence interval; HR: hazard ratio; PPI: proton pump inhibitor
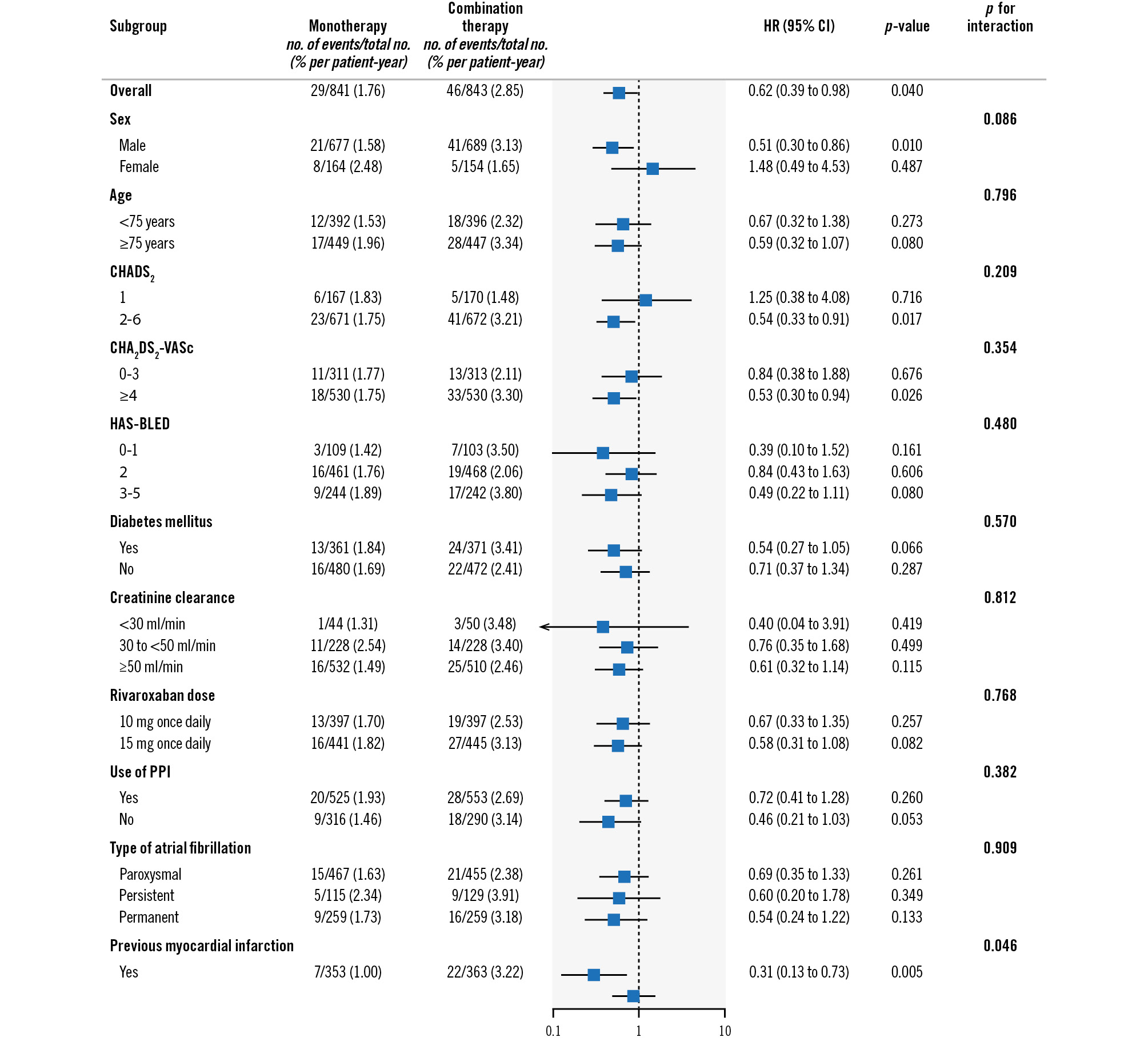
Figure 5. Primary safety endpoint among patients with prior revascularisation history. Hazard ratios for the primary safety endpoint of major bleeding in the two trial groups among patients with a history of prior revascularisation. CI: confidence interval; HR: hazard ratio; PPI: proton pump inhibitor
INDIVIDUAL COMPONENTS OF EFFICACY AND SAFETY ENDPOINTS
Table 2 shows the incidence of the individual components of the efficacy and safety endpoints among patients with versus without prior revascularisation as secondary endpoints. Among the patients with prior revascularisation, all-cause mortality was significantly lower in patients receiving rivaroxaban monotherapy versus combination therapy (event rates of 1.72% and 3.53%, respectively; HR 0.49, 95% CI: 0.31-0.77; p=0.0013). As for cardiovascular death, this occurred in 1.01% of the patients receiving rivaroxaban monotherapy and in 2.10% of those receiving combination therapy per patient-year (HR 0.48, 95% CI: 0.27-0.86; p=0.011). The event rate for any bleeding was significantly lower in the rivaroxaban monotherapy versus combination therapy group (7.5% vs 13.2% per patient-year, respectively; HR 0.57, 95% CI: 0.46-0.72; p<0.0001).
Regarding the incidence of the individual components of the efficacy and safety endpoints among patients without prior revascularisation, there were no significant differences in any parameters of the efficacy endpoints, including death from any cause and cardiovascular death. However, the incidence of any bleeding was significantly lower in the rivaroxaban monotherapy versus the combination therapy group among the patients without a history of prior revascularisation (6.4% vs 11.1% per patient-year, respectively; HR 0.59, 95% CI: 0.38-0.93; p=0.022).
Rivaroxaban monotherapy was superior to combination therapy regarding NACE among patients with a history of prior revascularisation (event rates of 3.96% and 6.67% per patient-year, respectively; HR 0.60, 95% CI: 0.44-0.81; p=0.0009), whereas no significant difference was noted regarding NACE in patients receiving rivaroxaban monotherapy versus combination therapy among the 518 patients without a history of prior revascularisation (event rates of 3.70% and 4.99% per patient-year, respectively; HR 0.74, 95% CI: 0.41-1.36; p=0.336). There was no interaction in NACE between prior revascularisation and antithrombotic therapy (p=0.535).
Table 2. Primary and secondary endpoints among patients with versus without prior revascularisation.
| Patients with prior revascularisation | Patients without prior revascularisation | p for interaction | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Endpoints | Rivaroxaban monotherapy (n=847)(per patient-year) | Combination therapy (n=850)(per patient-year) | Hazard ratio(95% CI) | p-value | Rivaroxaban monotherapy (n=260)(per patient-year) | Combination therapy (n=258)(per patient-year) | Hazard ratio(95% CI) | p-value | |
| Efficacy endpoints | |||||||||
| Primary efficacy endpoint | 63 (3.83) | 100 (6.18) | 0.62 (0.45-0.85) | 0.003 | 26 (5.14) | 21 (4.34) | 1.19 (0.67-2.12) | 0.554 | 0.055 |
| Death from any cause | 29 (1.72) | 59 (3.53) | 0.49 (0.31-0.77) | 0.001 | 12 (2.28) | 14 (2.84) | 0.80 (0.37-1.73) | 0.565 | 0.289 |
| Cardiovascular death | 17 (1.01) | 35 (2.10) | 0.48 (0.27-0.86) | 0.011 | 9 (1.71) | 8 (1.62) | 1.05 (0.40-2.71) | 0.925 | 0.172 |
| Ischaemic stroke | 14 (0.84) | 22 (1.33) | 0.63 (0.32-1.23) | 0.171 | 7 (1.35) | 6 (1.23) | 1.10 (0.37-3.28) | 0.860 | 0.392 |
| Haemorrhagic stroke | 2 (0.12) | 12 (0.72) | 0.17 (0.04-0.74) | 0.007 | 2 (0.38) | 1 (0.20) | 1.90 (0.17-20.98) | 0.593 | 0.092 |
| Myocardial infarction | 10 (0.60) | 6 (0.36) | 1.66 (0.60-4.57) | 0.320 | 3 (0.58) | 2 (0.41) | 1.45 (0.24-8.66) | 0.684 | 0.890 |
| Unstable angina requiring revascularisation | 9 (0.54) | 16 (0.97) | 0.55 (0.25-1.25) | 0.150 | 4 (0.77) | 2 (0.41) | 1.93 (0.35-10.54) | 0.440 | 0.197 |
| Systemic embolism | 1 (0.06) | 0 (0) | - | - | 1 (0.19) | 1 (0.20) | 0.96 (0.06-15.29) | 0.975 | 0.995 |
| Safety endpoints | |||||||||
| Primary safety endpoint | 29 (1.76) | 46 (2.85) | 0.62 (0.39-0.98) | 0.042 | 6 (1.17) | 12 (2.48) | 0.47 (0.18-1.26) | 0.134 | 0.633 |
| Any bleeding | 116 (7.50) | 191 (13.20) | 0.57 (0.46-0.72) | <0.001 | 31 (6.41) | 48 (11.14) | 0.59 (0.38-0.93) | 0.022 | 0.893 |
| Non-major bleeding | 97 (6.17) | 162 (10.89) | 0.57 (0.44-0.73) | <0.001 | 25 (5.09) | 37 (8.43) | 0.62 (0.38-1.04) | 0.066 | 0.760 |
| Data are expressed as n (%). CI: confidence interval | |||||||||
Discussion
In this post hoc analysis of the AFIRE trial, the major findings were as follows: 1) rivaroxaban monotherapy had a greater impact on efficacy and safety endpoints in patients with versus without a history of prior revascularisation; 2) there was borderline interaction between the primary efficacy outcomes of prior revascularisation and antithrombotic therapy depending on the randomised treatment assignment; and 3) the incidence of any bleeding was significantly lower in the monotherapy versus the combination therapy group among patients without a history of prior revascularisation.
The AFIRE trial findings indicated that rivaroxaban monotherapy was non-inferior to combination therapy with rivaroxaban plus a single antiplatelet agent regarding efficacy and superior for safety endpoints in patients with AF and stable CAD8. In this subanalysis of the AFIRE trial, we found that the benefit of rivaroxaban monotherapy regarding primary efficacy and safety endpoints among the patients with a history of prior revascularisation was consistent with that of the whole population of the AFIRE trial. Compared to patients with AF and stable CAD without a history of prior revascularisation, rivaroxaban monotherapy demonstrated more favourable safety and efficacy outcomes than combination therapy. These results may be related to baseline characteristics, especially the higher thrombotic and bleeding risks among patients with AF and stable CAD with a history of prior revascularisation. The HAS-BLED scores were higher in patients with prior revascularisation than in those without. Because bleeding is strongly correlated with subsequent cardiovascular events in patients with CAD10, it is important to suppress bleeding events in the setting of antithrombotic therapy. An additional analysis of the AFIRE trial results showed that the rate of cardiovascular events was high in patients with major bleeding and that major bleeding events were associated with high morbidity and mortality rates11. A recent subanalysis of the AFIRE trial consistently demonstrated that the safety benefit of monotherapy was even greater in patients receiving PCI for multivessel disease or left main trunk lesions than in those with single-vessel disease12. Moreover, there was a statistically significant interaction of the primary safety endpoint between patients with versus without previous myocardial infarction among patients with prior revascularisation, which may indicate that rivaroxaban monotherapy resulted in more favourable safety outcomes than combination therapy in severely high-risk thrombosis patients with a history of previous myocardial infarction. A post hoc analysis of the AFIRE trial consistently reported that rivaroxaban monotherapy significantly reduced net adverse events compared to combination therapy in patients with atrial fibrillation, CAD, and prior atherothrombotic disease (those with a history of myocardial infarction, stroke, and/or peripheral artery disease)13.
Among the patients without a history of prior revascularisation, in whom the median HAS-BLED score was 1.7, there were no significant differences in any parameters of the efficacy endpoints; however, the incidence of any bleeding was significantly lower in the monotherapy group than in the combination therapy group. Despite the relatively lower thrombotic and bleeding risks, anticoagulant monotherapy would be a better choice, as it is associated with less bleeding and offers a potential net clinical benefit.
Limitations
This study had several limitations as previously reported8. The post hoc design was a limitation of the study. We divided the entire cohort into several groups; therefore, the number of patients in these analyses was relatively small, which may have influenced the results, especially the borderline interaction between the primary outcomes of prior revascularisation and antithrombotic therapy according to the randomised treatment allocations. Second, the trial participants consisted only of Japanese patients who received the rivaroxaban dose approved in Japan (10 or 15 mg once daily, according to the patient’s creatinine clearance) rather than a globally approved once-daily dose of 15 or 20 mg. In addition, the selection of an antiplatelet agent, either aspirin or a P2Y12 inhibitor, was made at the discretion of the treating physicians. However, no significant differences were found in efficacy and safety outcomes between the P2Y12 inhibitor and aspirin groups in a post hoc analysis of the AFIRE trial14. With respect to the limited number of patients, further studies of patients with prior CABG, generally characterised as having multivessel or left main trunk lesions, are needed to determine whether oral anticoagulant monotherapy is the preferred treatment strategy.
Conclusions
In this post hoc subgroup analysis of the AFIRE trial, among high-risk thrombosis patients with a history of prior PCI or CABG, rivaroxaban monotherapy consistently resulted in favourable safety and efficacy outcomes compared to combination therapy. Among patients without revascularisation, the incidence of bleeding was significantly lower in the monotherapy versus combination therapy group, indicating a potential net clinical benefit.
Impact on daily practice
This post hoc subgroup analysis of high-risk thrombosis patients with a history of prior revascularisation with percutaneous coronary intervention or coronary artery bypass grafting demonstrated that rivaroxaban monotherapy consistently resulted in favourable safety and efficacy outcomes versus combination therapy. Further clinical trials are needed to determine whether anticoagulant monotherapy could be applicable to patients who are at particularly high risk of thrombosis, including those with previous stent thrombosis, severe diffuse coronary artery disease, or extensive complex coronary stenting.
Funding
This work was supported by the Japan Cardiovascular Research Foundation based on a contract with Bayer Yakuhin Ltd., which had no role in the study design, data collection and analysis, results interpretation, or manuscript writing.
Conflict of interest statement
T. Noda reports grants-in-aid for Scientific Research (22K08092) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan; and personal fees from Bayer Yakuhin, Medtronic Japan, and Biotronik Japan. K. Nochioka reports grants from the Japan Agency for Medical Research and Development (AMED, 21ek0210136h0003). K. Kaikita reports trust research/joint research funds from Bayer Yakuhin and Daiichi Sankyo; scholarship funds from Abbott; and personal fees from Bayer Yakuhin, Daiichi Sankyo, Novartis Pharma AG, and Otsuka Pharmaceutical Co. M. Akao reports grants from the Japan AMED; personal fees from Bristol-Myers Squibb and Nippon Boehringer Ingelheim; and grants and personal fees from Bayer Yakuhin and Daiichi Sankyo. J. Ako reports personal fees from Bayer Yakuhin and Sanofi; and grants and personal fees from Daiichi Sankyo. T. Matoba reports grants from the Japan Cardiovascular Research Foundation; and personal fees from Nippon Boehringer Ingelheim, Daiichi Sankyo, AstraZeneca, and Bayer Yakuhin. M. Nakamura reports grants and personal fees from Bayer Yakuhin, Daiichi Sankyo, and Sanofi; and personal fees from Bristol-Myers Squibb and Nippon Boehringer Ingelheim. K. Miyauchi reports personal fees from Amgen Astellas BioPharma, Astellas Pharma, Merck & Co., Bayer Yakuhin, Sanofi, Takeda Pharmaceutical, Daiichi Sankyo, Nippon Boehringer Ingelheim, and Bristol-Myers Squibb. N. Hagiwara reports grants and personal fees from Bayer Yakuhin; grants from Nippon Boehringer Ingelheim; and personal fees from Bristol-Myers Squibb. K. Kimura reports grants from the Japan Cardiovascular Research Foundation; grants and personal fees from Bayer Yakuhin, Daiichi Sankyo, Sanofi, Merck & Co., and AstraZeneca; and personal fees from Bristol-Myers Squibb and Nippon Boehringer Ingelheim. A. Hirayama reports grants and personal fees from Boston Scientific Japan, Otsuka Pharmaceutical, Sanofi, Amgen Astellas Pharma, Bristol-Myers Squibb, Daiichi Sankyo, and Bayer Yakuhin; grants from Fukuda Denshi, Abbott Japan, Japan Lifeline, Takeda Pharmaceutical, and Sumitomo Dainippon Pharma; and personal fees from Toa Eiyo, Nippon Boehringer Ingelheim, Amgen Astellas BioPharma, and AstraZeneca. H. Ogawa reports personal fees from Abbott Medical Japan, Bayer Yakuhin, Daiichi Sankyo, Eisai, Kowa, Takeda Pharmaceutical, and Teijin. S. Yasuda reports grants from Takeda Pharmaceutical, Abbott, and Boston Scientific; and personal fees from Daiichi Sankyo and Bristol-Myers Squibb. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

