
Up to 15% of patients with atrial fibrillation (AF) will ultimately undergo percutaneous coronary intervention (PCI) or have acute coronary syndrome1. Management of these patients is challenging: long-term treatment with an oral anticoagulant (OAC) is necessary to prevent stroke and systemic embolisation related to AF, but dual antiplatelet therapy with aspirin plus a P2Y12 inhibitor represents the standard of care to prevent stent thrombosis and recurrent myocardial infarction. The rate of major bleeding in patients treated with triple antithrombotic therapy (OAC plus aspirin plus a P2Y12 inhibitor) approaches 10% per year, a rate twofold to threefold higher than that of patients treated with OAC alone2. Despite the high bleeding risk associated with triple antithrombotic therapy, for a long time there was limited evidence supporting alternative strategies for managing these patients. Recently, four randomised controlled trials have compared double antithrombotic regimens including a non-vitamin K antagonist oral anticoagulant (NOAC) plus a P2Y12 inhibitor (without aspirin) against triple antithrombotic therapy3,4,5,6. A meta-analysis of these trials showed that these double antithrombotic therapy regimens reduce the risk of bleeding compared with triple antithrombotic therapy while maintaining efficacy for the prevention of ischaemic events7.
However, there are nearly limitless ways to combine the available OACs plus P2Y12 inhibitors plus aspirin. Among the alternatives to double antithrombotic therapy with an OAC plus P2Y12 inhibitor are regimens that drop the P2Y12 inhibitor after a short duration in favour of an OAC plus aspirin. The type of regimen was tested in the ISAR-TRIPLE (Triple Therapy in Patients on Oral Anticoagulation After Drug Eluting Stent Implantation) trial, which randomised 614 patients with an indication for OAC undergoing PCI to warfarin plus aspirin plus either six weeks or six months of therapy with the P2Y12 inhibitor clopidogrel. In that study, the authors found no difference in the primary composite outcome, a combination of ischaemic events and Thrombolysis In Myocardial Infarction (TIMI) major bleeding, which occurred in 9.8% of patients in the six-week clopidogrel group and 8.8% of patients in the six-month group. ISAR-TRIPLE was a small trial, powered only to detect a 60% reduction in events. Moreover, it enrolled patients between 2008 and 2013, before the availability of all NOACs and more potent P2Y12 inhibitors; its warfarin-based regimen is less relevant in the NOAC era.
The SAFE-A (Short-duration triple antithrombotic therapy for atrial fibrillation patients who require coronary stenting) trial, published in this edition of EuroIntervention8, builds on the design of ISAR-TRIPLE.
SAFE-A randomised patients with AF undergoing PCI to either one month or six months of P2Y12 inhibitor therapy (clopidogrel or prasugrel) on a background of apixaban plus aspirin. The authors intended to enrol 600 patients, giving them 80% power to detect a 50% reduction in the primary outcome of any bleeding, but the trial was stopped early due to slow recruitment after 210 patients were enrolled. The primary endpoint occurred in 12/102 patients in the one-month P2Y12 inhibitor arm and 17/106 patients in the six-month P2Y12 inhibitor arm (11.8 vs 16.0%; hazard ratio [HR] 0.70, 95% confidence interval [CI]: 0.33-1.47). Ten patients in the one-month arm had an ischaemic event (death, myocardial infarction, stroke, or systemic embolism) compared with three in the six-month arm (9.8 vs 2.8%; HR 3.00, 95% CI: 0.82-10.94).
The SAFE-A approach to antithrombotic therapy is worthy of consideration. Despite the results of clinical trials, many PCI operators remain concerned that dropping aspirin following PCI increases the risk of stent thrombosis. Stent thrombosis is rare enough that clinical trials have not been powered to detect a change in its incidence with less aggressive antithrombotic therapy, but it can be very serious or fatal to individual patients. The great majority of stent thrombosis events occur in the first month after PCI9, and one month of P2Y12 inhibitor therapy appears safe in high-bleeding risk patients undergoing PCI with third-generation drug-eluting stents10, providing rationale for a strategy that employs a short duration of triple antithrombotic therapy. After the first month, whether single antiplatelet therapy with aspirin or a P2Y12 inhibitor provides the optimal balance of protection from ischaemic events and bleeding events has not been tested in randomised controlled trials. However, though the rate of bleeding and ischaemic events in SAFE-A is similar to that in other trials evaluating double antithrombotic therapy regimens (Figure 1), SAFE-A has several limitations that make its interpretation challenging. It was stopped early with one third of the intended sample size, and consequently had a small number of events. It enrolled patients in a single country and used a dose of prasugrel (3.75 mg daily) that is not available in most parts of the world. It used a bleeding definition that combined different bleeding scales and different bleeding severities, which is not typical for other trials in this area and is difficult to interpret.
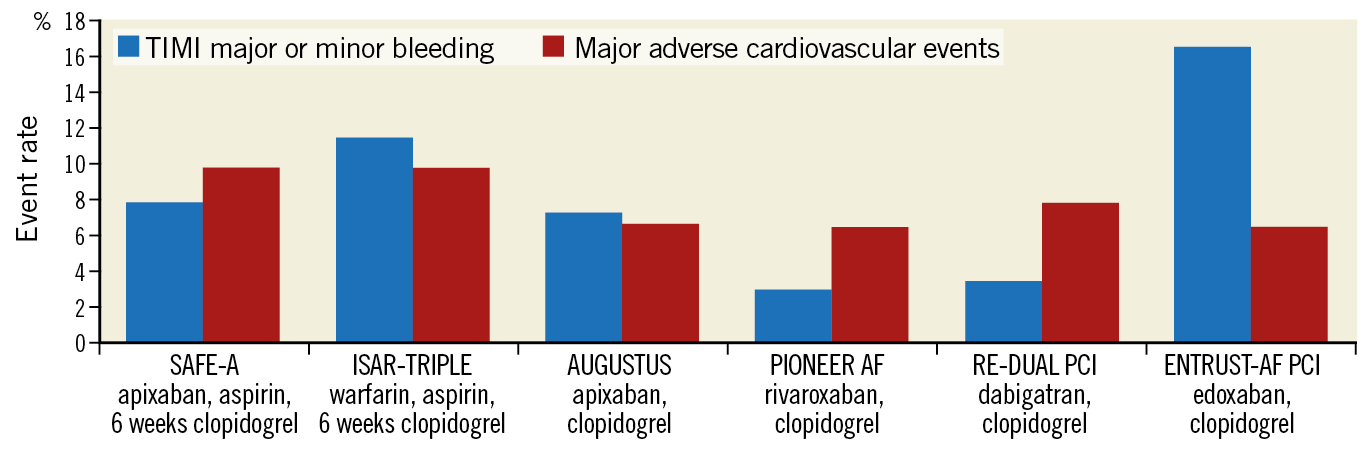
Figure 1. Ischaemic and major bleeding events in the double antithrombotic therapy arms of clinical trials enrolling patients with atrial fibrillation and either acute coronary syndrome or undergoing percutaneous coronary intervention. Rates of bleeding and major adverse cardiovascular events were relatively similar in the double antithrombotic arms of all trials. Major adverse cardiovascular events include all-cause or cardiovascular death, myocardial infarction, and stroke or systemic embolism. TIMI major and minor bleeding was selected for display as it was the only bleeding outcome reported by all trials. WOEST did not report the composite of death, myocardial infarction, and stroke or systemic embolism and is not included.
For these reasons, the SAFE-A trial is unable to inform physicians how to treat patients with AF and PCI. Until we have an appropriately powered trial testing the SAFE-A regimen, physicians should use a NOAC plus a P2Y12 inhibitor for patients with AF undergoing PCI. This has been shown in four appropriately powered trials to represent the antithrombotic sweet spot in the prevention of ischaemic events at a minimal cost of bleeding events in this high-risk, challenging population.
Conflict of interest statement
R. Lopes reports research grants from Bristol-Myers Squibb, GlaxoSmithKline, Medtronic, Pfizer, and Sanofi; and personal fees from Amgen, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, GlaxoSmithKline, Medtronic, Pfizer, and Sanofi. A. Fanaroff reports a career development grant from the American Heart Association, grant funding from Boston Scientific, and honoraria from the American Heart Association.
Supplementary data
To read the full content of this article, please download the PDF.

