
Antithrombotic drugs are used to prevent ischaemic complications in patients with coronary artery disease and/or atrial fibrillation. Randomised trials showed that, after coronary stenting, dual antiplatelet therapy (DAPT) with aspirin and a P2Y12 inhibitor was superior to aspirin and oral anticoagulation in preventing ischaemic events, with a reduced or comparable risk of bleeding1,2. Similarly, in the case of patients with atrial fibrillation, oral anticoagulation with a vitamin K antagonist (VKA) is superior to DAPT in preventing ischaemic events, albeit at the cost of increased bleeding3,4. As a rule of thumb, such trials have evaluated new antithrombotic drugs or regimens based on primary endpoints indicating efficacy, with safety representing a co-primary or secondary endpoint.
Patients with atrial fibrillation who present with acute coronary syndrome (ACS) or undergo percutaneous coronary intervention (PCI) are frequently encountered in daily practice. In principle, such patients have an indication for both oral anticoagulation and DAPT – so-called triple therapy – in order to prevent ischaemic events. This presents a challenge for clinical decision making due to the increased risk of bleeding associated with combination therapy5. For a long time, we had little evidence from randomised clinical trials to guide our decision making in this situation, with only two modest-sized investigator-initiated trials examining different antithrombotic strategies6,7. In recent years, however, three larger clinical trials have added to the evidence base8-10, each comparing a non-VKA oral anticoagulant (NOAC) plus P2Y12 inhibitor monotherapy (with omission of aspirin) – so-called dual therapy – with triple therapy (including aspirin). Notably, in contrast to the design of previous trials on antithrombotic drugs/regimens, each of these trials primarily tested bleeding reduction rather than efficacy in preventing ischaemic events. The results have led some experts to dispute the role of aspirin in these patients. In our opinion, this interpretation is open to question. Moreover, as a community, we should ask critically if this is the correct way to test antithrombotic therapy and why the goalposts seem to have moved?
A total of four clinical trials – WOEST included – compared aspirin omission (i.e., dual therapy) with triple therapy in patients receiving oral anticoagulation, who underwent stenting or presented with ACS6,8-10. In terms of the primary endpoint, bleeding in all cases, the results should come as no surprise. Low-dose aspirin is used for its antiplatelet effect, achieved through inhibition of platelet thromboxane, and thus predisposes to bleeding. Obviously, its omission would be expected to reduce bleeding risk, all other things being equal. These expectations are borne out in all four trials. Taken together, these data confirm a clear reduction in bleeding if aspirin is omitted, albeit the difference is less marked when only major bleeding is considered. Simple pooling of events shows that omitting aspirin results in fewer Thrombolysis In Myocardial Infarction (TIMI) major bleeding events: 84/4,988 (1.7%) versus 142/4,936 (2.9%) (Figure 1). However, when evaluating the clinical benefit of antithrombotic treatment, surely its efficacy in preventing thrombotic events must be considered in the primary hypothesis testing? This is especially important in these patients who, after all, are at increased risk of both cerebrovascular and coronary thrombotic events. In this respect, a closer look at the data from these trials provides some pause for thought.
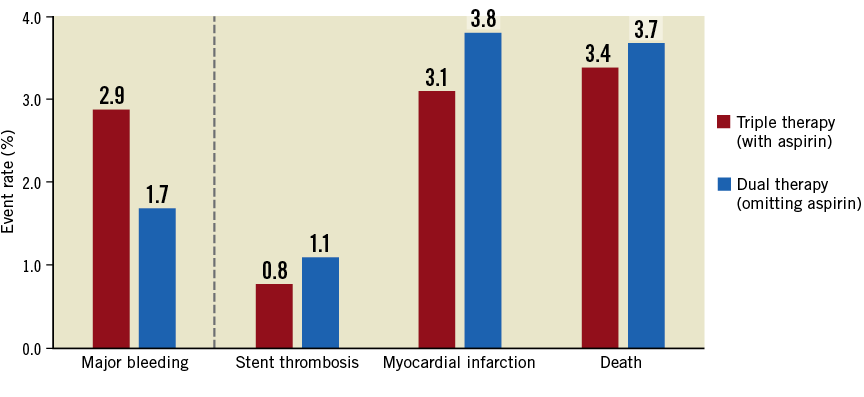
Figure 1. Rates of major clinical endpoints in trials comparing aspirin omission (dual therapy) versus aspirin administration (triple therapy). Rates of TIMI major bleeding, stent thrombosis, myocardial infarction and death based on simple pooling of events from the four randomised trials – WOEST, PIONEER-AF, RE-DUAL PCI and AUGUSTUS – testing aspirin omission (dual therapy) versus aspirin administration (triple therapy) in patients receiving oral anticoagulation who present with acute coronary syndrome or undergo percutaneous intervention6,8-10.
Prevention of stent thrombosis – the most specific target of antiplatelet therapy – is apparently less effective after omission of aspirin: 55/5,024 (1.1%) versus 38/4,971 (0.8%) (Figure 1). The risk of myocardial infarction follows a similar pattern with omission of aspirin: 191/5,024 (3.8%) versus 154/4,971 (3.1%). Although a formal meta-analysis is beyond the scope of this editorial, there appears to be significant heterogeneity for both of these endpoints, introduced by the results of the WOEST trial. In that trial, for reasons not fully explained, the rate of stent thrombosis was twice as high in patients receiving aspirin compared with those without. In fact, omitting this trial, the evidence indicating an increased risk of stent thrombosis and myocardial infarction for aspirin omission is stronger. Finally, total mortality as a marker of net clinical benefit between the two strategies is also numerically higher in patients in whom aspirin is omitted: 187/5,024 (3.7%) versus 168/4,971 (3.4%) (Figure 1).
One issue clarified by the most recently published trial, AUGUSTUS10, relates to the direct comparison between NOAC and VKA. Prior trials compared a strategy of dual therapy with NOAC and a P2Y12 inhibitor (omitting aspirin) against triple therapy with VKA (including aspirin)8,9. This meant that it was unclear whether the bleeding reductions observed were due to omission of aspirin or to the replacement of VKA with NOAC. The factorial design of AUGUSTUS allows us to better tease out differences attributable to individual therapies. The finding that bleeding was significantly lower with NOAC as compared with VKA supports the observations from the pivotal approval trials of NOAC for stroke prevention in atrial fibrillation, though we should remember that gastrointestinal bleeding may be higher with NOAC11.
New antithrombotic treatment strategies should not be tested based primarily on bleeding risk reduction. Taking this approach, for example, more potent P2Y12 inhibitors might never have been approved, neither would we accept oral anticoagulation for stroke prevention. Surveying the evidence from the four available clinical trials, we are left with a fragmented picture, with data derived from trials, each of which was individually powered for bleeding rather than efficacy. The charge that the goalposts were moved because it was not feasible to design trials powered for efficacy does not hold true. In actual fact, it was possible to power the individual trials for bleeding only because liberal bleeding definitions were chosen – the primary endpoint in WOEST, for example, was any bleeding. If similar creativity were used for thrombotic endpoints, a trial powered to show comparable efficacy (non-inferiority hypothesis) with reduced bleeding (superiority hypothesis) in hierarchical fashion could be pursued. Until such a trial is carried out, clinical equipoise will remain.
In the meantime, systematic adoption of dual therapy for all patients receiving oral anticoagulation with ACS or undergoing coronary intervention would seem ill-advised, as we cannot be sure that omitting aspirin does not cause harm. At a minimum, it would seem pertinent that the period of highest risk for stent thrombosis should be covered by DAPT. This might be for a duration of one month after intervention (or even less), or as long as six months. Indeed, the totality of the evidence provides a rationale for individualisation of therapy. Where ischaemic risk predominates, a longer duration of triple therapy may be preferred; where bleeding risk predominates, a shorter duration might be chosen, or aspirin may be omitted completely in favour of dual therapy. This personalised approach is supported by current European clinical practice guideline recommendations12. The existing evidence is not strong enough to conclude otherwise.
Conflict of interest statement
R. Byrne reports lecture fees/honoraria from B. Braun Melsungen AG, Biotronik, Boston Scientific and Micell Technolgies, and research grants to the institution from Boston Scientific and CeloNova Biosciences. The other authors have no conflicts of interest to declare.

