
There is something going on in the field of long-term antithrombotic therapy for patients with a recent acute coronary syndrome (ACS), where twelve months of dual antiplatelet therapy (DAPT) with low-dose aspirin and a potent P2Y12 inhibitor is regarded as the standard of care1. The superiority of prasugrel and ticagrelor over the earlier-generation clopidogrel is undisputed, but some problems remain2. For example, in TRITON and PLATO, the residual rates of ischaemic events were still as high as 9.9% at 15 months with prasugrel and 9.8% at 12 months with ticagrelor3,4. In addition, both drugs increased the rate of major bleeding unrelated to coronary artery bypass grafting as compared with clopidogrel. In aggregate, these findings underscore that, even in the era of potent P2Y12 inhibitors, there is room for improvement with respect to both efficacy and safety.
When we target platelets to reduce thrombus formation, we tackle only one part of the problem (Figure 1). Aspirin inhibits the cyclooxygenase 1, thus restraining the production of thromboxane A2, a stimulator of platelet activation and aggregation. Prasugrel, ticagrelor and clopidogrel block the platelet receptor for adenosine diphosphate, another potent agonist promoting activation and aggregation, and also modulate indirectly the production of thromboxane A2. The other part of the problem is fibrin. Polymerised fibrin contributes together with platelets to thrombus formation, which calls the coagulation cascade into question. In a modern view, platelets and coagulation factors are no longer discerned as separate players in the pathophysiological mechanism that leads to ACS, and their interplay is emphasised (Figure 1). Where the link between the two pathways becomes apparent is especially at the level of thrombin, which is not only the activated coagulation factor that converts fibrinogen into fibrin, but also a potent platelet activator itself through the PAR-1 and PAR-4 receptors.
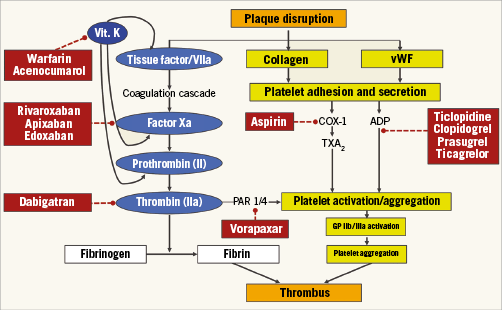
Figure 1. Schematic representation of factors involved in thrombus formation and targets of oral antithrombotic drugs. ADP: adenosine diphosphate; COX-1: cyclooxygenase 1; PAR 1/4: platelet-activated receptors 1 and 4; TXA2: thromboxane A2; Vit. K: vitamin K; vWF: von Willebrand Factor
Can we use approved drugs to reduce thrombin-mediated platelet activation, and hit the sweet spot of improved efficacy and acceptable safety? One attempt was using vorapaxar, a blocker of the PAR-1 receptor, but this strategy proved ineffective and harmful in the large (N=12,944) TRACER trial5. Another chance is decreasing the generation of thrombin, or blocking thrombin directly, by means of oral anticoagulants. Indeed, the idea of using oral anticoagulants in ACS patients is not that new. In 2006, a meta-analysis of 7,836 ACS patients from 10 studies suggested that adding warfarin with an international normalised ratio 2-3 to aspirin decreases the composite of death, myocardial infarction and stroke by 28% at the price of a 2.3-fold increased risk of bleeding6. Non-vitamin K antagonist oral anticoagulants (NOACs) are valuable alternatives to warfarin, with a favourable safety profile demonstrated in settings such as venous thromboembolism and atrial fibrillation. ESTEEM, a phase 2 trial of the direct thrombin inhibitor ximelagatran, showed promise in ACS, with no increase in bleeding and a significant reduction in ischaemic events7. Ximelagatran, however, has been withdrawn pending applications for marketing approval (and its distribution discontinued in countries where the drug had been approved) after reports of hepatotoxicity. Three more phase 2 trials of dabigatran (RE-DEEM), another thrombin inhibitor, and the factor X inhibitors darexaban (RUBY-1) and letaxaban (AXOM-ACS) failed to suggest any meaningful ischaemic benefit, and were associated with a dose-dependent increase in bleeding8-10.
The only NOACs whose clinical development progressed to a phase 3 ACS trial – following satisfactory results in phase 2 investigations – are apixaban and rivaroxaban11. In the phase 2 APPRAISE-1 trial, apixaban was investigated at escalating doses in 1,715 patients with recent ACS12. The 2.5 mg twice daily dose was selected for testing in the subsequent and larger (N=7,392) APPRAISE-2 study, where nevertheless no evidence of efficacy was demonstrated, and bleeding increased by 2.6-fold compared with placebo13. Rivaroxaban was first tested in 3,491 ACS patients from the phase 2 ATLAS-ACS trial14. Two low doses, 2.5 mg twice daily and 5 mg twice daily, were selected for phase 3 testing due to their potential of reducing ischaemic events in both patients on aspirin alone and those on DAPT with aspirin and clopidogrel, and despite some nominal increase in bleeding compared with placebo14. In ATLAS-ACS 2 (N=15,526), both doses of rivaroxaban reduced the primary ischaemic endpoint, but the 2.5 mg twice daily dose also reduced cardiovascular and overall mortality, a survival benefit that was missed by the 5 mg twice daily dose15. These results – along with a more favourable safety profile – led to the regulatory approval of the rivaroxaban 2.5 mg twice daily dose for ACS, and a IIb recommendation in clinical guidelines. This cautious recommendation reflects the significant increase in major and intracranial (albeit non-fatal) bleeding observed in ATLAS-ACS 2, even with the lower dose of rivaroxaban.
Thus, cardiologists are confronted with different potential strategies for long-term antithrombotic therapy management of patients with ACS, and there is a paucity or lack of comparisons to facilitate clinical decisions in daily practice. A recent randomised comparison of DAPT with prasugrel and ticagrelor in patients with ST-segment elevation ACS showed no differences between these regimens, but the study conclusions were limited by low statistical power16. Triple therapy with DAPT and the low dose of rivaroxaban has shown the largest reduction in cardiovascular and total mortality so far reported in post-ACS patients, but the uptake of this strategy is hampered by multiple considerations, including the modest appeal of “add-on” antithrombotic strategies in general for both patients and physicians, the increased rates of major and intracranial bleeding shown in ATLAS-ACS 2, and the lack of comparisons versus modern DAPT with prasugrel or ticagrelor. Simplification of the ATLAS-ACS 2 strategy has recently been attempted in GEMINI-ACS 1, a phase 2 investigation of combining low-dose rivaroxaban with a P2Y12 inhibitor (ticagrelor or clopidogrel) for the treatment of patients with stabilised ACS17. This strategy (which essentially replaces aspirin with a low dose of rivaroxaban after a short period of DAPT) showed similar risks of clinically significant bleeding compared with standard DAPT, but no plan for a phase 3 investigation has been communicated so far.
DAPT has been the mainstay of ACS pharmacological strategies for decades1. This practice stems from the paradigm that arterial thrombosis is a platelet-mediated process, which is essentially true in the acute phase of ACS but could be different when transitioning to the longer period. Should we broaden our view on different paradigms? While rivaroxaban struggles to find its clinical way in ACS, current understanding that blocking the thrombin pathway on top of inhibiting platelets may translate into the survival advantage displayed by ATLAS-ACS 2 is worthy of more investigation. NOACs decrease bleeding compared with warfarin in patients with atrial fibrillation, but this might not be true when these drugs are added on top of DAPT in patients with ACS. Removing aspirin from the drug cocktail, not necessarily in the acute phase, is an option to decrease bleeding, but whether this strategy is also effective was not addressed by the relatively small GEMINI-ACS 1, and its net clinical benefit remains uncertain. Also unclear is whether strategies modulating the intensity of DAPT (i.e., de-escalation from prasugrel or ticagrelor to clopidogrel after the acute phase) confer the same vascular protection on top of the expected decrease in bleeding complications18,19. If proven so, one might also envisage future scenarios where the acute phase of ACS will be treated with more potent antiplatelet therapy (i.e., DAPT with ticagrelor or prasugrel) and the chronic phase with less antiplatelet intensity (i.e., the 60 mg or 90 mg twice daily dose of ticagrelor alone, or DAPT with aspirin and clopidogrel) combined with a “touch” of antithrombin effect (i.e., the low dose of rivaroxaban). Recently, among patients with stable atherosclerotic vascular disease, a strategy of aspirin plus low dose rivaroxaban showed better cardiovascular outcomes than the use of aspirin alone20. Head-to-head outcome trials of “dual antiplatelet” versus “dual-pathway” strategies are of utmost interest and welcome in ACS.

