
Introduction to the session: the trial headlines
The aim of the article is to capture the session at EuroPCR 2017 covering the PIONEER AF-PCI trial, communicate the analysis of the trialists, and report the views expressed in the interactive discussion. This article does not constitute an independent review of the topic by the authors.
Gilles Montalescot as co-chair (with Marco Valgimigli) introduced the session. In patients with atrial fibrillation (AF) undergoing percutaneous coronary intervention (PCI) on anticoagulation, additional dual antiplatelet therapy (DAPT) may reduce the risk for stent thrombosis and ischaemic events but increases the risk of bleeding. Although the WOEST trial showed that elimination of aspirin was associated with lower bleeding rates compared to triple therapy, the study was too small to ensure safety of this regimen1. The PIONEER AF-PCI trial demonstrated a reduction of bleeding events in these patients treated either with a P2Y12 inhibitor and rivaroxaban (RIV) 15 mg QD or with a DAPT consisting of a P2Y12 inhibitor and aspirin and very low dose rivaroxaban (2.5 mg BID) compared to triple therapy with a vitamin K antagonist (VKA) and DAPT2. In the light of this evidence, the antiplatelet and anticoagulation strategy after PCI in patients with AF was discussed at EuroPCR 2017 in a “Will this trial change my practice?” session.
Case presentation: how should I treat?
Sergio Leonardi presented a case of a 71-year-old male patient with paroxysmal AF (CHA2DS2-VASc score 4) first treated with VKA. Echocardiography showed reduced left ventricular function (ejection fraction 30%) with inferior and posterolateral akinesia. Coronary angiography was performed showing three-vessel disease with PCI of the mid and distal LAD as well as the distal RCA. The patient was enrolled in PIONEER AF-PCI and randomised one day after PCI to rivaroxaban 15 mg OD and clopidogrel 75 mg OD. After three days, the patient had ventricular fibrillation requiring cardiopulmonary resuscitation, with an ECG demonstrating ST elevation in the anterior leads after restoring sinus rhythm. Urgent coronary angiography revealed acute stent thrombosis of the LAD and RCA. Thrombus aspiration and re-PTCA of both vessels were performed. After primary PCI, platelet reactivity on clopidogrel was assessed via VerifyNow® (Accriva Diagnostics, San Diego, CA, USA), resulting in a low inhibition rate of 3%. Antithrombotic therapy was then switched from the randomised treatment to aspirin 100 mg OD and ticagrelor 90 mg BID. Left ventricular ejection fraction determined by echocardiography was further reduced to 25% with new akinesia of the apex, septum and anterolateral wall. During hospital stay and after two and six months, no recurrence of AF was detected and the patient stayed on DAPT with aspirin and ticagrelor.
Background: what was known before the trial
Tullio Palmerini reviewed PCI studies among patients with AF before the PIONEER trial. About 5-8% of patients undergoing PCI have concomitant AF3. The dilemma in these patients consists of having an effective antithrombotic therapy to minimise the risk for stent thrombosis and thromboembolism, which should also be safe concerning bleeding events. Dual antiplatelet therapy (DAPT) with aspirin and clopidogrel is associated with higher bleeding rates (HR 1.66, 95% CI: 1.34-2.04) compared to monotherapy with either VKA, aspirin, or clopidogrel. In case of triple therapy (TT) consisting of VKA and DAPT, bleeding risk is profoundly increased (HR 3.7, 95% CI: 2.89-4.76)4. In patients with AF, oral anticoagulation is clearly superior to DAPT for prevention of thromboembolic events5. The non-vitamin K oral anticoagulants (NOAC) have been shown to have a favourable risk-benefit profile for reduction of stroke or mortality with similar bleeding events compared to VKA6. In non-AF patients after an acute coronary syndrome, the addition of a NOAC to antiplatelet therapy results in a modest reduction in cardiovascular events but a substantial increase in bleeding events7. Therefore, the net clinical benefit was unfavourable for NOAC in combination with either single antiplatelet therapy (SAPT) or DAPT in these patients. The WOEST study enrolling patients with AF showed a significant reduction of the primary endpoint of any TIMI bleeding after PCI treated with warfarin and clopidogrel (double therapy) compared to those treated with warfarin, clopidogrel, and aspirin (triple therapy)1. However, the primary endpoint was mainly driven by significant differences in minor bleeding events, and the study was underpowered to prove differences concerning stent thrombosis. Tullio Palmerini remarked that a trial with bleeding as an endpoint is more feasible than a trial with stent thrombosis or stroke as an endpoint due to the low event rates (trial sample size should be approximately 14,000 patients considering a one-year rate of stent thrombosis of 1%). Additionally, triple therapy in the WOEST study was performed for 12 months, which may in part explain the excess of bleeding events in this group. The ISAR-TRIPLE trial investigated the effect of a clopidogrel therapy for six weeks compared to six months in addition to VKA and aspirin in patients with AF after DES implantation8. There was no significant difference between the two treatment arms concerning the primary composite endpoint consisting of death, myocardial infarction, stent thrombosis, stroke and bleeding. However, a post hoc landmark analysis of any BARC bleeding after six weeks revealed a higher event rate in the six-month group. The PIONEER AF-PCI trial as well as the ongoing REDUAL-PCI, ENTRUST-AF-PCI, and the AUGUSTUS studies aim to investigate the value of NOAC in combination with SAPT or DAPT in AF patients after PCI. The primary endpoint of these trials is safety (bleeding events) but not efficacy (such as prevention of thromboembolism or stent thrombosis). Tullio Palmerini concluded that the evidence before the PIONEER trial suggests that triple therapy is associated with significantly higher rates of bleeding events compared to double therapy. These studies were not powered for ischaemic outcomes such as stent thrombosis, and therefore the efficacy of these treatment strategies is still uncertain.
Trial analysis: summary of the trialists’ critical review
Alexandra Lansky reviewed the PIONEER AF-PCI trial design and outcomes. The ATLAS ACS study previously tested a low dose of rivaroxaban (2.5 mg) in acute coronary syndrome and demonstrated a reduction in cardiovascular death, myocardial infarction and stroke9. PIONEER AF-PCI enrolled 2,124 patients with non-valvular AF after coronary artery stenting and randomised patients within 72 hours after sheath removal into three treatment groups: the first group with rivaroxaban 15 mg QD and a P2Y12 inhibitor QD (SAPT+RIV 15 mg), the second group with rivaroxaban 2.5 mg BID, a P2Y12 inhibitor and aspirin 75-100 mg QD for 1-12 months followed by rivaroxaban 15 mg and aspirin 75-100 mg QD (DAPT+RIV 2.5 mg), and the third group with VKA, a P2Y12 inhibitor and aspirin 75-100 mg QD for 1-12 months, followed by VKA and aspirin 75-100 mg QD (DAPT+VKA)2. Lansky raised the issue that rivaroxaban in a dosage of 15 mg QD is not approved for treatment of either ACS or AF (renal dose) and in a dosage of 2.5 mg QD is approved in Europe for ACS but not even tested for AF. Patients in the PIONEER AF-PCI trial mostly represented daily practice with a median age of 71 years and median CHA2DS2-VASc score of 4. DES were used in one third of all cases and clopidogrel was chosen as P2Y12 inhibitor in 93%. The primary safety endpoint was the occurrence of clinically significant bleeding, which was a composite of TIMI major or minor bleeding or bleeding requiring medical attention. Patients treated with DAPT and VKA reached the primary safety endpoint significantly more often compared to both groups treated with low dose rivaroxaban (Figure 1). The number needed to treat (NNT) to prevent a clinically relevant bleeding was 12 in the DAPT+RIV 2.5 mg group and 11 in the SAPT+RIV 15 mg group compared to DAPT+VKA. However, the primary endpoint was mainly driven by bleedings requiring medical attention, whereas the rate of TIMI major or minor bleedings was not significantly different between the groups. A secondary endpoint analysis revealed that these reductions of bleeding events were irrespective of the duration of DAPT (1-12 months). There was no significant difference in the composite of cardiovascular death, myocardial infarction and stroke between the groups. However, the study was not powered to show superiority and therefore leaves uncertainty as to the effectiveness of preventing these events by low dose rivaroxaban. As a further secondary endpoint, reduced dose rivaroxaban reduced hospitalisation for bleeding (NNT 20-25) and hospitalisation for cardiovascular events (NNT 13). Lansky concluded that the PIONEER AF-PCI study has shown that a reduced dose of rivaroxiban (SAPT+RIV 15 mg or DAPT+RIV 2.5 mg) reduces clinically significant bleeding compared with conventional triple therapy of VKA+DAPT by about 40%. Furthermore, hospitalisations for bleedings and cardiovascular events were reduced by rivaroxaban, which may have cost implications. The rate of the secondary endpoint death, myocardial infarction, and stroke was low with no significant differences between the treatment groups. Hence, the major limitation of PIONEER AF-PCI is that it was not powered for efficacy in cardiovascular events, which leaves uncertainty about the effectiveness of these regimens on outcomes, much like the case after WOEST.
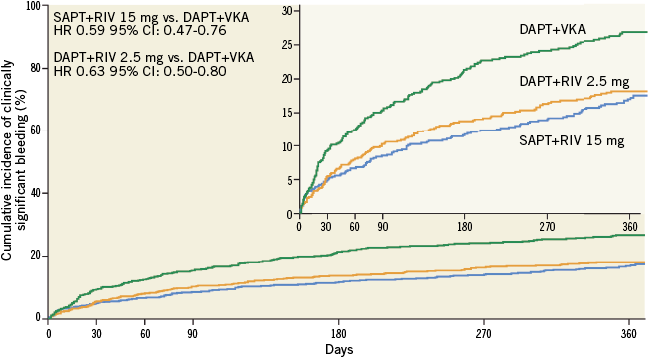
Figure 1. Primary safety endpoint (clinically significant bleeding) of PIONEER AF-PCI for each treatment group (modified from Gibson et al2).
“Will this trial change my practice?” discussion
Sergio Leonardi gave the conclusion of the case based on the data presented. He highlighted the importance of combination triple therapy for at least one month after PCI in patients with AF. In PIONEER AF-PCI, the rate of stent thrombosis, which was part of the efficacy evaluation, was too low to show any differences between the groups. The bleeding risk of patients on triple therapy is grossly time independent, while the risk for stent thrombosis diminishes over time. Sergio Leonardi mentioned that, in particular, prior bleeding, age, and renal function, all of which are part of established bleeding risk scores such as HAS-BLED, could help to assess bleeding risk in patients undergoing PCI. Routine platelet reactivity testing is not recommended and should only be considered in specific or high-risk situations (as prior history of stent thrombosis or compliance issues)10. Based on the findings of the PIONEER AF-PCI study and the clinical case presented, Sergio Leonardi concluded that, if dual therapy with rivaroxaban and a SAPT is selected, especially early after PCI, it is essential that the patient responds to that antiplatelet agent. Hence, platelet function testing may be considered in selected high-risk patients to assess clopidogrel (and/or aspirin) response before starting single antiplatelet therapy. After high-risk PCI in patients requiring OAC, immediate (<48 hours post PCI) discontinuation of aspirin should probably be considered only if the bleeding risk is very high.
The Chairperson’s conclusion: where do we stand now?
Marco Valgimigli began by asking for a poll as to which treatment strategy the audience preferred. Only a small minority agreed with rivaroxaban in the very low dose (2.5 mg BID) and DAPT after PCI, probably due to insufficient stroke prevention. The majority of the audience would choose rivaroxaban 15 mg QD in combination with SAPT in patients with a high bleeding risk. Otherwise, one month of triple therapy, then de-escalation according to current guidelines was the preferred treatment strategy. Marco Valgimigli agreed that PIONEER AF-PCI would change his practice as it confirms and extends to rivaroxaban the findings of WOEST. However, he underlined that the data for dual therapy after PCI in AF patients are insufficient concerning safety aspects and prevention of thromboembolism and stent thrombosis. Additionally, the benefits on bleeding events might be overestimated due to the long duration of dual antiplatelet therapy (up to 12 months). Three additional studies (REDUAL-PCI, ENTRUST-AF-PCI, AUGUSTUS) are ongoing and results will be reported soon.
| Summary ARGUMENTS FOR A CHANGE IN PRACTICE – Single antiplatelet therapy in combination with reduced oral anticoagulation reduces the rate of bleeding events in comparison to triple therapy after PCI in patients with atrial fibrillation (PIONEER AF-PCI, WOEST). ARGUMENTS AGAINST A CHANGE IN PRACTICE – Current trials are underpowered to assess the risk of ischaemic events adequately, especially stent thrombosis. – Differences in bleeding events are mainly driven by minor bleedings. – Patients must be responders to the agent used in case of single antiplatelet therapy in combination with oral anticoagulation. Results of further ongoing trials (REDUAL-PCI, ENTRUST-AF-PCI, AUGUSTUS) are expected soon. |
Conflict of interest statement
C. Ukena has received speaker’s honoraria from Bayer, Boehringer Ingelheim, and Daiichi Sankyo. S. Leonardi has received advisory board honoraria from AstraZeneca, Chiesi, The Medicines Company, and Ely Lilly, and institutional grant support from Daiichi Sankyo and AstraZeneca. G. Montalescot has received grants, personal fees and non-financial support from Amgen, grants, personal fees and non-financial support from AstraZeneca, grants, personal fees and non-financial support from Bayer, grants and personal fees from Boehringer Ingelheim, grants, personal fees and non-financial support from Bristol-Myers Squibb, grants from Celladon, grants and personal fees from Daiichi Sankyo, grants and personal fees from Eli Lilly, grants from ICAN, grants from Fédération Française de Cardiologie, grants and personal fees from Medtronic, grants, personal fees and non-financial support from MSD, grants, personal fees and non-financial support from Pfizer, grants, personal fees and non-financial support from Sanofi-Aventis, personal fees from Beth Israel Deaconess Medical, personal fees from Brigham Women’s Hospital, non-financial support from Cardiovascular Research Foundation, personal fees from CME Resources, personal fees and non-financial support from Europa, personal fees from Elsevier, personal fees from Lead-Up, personal fees from Menarini, personal fees from TIMI Study Group, personal fees from WebMD, grants and personal fees from Servier, personal fees and non-financial support from Actelion, personal fees from CCC, personal fees from INSERM, outside the submitted work. M. Valgimigli has received advisory board honoraria from Bayer and Daiichi Sankyo. The other authors have no conflicts of interest to declare.

