Abstract
Background: Little is known about the optimal antithrombotic therapy in patients with atrial fibrillation undergoing PCI for ST-elevation myocardial infarction (STEMI).
Aims: The aim of this study was to investigate the safety and efficacy of dabigatran dual therapy (110 or 150 mg twice daily, plus clopidogrel or ticagrelor) versus warfarin triple therapy in patients with atrial fibrillation and STEMI.
Methods: In the RE-DUAL PCI trial, 305 patients with STEMI were randomised to dabigatran 110 mg (n=113 versus 106 warfarin) or 150 mg (n=86 versus 84 warfarin). The primary endpoint was the time to first major/clinically relevant non-major bleeding event (MBE/CRNMBE). The thrombotic endpoint was a composite of death, thromboembolic events, or unplanned revascularisation.
Results: In STEMI patients, dabigatran 110 mg (HR 0.39, 95% CI: 0.20-0.74) and 150 mg (0.43, 0.21-0.89) dual therapy reduced the risk of MBE/CRNMBE versus warfarin triple therapy (p for interaction vs all other patients=0.31 and 0.16). The risk of thrombotic events for dabigatran 110 mg (HR 1.61, 95% CI: 0.85-3.08) and 150 mg (0.56, 0.20-1.51) had p interactions of 0.20 and 0.33, respectively. For net clinical benefit, the HRs were 0.74 (95% CI: 0.46-1.17) and 0.49 (0.27-0.91) for dabigatran 110 and 150 mg (p for interaction=0.80 and 0.12), respectively.
Conclusions: After PCI for STEMI, patients on dabigatran dual therapy had lower risks of bleeding events versus warfarin triple therapy with similar risks of thromboembolic events, supporting dabigatran dual therapy even in patients with high thrombotic risk.
Introduction
Approximately 6-10% of patients with ST-elevation myocardial infarction (STEMI) have atrial fibrillation (AF)1,2,3,4. Current guidelines recommend early reperfusion therapy with primary percutaneous coronary intervention (PCI) in patients with STEMI5. Notably, these patients have an increased risk of thromboembolic and ischaemic events compared with those undergoing elective PCI. Additionally, patients with STEMI and AF have higher rates of complications including bleeding3,4,6,7.
The use of anticoagulation in patients with AF has been transformed by the availability of non-vitamin K oral anticoagulants (NOACs), which offer superior safety and similar efficacy compared with vitamin K antagonists (VKAs)8,9,10.
Dabigatran is a direct thrombin inhibitor approved worldwide at 110 mg (excluding the USA) or 150 mg twice daily doses for the prevention of stroke in AF11. The Randomized Evaluation of Dual Antithrombotic Therapy with Dabigatran versus Triple Therapy with Warfarin in Patients with Nonvalvular Atrial Fibrillation Undergoing Percutaneous Coronary Intervention (RE-DUAL PCI) trial (NCT02164864) assessed the safety and efficacy of dabigatran dual therapy at doses of 110 or 150 mg with a P2Y12 inhibitor (either clopidogrel or ticagrelor) versus warfarin plus aspirin (ASA) and a P2Y12 inhibitor (either clopidogrel or ticagrelor) in patients with AF undergoing PCI12. Both dabigatran strategies significantly reduced the primary endpoint of International Society on Thrombosis and Haemostasis (ISTH) major bleeding events (MBEs) or clinically relevant non-major bleeding events (CRNMBEs) without any increase in the composite endpoint of death, thromboembolic events (DTE: myocardial infarction, stroke or systemic embolism) or unplanned revascularisation12. However, there is still some uncertainty about the efficacy of dual therapy in the patients with the highest thrombotic risk, including those with STEMI. Therefore, we performed a non-pre-specified subgroup analysis in RE-DUAL PCI by distinguishing between patients with STEMI and the remaining patients with non-ST-segment elevation myocardial infarction (NSTEMI), unstable angina (UA) or an elective PCI.
Methods
TRIAL DESIGN AND TREATMENT
The design and the results of the RE-DUAL PCI trial have been reported previously12. In brief, RE-DUAL PCI randomly assigned 2,725 patients to receive dual therapy comprising dabigatran 110 or 150 mg twice daily plus either clopidogrel or ticagrelor, or to receive triple therapy with warfarin (adjusted to achieve an international normalised ratio of 2.0-3.0) plus ASA (≤100 mg daily) and either clopidogrel or ticagrelor. Outside the USA, patients aged ≥80 years (≥70 years in Japan) were randomised only to the 110 mg dabigatran dose versus warfarin. All patients were to receive clopidogrel (75 mg daily) or ticagrelor (90 mg twice daily) for ≥12 months after randomisation. In the warfarin triple therapy group, ASA was discontinued after one month in patients implanted with a bare metal stent (BMS) and after three months in patients with a drug-eluting stent (DES).
PATIENTS
Men and women aged ≥18 years with non-valvular AF who had undergone PCI with a BMS or DES within the previous 120 hours were eligible for study inclusion. The indication for PCI could be either acute coronary syndrome (ACS) or stable coronary artery disease. Patients with bioprosthetic or mechanical heart valves, severe renal insufficiency (creatinine clearance <30 ml/min at screening, calculated by the Cockcroft-Gault equation) or other major coexisting conditions were excluded.
ASSESSMENTS
The primary endpoint was the time to a first ISTH MBE or CRNMBE during follow-up (mean 14 months). The time to the composite endpoint of thromboembolic events (myocardial infarction, stroke or systemic embolism), death or unplanned revascularisation (first event) was also evaluated. In addition, the time to first definite stent thrombosis was evaluated. Furthermore, the net clinical benefit (NCB) endpoint comprising ISTH MBE or CRNMBE or DTE or unplanned revascularisation was investigated13.
STATISTICAL ANALYSIS
For the comparison of dabigatran 110 mg dual therapy versus warfarin triple therapy within the two subgroup categories (STEMI vs NSTEMI/UA/elective PCI), stratified Cox proportional hazards regression models were applied, which included age as a stratifying factor (non-elderly vs elderly: <70 versus ≥70 years in Japan and <80 vs ≥80 years elsewhere) and treatment arm. For the dabigatran 150 mg dual therapy versus warfarin triple therapy comparison, corresponding unstratified Cox proportional hazards regression models were applied (excluding patients aged ≥80 years [≥70 years in Japan] outside the USA). Corresponding hazard ratios (HRs) and two-sided 95% Wald confidence intervals (CIs) for HRs were calculated for each subgroup category. Exploratory treatment by subgroup interaction p-values resulting from Cox proportional hazard regression models were provided.
Results
PATIENT CHARACTERISTICS
A total of 2,725 patients were randomised in the RE-DUAL trial; 305 of these patients (11.2%) underwent PCI for STEMI (dabigatran 110 mg, n=113 versus n=106 warfarin) or dabigatran 150 mg (n=86 versus n=84 warfarin), whereas 2,393 (87.8%) patients had a PCI for NSTEMI, UA or underwent an elective PCI. For another 27 (1.0%) patients, the ACS type or the reason for PCI was missing. Patient characteristics for the 2,698 patients included in this subgroup analysis (STEMI vs rest) are shown in Supplementary Table 1. Overall rates of male sex, diabetes, median time between PCI and randomisation and mean HAS-BLED scores were similar between the two groups.
BLEEDING EVENTS
The risk of MBE and CRNMBE was reduced with both dabigatran 110 mg and 150 mg dual therapy compared with warfarin triple therapy (Figure 1, Supplementary Figure 1A, Supplementary Figure 1B), regardless of whether the patient had STEMI or underwent the PCI for a different reason (interaction p-value: 0.31). In STEMI patients, dabigatran 110 mg dual therapy had an 11.5% rate of MBE/CRNMBE compared with 29.2% in the warfarin triple therapy group (HR 0.39, 95% CI: 0.20-0.74). Similarly, the dabigatran 150 mg dual therapy group had lower risks of MBE/CRNMBE compared with the respective warfarin triple therapy group, regardless of whether the patient had STEMI or not, with an interaction p-value of 0.1560 (12.8% vs 28.6%, respectively; HR 0.43, 95% CI: 0.21-0.89 for STEMI).
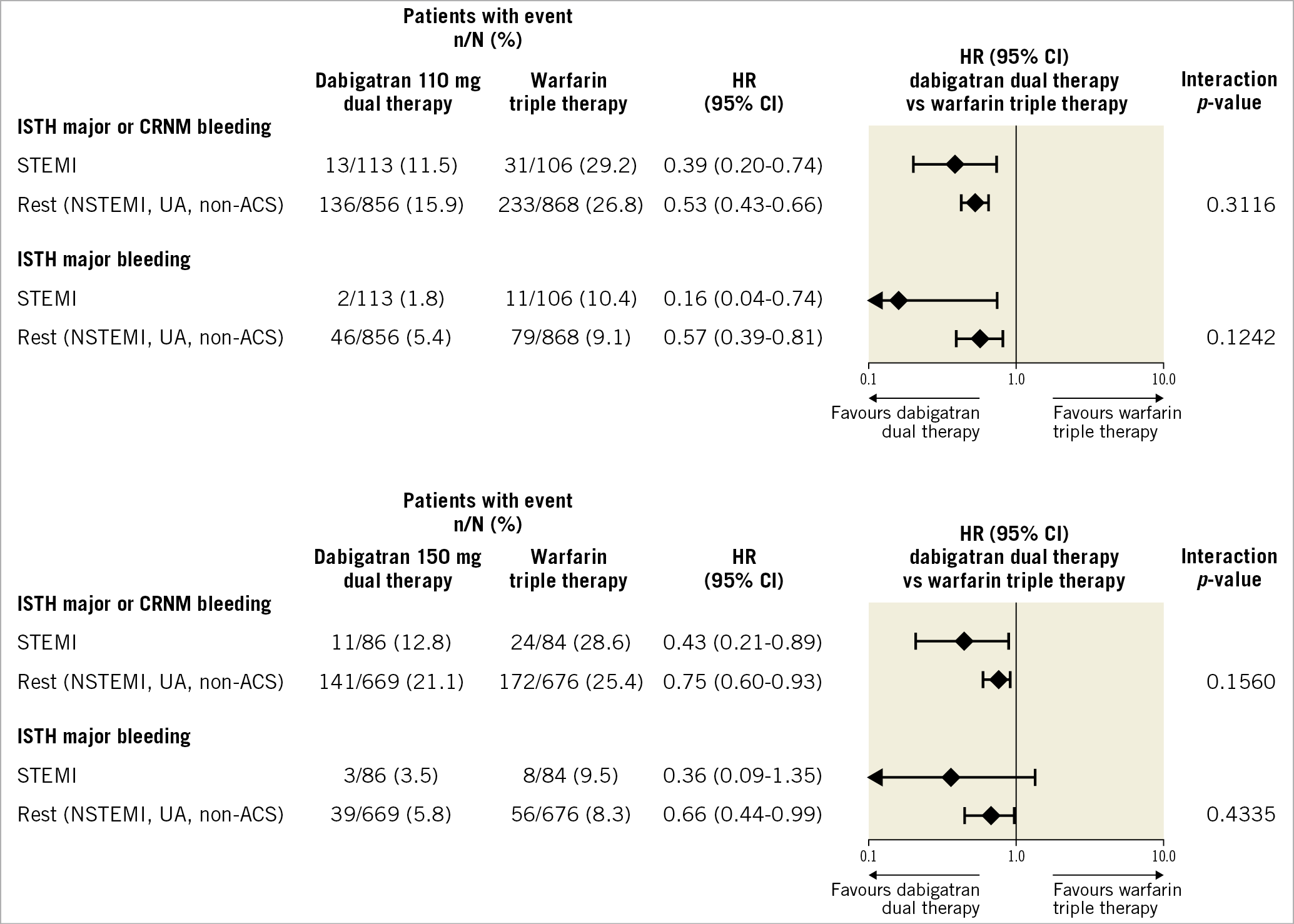
Figure 1. Bleeding endpoints in patients with STEMI versus others treated with dabigatran dual therapy versus warfarin triple therapy. HRs and Wald CIs from Cox proportional hazards model. For the comparison dabigatran 110 mg dual therapy versus warfarin triple therapy, the model is stratified by age, non-elderly versus elderly (<70 or ≥70 years in Japan and <80 or ≥80 years elsewhere). For the comparison dabigatran 150 mg dual therapy versus warfarin triple therapy, an unstratified model is applied and elderly patients outside the USA were excluded. Exploratory interaction p-values for the interaction between treatment and subgroup. ACS: acute coronary syndrome; CI: confidence interval; CRNM: clinically relevant non-major; HR: hazard ratio; ISTH: International Society on Thrombosis and Haemostasis; NSTEMI: non-ST-segment elevation myocardial infarction; STEMI: ST-elevation myocardial infarction; UA: unstable angina
When ISTH MBEs only were investigated, patients in the dabigatran 110 mg dual therapy group had lower risks compared with warfarin triple therapy, regardless of whether the patient had STEMI or underwent the PCI for a different reason (interaction p-value: 0.12; 1.8% vs 10.4%, respectively; HR 0.16, 95% CI: 0.04-0.74 for STEMI). Additionally, for the comparison of dabigatran 150 mg dual therapy versus warfarin triple therapy, the interaction p-value was statistically not significant (p=0.43), which suggests a consistent bleeding risk reduction regardless of whether the patient had STEMI or not (3.5% vs 9.5%, respectively; HR 0.36, 95% CI: 0.09-1.35 for STEMI; 5.8% vs 8.3%, respectively; HR 0.66, 95% CI: 0.44-0.99 for remaining patients) (Figure 1).
THROMBOEMBOLIC EVENTS
The overall risk of the composite ischaemic endpoint was 14.8% in patients with STEMI, 17.9% in patients with NSTEMI, 10.8% in patients with UA and 12.4% in patients with elective PCI. The risk of the composite endpoint of DTE or unplanned revascularisation was in general similar between the dabigatran dual therapy and warfarin triple therapy groups regardless of whether the patient had STEMI or underwent the PCI for a different reason (Figure 2). When looking at the dabigatran 150 mg dual therapy versus warfarin triple therapy comparison, an HR of 0.56 (95% CI: 0.20-1.51) for STEMI and 0.92 (95% CI: 0.68-1.24) for the remaining patients together with an interaction p-value of 0.3292 suggest consistent results as in the overall population. Numerically, however, the HRs slightly tended towards a value of >1.0 for the dabigatran 110 mg dual therapy versus warfarin triple therapy comparison (STEMI group HR 1.61, 95% CI: 0.85-3.08, remaining patients HR 1.06, 95% CI: 0.82-1.36, p-value for interaction 0.1990), whereas the HRs for dabigatran 150 mg dual therapy versus corresponding warfarin triple therapy did not. The Kaplan-Meier curves for the combined ischaemic endpoint are shown in Supplementary Figure 1C and Supplementary Figure 1D.
There were very few stent thromboses, limiting our ability to assess this endpoint. Figure 2 provides the results, where, in both STEMI and all other patients, there were numerically higher rates in the 110 mg dabigatran group compared with warfarin; in the 150 mg dabigatran dual therapy group, none of the STEMI patients had stent thrombosis and, in the other patients, the rates of stent thrombosis were similar (p interactions of 0.99 for both doses) (Figure 2).
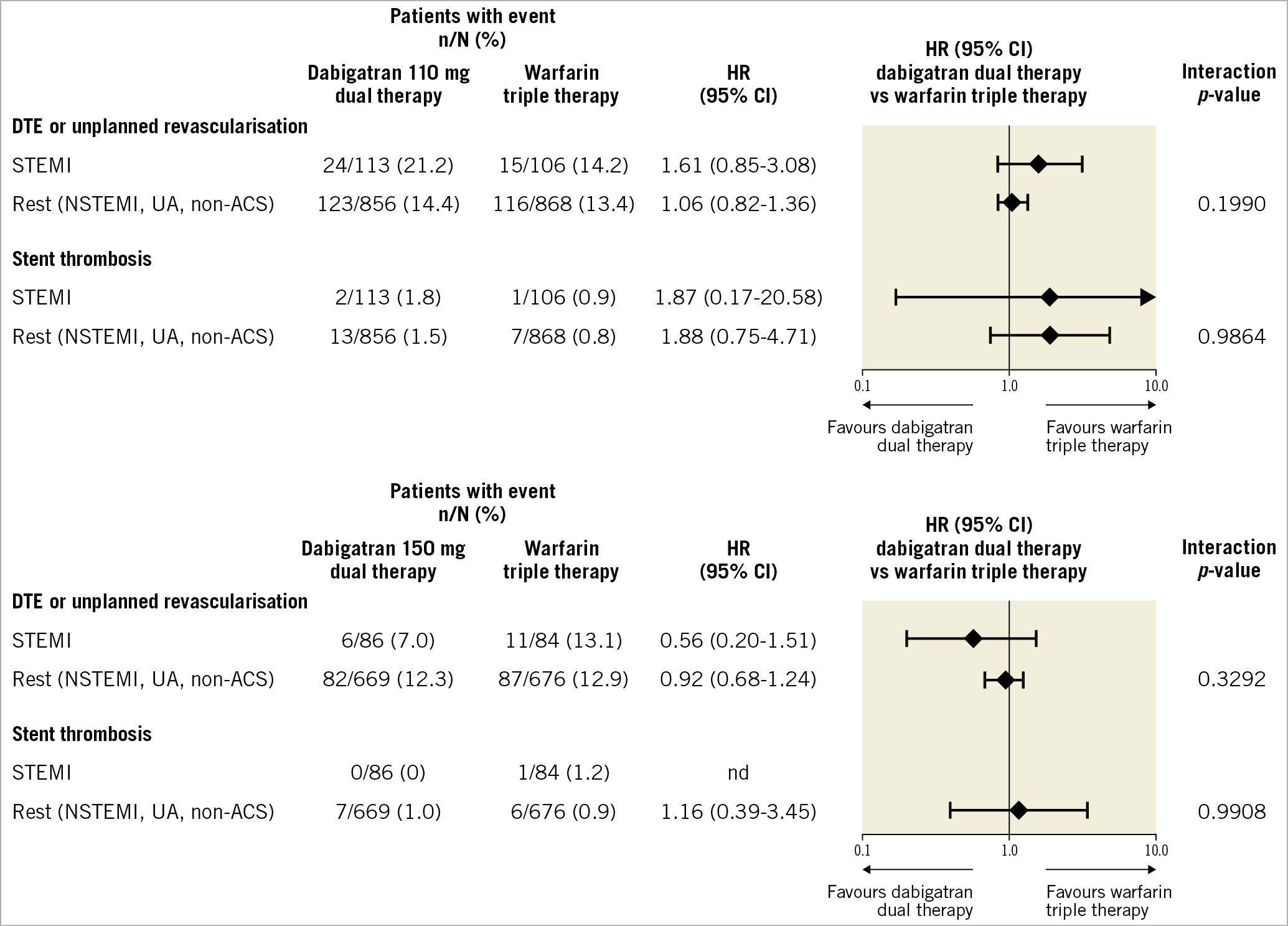
Figure 2. Thrombotic endpoints in patients with STEMI versus others treated with dabigatran dual therapy versus warfarin triple therapy. HRs and Wald CIs from Cox proportional hazards model. For the comparison dabigatran 110 mg dual therapy versus warfarin triple therapy, the model is stratified by age, non-elderly versus elderly (<70 or ≥70 years in Japan and <80 or ≥80 years elsewhere). For the comparison dabigatran 150 mg dual therapy versus warfarin triple therapy, an unstratified model is applied and elderly patients outside the USA were excluded. Exploratory interaction p-values for the interaction between treatment and subgroup. ACS: acute coronary syndrome; CI: confidence interval; DTE: death, thromboembolic events; HR: hazard ratio; nd: not determined; NSTEMI: non-ST-segment elevation myocardial infarction; STEMI: ST-elevation myocardial infarction; UA: unstable angina
NET CLINICAL BENEFIT
The risk of an NCB event in the STEMI patients was reduced with both dabigatran 110 mg (28.3% vs 38.7%, respectively; HR 0.74, 95% CI: 0.46-1.17) and 150 mg (18.6% vs 35.7%, respectively; HR 0.49, 95% CI: 0.27-0.91) dual therapy compared with warfarin triple therapy (Figure 3) with no statistically significant interactions between the treatment and the subgroup (STEMI vs NSTEMI/UA/elective PCI); interaction p-values were 0.80 for dabigatran 110 mg versus warfarin, and 0.12 for dabigatran 150 mg versus warfarin (Figure 3).
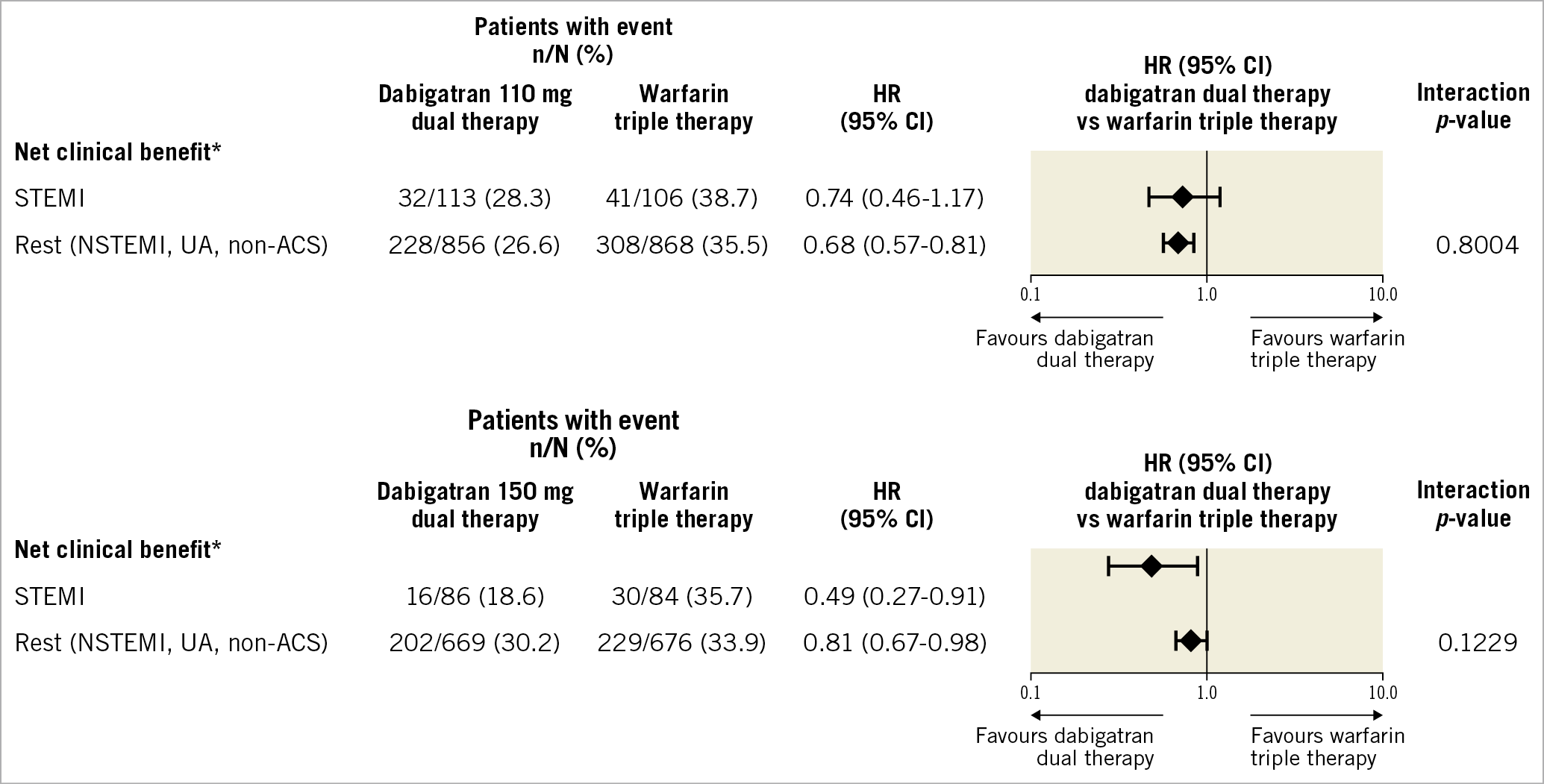
Figure 3. Net clinical benefit in patients with STEMI versus others treated with dabigatran dual therapy versus warfarin triple therapy. * ISTH major bleeding/clinically relevant non-major bleeding/DTE/unplanned revascularisation. HRs and Wald CIs from Cox proportional hazards model. For the comparison dabigatran 110 mg dual therapy versus warfarin triple therapy, the model is stratified by age, non-elderly versus elderly (<70 or ≥70 years in Japan and <80 or ≥80 years elsewhere). For the comparison dabigatran 150 mg dual therapy versus warfarin triple therapy, an unstratified model is applied and elderly patients outside the USA were excluded. Exploratory interaction p-values for the interaction between treatment and subgroup. ACS: acute coronary syndrome; CI: confidence interval; DTE: death, thromboembolic events; HR: hazard ratio; ISTH: International Society on Thrombosis and Haemostasis; NSTEMI: non-ST-segment elevation myocardial infarction; STEMI: ST-elevation myocardial infarction; UA: unstable angina
Discussion
In patients with AF and STEMI the risk of complications such as stroke, mortality and severe bleeding events is increased3,4. Whilst anticoagulation is indicated in AF to prevent stroke and other thromboembolic events, warfarin use in the STEMI population is associated with worse outcomes, especially more bleeding in patients with STEMI6,7. The advent of the introduction of NOACs for antithrombotic management of AF brought on alternatives to warfarin that carry lower bleeding risks. Rivaroxaban and apixaban, direct factor Xa inhibitors, were shown to decrease bleeding risk compared with warfarin in patients undergoing PCI, whilst having no difference in thrombotic complications in the PIONEER AF and AUGUSTUS trials, respectively14,15. Additionally, dual therapy (anticoagulation in addition to P2Y12 inhibitor) has been shown to decrease bleeding risk whilst also having no difference in thrombotic risk compared with triple therapy (anticoagulation in addition to P2Y12 inhibitor plus ASA)14,15,16,17,18. RE-DUAL PCI showed a decrease in bleeding with no effect on thrombotic complications with dabigatran dual therapy compared with warfarin triple therapy.
This subgroup analysis examined a high thrombotic risk group of patients, those with STEMI versus all other patients with ACS or elective PCI. A consistent reduction in bleeding events with no difference in thrombotic events for patients treated with dabigatran dual therapy was observed for both subgroup categories. When looking at the two doses of dabigatran used in the dual therapy strategies, the 150 mg dose looks very appealing in this high thrombotic risk group of STEMI patients. It has considerably lower bleeding risks, but also no increased (and actually a numerically lower) risk of thrombotic events. This parallels the main trial results in the overall population. There the risk of bleeding was significantly lower (HR 0.72, 95% CI: 0.58-0.88), and the risk of thrombotic events was not inferior (HR 0.89, 95% CI: 0.67-1.19). In addition, the risk of stent thrombosis seemed to be similar in the dabigatran 150 mg dual therapy group compared with the warfarin triple therapy group (HR 0.99, 95% CI: 0.35-2.81)12. Finally, the risk of an NCB event was also reduced with dabigatran 150 mg dual therapy compared with warfarin triple therapy (HR 0.79, 95% CI: 0.66-0.94)13.
There have been concerns about a higher risk of stent thrombosis from prior studies. In an analysis of the AUGUSTUS trial, the rate of patients with definite or probable stent thrombosis at six months was 13 (0.74%) for apixaban and 17 (0.97%) for VKA (HR 0.76, 95% CI: 0.37-1.56), and 11 (0.63%) for aspirin and 19 (1.08%) for placebo (HR 0.58, 95% CI: 0.28-1.22)19. Kaplan-Meier curves show that many of these early events occur in the first 30 days, the highest risk period. Similarly, in a meta-analysis that pooled four large trials (AUGUSTUS, RE-DUAL PCI, PIONEER-AF and WOEST), there was a trend towards more stent thrombosis in the NOAC plus P2Y12 inhibitor group compared with the VKA plus DAPT reference group, but this did not reach statistical significance17. Unfortunately, the number of stent thromboses was too small to draw any reliable conclusion. However, our results suggest no difference in thromboembolic events or stent thrombosis between dabigatran 150 mg dual therapy compared with warfarin triple therapy, in the overall trial or in the high thrombotic risk group of STEMI patients. As such, the results of this subgroup analysis and those of the trial overall suggest that dabigatran 150 mg dual therapy may be an ideal strategy for this high thrombotic risk population.
Limitations
As in any exploratory subgroup analysis, this subgroup analysis is not powered, so no formal statistical conclusion can be drawn. Also, the sample sizes of the two subgroup categories are quite unbalanced, so that the group of STEMI patients is quite small, which results in wide confidence intervals. Therefore, all results should be regarded as exploratory and interpreted with caution. However, this is a subgroup of interest to practising cardiologists. Thus, it is worth looking at the specific data. The findings are consistent with the overall trial, which support the results.
Conclusions
This post hoc analysis from the RE-DUAL PCI trial suggests that dabigatran dual therapy reduces bleeding events compared with warfarin triple therapy in patients with AF regardless of whether the patient had STEMI or underwent PCI for a different reason. Thromboembolic events occurred at similar risks, consistent with the main study outcomes. These results support the use of a dabigatran dual therapy even in the high-risk subgroup of patients with STEMI. The 150 mg dose especially seems to provide a favourable risk-benefit profile in the non-elderly STEMI population.
|
Impact on daily practice Dual therapy with dabigatran plus a P2Y12 inhibitor can be recommended as standard of care after PCI for STEMI in patients with atrial fibrillation. |
Acknowledgements
Editorial assistance limited to formatting and figure preparation was provided by Lisa Jolly, PhD, at Parexel, with funding from Boehringer Ingelheim International GmbH.
Funding
Boehringer Ingelheim International GmbH.
Conflict of interest statement
U. Zeymer, personal fees: Amgen, Abiomed, AstraZeneca, Bayer, Boehringer Ingelheim, BMS, Pfizer, Novartis, Sanofi, Idorsia, Lilly, The Medicines Company. S.H. Hohnloser, personal fees: Bayer HealthCare, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, Medtronic, Pfizer, SJM, ZOLL. P.G. Steg, research grants: Amarin, Bayer, Sanofi, Servier; speaking/consulting fees: Amarin, Amgen, AstraZeneca, Bayer/Janssen, Boehringer Ingelheim, Bristol-Myers Squibb, Idorsia, Lilly, Merck, Novartis, Novo Nordisk, Pfizer, Regeneron, Sanofi, Servier. J. Oldgren, fees to his institution: AstraZeneca, Bayer HealthCare, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, Pfizer, Portola, Roche Diagnostics, Sanofi. R.G. Kiss, honoraria as a speaker: Bayer, Boehringer Ingelheim, MSD, Pfizer. Z. Ongen, advisory/speaker fees: Bayer, Boehringer Ingelheim, Daiichi Sankyo. J. Navarro Estrada, advisory/speaker fees: AstraZeneca, Boehringer Ingelheim, Sanofi. T. Oude Ophuis, advisory/consulting/speaker fees: Amgen, AstraZeneca, Bristol-Myers Squibb, Medtronic. G.Y.H. Lip served as a consultant to: Bayer/Janssen, Bristol-Myers Squibb/Pfizer, Medtronic, Boehringer Ingelheim, Novartis, Verseon, Daiichi Sankyo; speaker: Bayer, Bristol-Myers Squibb/Pfizer, Boehringer Ingelheim, Daiichi Sankyo, Medtronic. No fees are personally received. M. Nordaby is an employee of Boehringer Ingelheim International GmbH. C. Miede is an employee of Mainanalytics GmbH, contracted by Boehringer Ingelheim International GmbH. J.M. ten Berg, advisory/consulting/speaker fees: Accumetrics, AstraZeneca, Bayer HealthCare, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, Eli Lilly, Ferrer, The Medicines Company, Pfizer; research grants: AstraZeneca, ZonMw. D.L. Bhatt discloses the following relationships – advisory board: Cardax, Cereno Scientific, Elsevier Practice Update Cardiology, Medscape Cardiology, PhaseBio and Regado Biosciences; honoraria: RE-DUAL PCI clinical trial steering committee funded by Boehringer Ingelheim; research funding: Abbott, Afimmune, Amarin, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Chiesi, CSL Behring, Eisai, Ethicon, Ferring Pharmaceuticals, Forest Laboratories, Fractyl, Idorsia, Ironwood, Ischemix, Lilly, Medtronic, PhaseBio, Pfizer, Regeneron, Roche, Sanofi Aventis, Synaptic and The Medicines Company. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

