Abstract
Aims: Coronary artery disease (CAD) and atrial fibrillation (AF) are major determinants of morbidity and mortality. A combined treatment with antiplatelet agents and vitamin K antagonists limits the risk of stent thrombosis and stroke while increasing the rate of bleeding. The objective of this study was to investigate the impact of atrial fibrillation (AF) on long-term clinical outcomes in patients with CAD undergoing percutaneous coronary intervention (PCI) with drug-eluting stents (DES).
Methods and results: Among 6,308 consecutive patients undergoing PCI with DES between 2002 and 2009, 323 (5.3%) patients were diagnosed with AF. We compared clinical outcomes between patients with and those without AF throughout four years. The primary endpoint was a composite of all-cause mortality, myocardial infarction (MI), ischaemic stroke, and BARC bleeding type 3b/3c/5a/5b. In adjusted analyses, the primary composite endpoint was more frequent among patients with AF (HR 1.59, 95% CI 1.26-2.00; p<0.001). Differences were driven by an increased risk of all-cause mortality (HR 1.67, 95% CI 1.27-2.20; p=0.003), ischaemic stroke (HR 3.09, 95% CI 1.45-6.56; p=0.003), and intracranial bleeding (HR 4.28, 95% CI 1.36-13.48; p=0.013). We observed a gradient of risk among patients with higher CHA2DS2-VASc scores and modified outpatient bleeding risk index.
Conclusions: Among patients with CAD undergoing revascularisation with DES, AF confers an increased risk of all-cause mortality, ischaemic stroke, and intracranial bleeding. The hazard imposed by AF correlates with the CHA2DS2-VASc score.
Introduction
Coronary artery disease (CAD) remains the leading cause of mortality in industrialised countries, while atrial fibrillation (AF) is a major risk factor for cerebrovascular accidents1 and is associated with a two to threefold increased risk of stroke among patients with CAD or heart failure1. CAD and AF coexist in up to 25% of patients with stable CAD or acute myocardial infarction (MI)2-7, which may be related to common risk factors including advanced age, diabetes, hypertension, and congestive heart failure8,9. Apart from cerebrovascular events, AF has been associated with a one-and-a-half to twofold increased risk of mortality in the general population after adjustment for pre-existing cardiovascular conditions8 as well as adverse in-hospital and long-term outcome after MI4,10.
Effective treatments of both AF and CAD have major prognostic implications for event-free survival in contemporary clinical practice. Vitamin K antagonists (VKA) reduce the risk of cerebrovascular events among patients with AF11. Similarly, percutaneous coronary interventions (PCI) reduce the risk of death and MI among patients with acute coronary syndromes while improving symptoms and quality of life among patients with stable CAD12. Dual antiplatelet therapy (DAPT) minimises the risk of stent thrombosis (ST) following implantation of stents and has become a mainstay in contemporary PCI practice. While the combination of antiplatelet agents with VKA aims to prevent thromboembolic events related to AF as well as ST, this therapeutic strategy imposes an increased risk of bleeding and malcompliance13. European and North American consensus documents provide a comprehensive review of clinical data investigating the delicate balance between stroke prevention, stent thrombosis and bleeding among patients with AF undergoing PCI. Since prospective clinical data are, however, limited, consensus documents are based substantially upon expert opinion and emphasise the need for further investigation14-16. Although drug-eluting stents (DES) have considerably reduced the risk of repeat revascularisation compared with bare metal stents, this benefit comes at the expense of a prolonged duration of DAPT and triple antithrombotic therapy in case of concomitant AF17. Despite the introduction of bleeding risk scores18, the balance between the risk of stroke on the one hand and haemorrhagic events on the other is based largely on clinical judgement, and little is known regarding the impact of AF on ischaemic and haemorrhagic outcomes among patients undergoing PCI with DES. The objective of the present analysis was therefore to assess the impact of AF among patients with CAD undergoing PCI with the unrestricted use of DES in routine clinical practice during long-term clinical follow-up through four years.
Methods
PATIENT POPULATION
All patients undergoing implantation of DES at Bern University Hospital, Switzerland, were prospectively entered into a dedicated database. Between April 2002 and March 2009, a total of 6,535 consecutive patients underwent PCI with the unrestricted use of early and newer-generation DES. Drug-eluting stents were used in more than 90% during the study period. Considering the registry character of the study, no formal exclusion criteria applied. Demographic and clinical characteristics, information on PCI, and hospital outcome data were systematically collected. The study was approved by the institutional ethics committee at Bern University Hospital, Switzerland, and complied with the Declaration of Helsinki. Written informed consent for prospective follow-up was obtained from all patients.
PROCEDURES
It was generally recommended that oral anticoagulation be discontinued in patients undergoing elective coronary angiography three days prior to intervention with an intended target INR <2 at the time of the procedure. In patients with acute coronary syndromes, percutaneous coronary intervention was performed without prior discontinuation of oral anticoagulation. Patients were loaded with a thienopyridine, and anticoagulation during the intervention was guided by measurement of activated clotting time (ACT). A radial access was chosen if the bleeding risk appeared excessive due to elevated INR values. Unfractionated heparin in a dose of 5,000 IU or 70-100 IU/kg was administered during the procedure to maintain an ACT >250 seconds. PCI was performed in accordance with current guidelines. The antiplatelet and antithrombotic regimen was installed upon the discretion of the operator. A 12-lead electrocardiogram was routinely obtained before and after the procedure and cardiac enzymes were assessed within 24 hours of the procedure. Creatinin kinase (CK), CK-MB and troponin T were repeated every six to eight hours until identification of the peak levels in patients with signs of ischaemia.
DATA COLLECTION
AF was not formally classified at baseline. All medical records of patients with AF at baseline were therefore evaluated by three investigators (TP, TZ, CP), including a systematic review of all electrocardiograms at the time of admission, during the hospitalisation and at discharge. Reasons for VKA other than AF (mechanic valve prosthesis, pulmonary embolism, venous thromboembolism, pulmonary hypertension [>50 mmHg], left ventricular ejection fraction <30%, left ventricular aneurysm or thrombus) were also recorded. All patients were actively followed up to ascertain major adverse cerebrovascular and cardiovascular events. Survival data for all patients were obtained from hospital records and municipal civil registries. A health questionnaire was sent to all living patients with questions on rehospitalisation and major adverse cardiac events19. In case of missing response, patients were contacted by telephone. General practitioners, referring cardiologists and patients were contacted as necessary for additional information. For patients who underwent treatment for major adverse cardiac events at other medical institutions, external medical records, discharge letters and coronary angiography documentation were systematically collected and reviewed. All major adverse cardiac events and bleeding events were adjudicated by a cardiologist, and all neurologic events by a neurologist.
DEFINITIONS
AF was defined as at least one ECG documented episode of irregularly irregular RR intervals consistent with AF any time before discharge. Patients with atrial flutter were also included in the analysis. CHADS2 scores and CHA2DS2-VASc scores were created for patients with AF by the addition of points assigned to individual risk factors for stroke. Congestive heart failure, history of hypertension, age ≥75 years, and diabetes contributed one point each to the CHADS2 score, whereas two points were added for previous cerebrovascular events20. The CHA2DS2-VASc score assigned one point each for congestive heart failure, hypertension, age 65-74 years, diabetes, vascular disease, and female gender, and two points for age ≥75 years and a history of previous cerebrovascular events, respectively21.
Bleeding risk was assessed using the modified Outpatients Bleeding Risk Index (mOBRI) which takes into account age ≥65 years, a history of stroke, a history of gastrointestinal bleeding, recent myocardial infarction, anaemia, renal insufficiency, and diabetes. Patients with none of the factors are assumed to have a low risk of bleeding, patients with one to two factors have an intermediate risk of bleeding, and patients with three to four factors are expected to have a high risk of bleeding22,23. We prespecified a composite primary endpoint consisting of all-cause mortality, non-fatal MI, ischaemic stroke, and BARC (Bleeding Academic Research Consortium) bleeding type 3b, 3c, and type 5a and 5b. Cardiac death was classified as death secondary to an immediate cardiac cause, procedure-related mortality, and death of unknown cause. MI was classified as Q-wave or non-Q-wave MI. Q-wave myocardial infarction was diagnosed in the circumstance of symptoms or signs of ischaemia in the presence of new pathological Q-waves in ≥2 contiguous leads. Non-Q-wave myocardial infarction was defined as an elevation in CK to ≥2x upper limit of normal (ULN) and a rise in CK-MB or troponin to ≥3x ULN in the presence of ischaemic symptoms or ischaemic ECG changes. Target vessel revascularisation (TVR) was defined as any repeat revascularisation within the vessel of the target lesion. Target lesion revascularisation (TLR) required revascularisation of a stenosis within the stent or the 5-mm borders adjacent to the stent. Stent thrombosis (ST) was defined according to the Academic Research Consortium (ARC) definitions24. Bleeding was classified in accordance with the definition by the BARC, as well as the TIMI and the GUSTO definition25. Since the BARC definition was only recently published, we refrained from reporting BARC bleeding types 1 and 2 due to difficulties in categorising objectively such events in a retrospective fashion. BARC bleeding type 3a was defined as an overt bleeding event plus a drop in haemoglobin of 3 to 5 g/dl or any transfusion in the setting of overt bleeding; type 3b was defined as overt bleeding plus haemoglobin drop ≥5 g/dl, cardiac tamponade, bleeding requiring surgical intervention for control, and bleeding requiring intravenous vasoactive agents; type 3c was documented in the presence of intracranial haemorrhage or intraocular bleeding. BARC bleeding type 4 compromised perioperative intracranial bleeding, reoperation after closure of sternotomy for the purpose of controlling bleeding, and transfusion of ≥5 units packed red blood cells within 48 hours, and was adjudicated by a cardiovascular surgeon (TC). Type 5a and type 5b were determined as probable and definite fatal bleeding, respectively. All cerebrovascular events were reviewed and adjudicated by two board certified neurologists (SJ, HM) and classified as ischaemic stroke, intracranial haemorrhage, transient ischaemic attack and unclear cerebrovascular events. Ischaemic stroke was diagnosed in case of a focal neurological deficit (motor or sensory deficit, dysarthria, aphasia, visual loss) with duration of ≥24 hours and/or imaging documentation of ischaemia and exclusion of intracranial bleeding. Transient ischaemic attack was defined as a neurological deficit with resolution of symptoms within 24 hours and without evidence of ischaemic brain injury from neuroimaging. An unclear neurologic event comprised all events reported by the patients without appropriate documentation to allow further classification into ischaemic stroke or transient ischaemic attack. In case of multiple events in one patient, only the first event in each event category was used for the purpose of this analysis.
STATISTICAL ANALYSIS
The baseline clinical and procedural characteristics and the medications at discharge were presented as counts and percentages (%) for categorical variables and as mean and standard deviation (SD) for continuous variables. Differences between the two groups (AF and absence of AF) were performed using chi-square test for categorical variables and Student’s t-test for continuous variables. Incidence rates (IR) per 100 patient years (PY) were reported rather than crude event rates to account for potential differences in follow-up time. We performed Cox proportional hazard models, with initial adjustment for type of stent and full adjustment in a multivariable analysis for age, gender, hypertension, hyperlipidaemia, diabetes, smoking, renal impairment, LVEF and ACS to compare the clinical outcomes between patients with and without AF at baseline. Hazard ratios and Kaplan-Meier curves with 95% confidence intervals were constructed for the time from index PCI until the end of four years. We further categorised the AF group according to onset (pre-existing AF vs. new onset periprocedurally), baseline risk according to CHA2DS2-VASc score, type of AF (permanent, persistent or paroxysmal), and compared the clinical outcomes of these groups, separately, to those presenting with no AF at baseline. Then, we performed landmark analyses according to a prespecified landmark point at one year (360 days) or six months (180 days) and estimated hazard ratios and cumulative IRs separately for events up to one year/six months, and from after one year/six months until the end of follow-up at four years. Stratified analyses were performed by prespecified baseline characteristics and χ2 test was used to assess the interaction between presence or absence of AF and these characteristics. Statistical analyses were performed using STATA release 11.2 (Stata Corp., College Station, TX, USA). All p-values are two-sided.
Results
Among 6,308 patients undergoing PCI with at least one DES between April 2002 and March 2009, 87 patients were excluded since they were not followed up after index PCI, and 180 patients were excluded due to indications for oral anticoagulation other than AF. Therefore, 6,041 patients were included in the analysis.
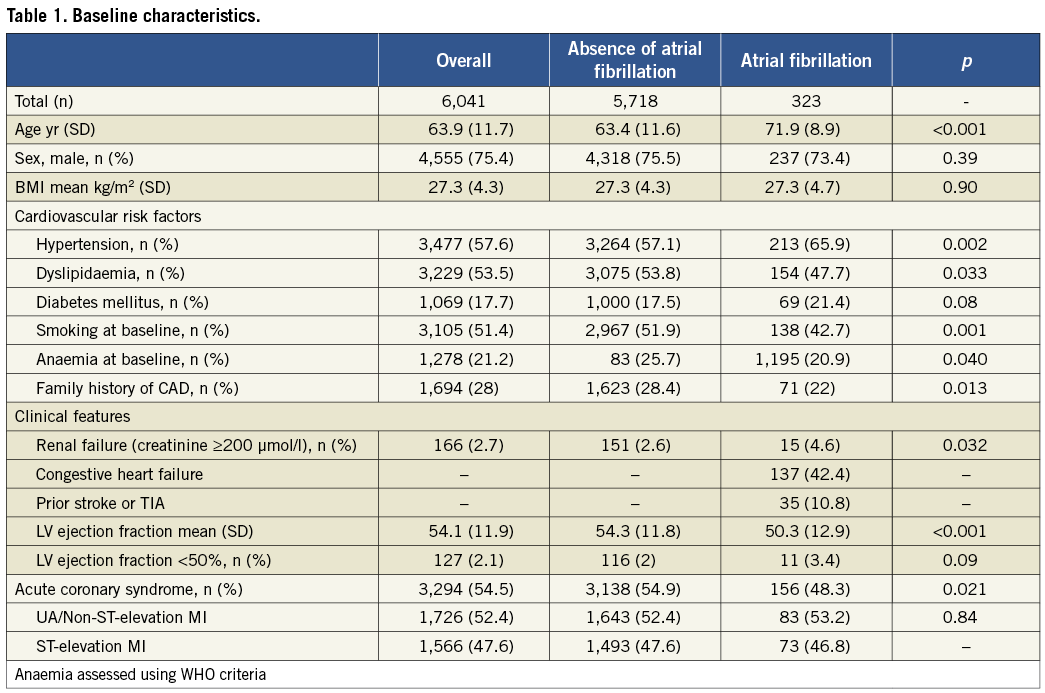
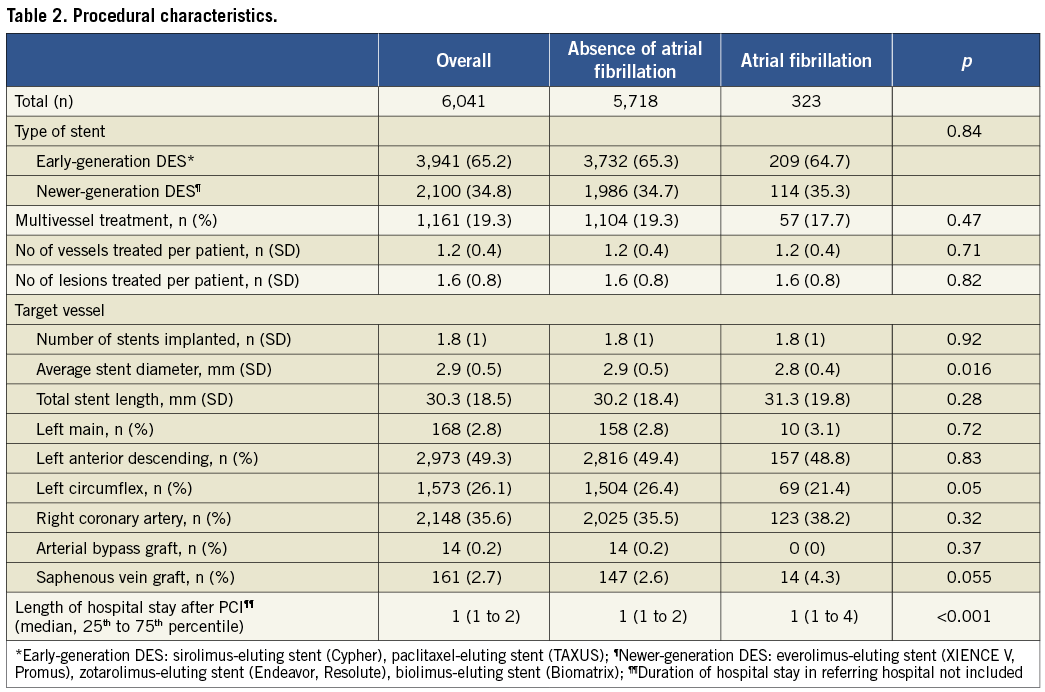
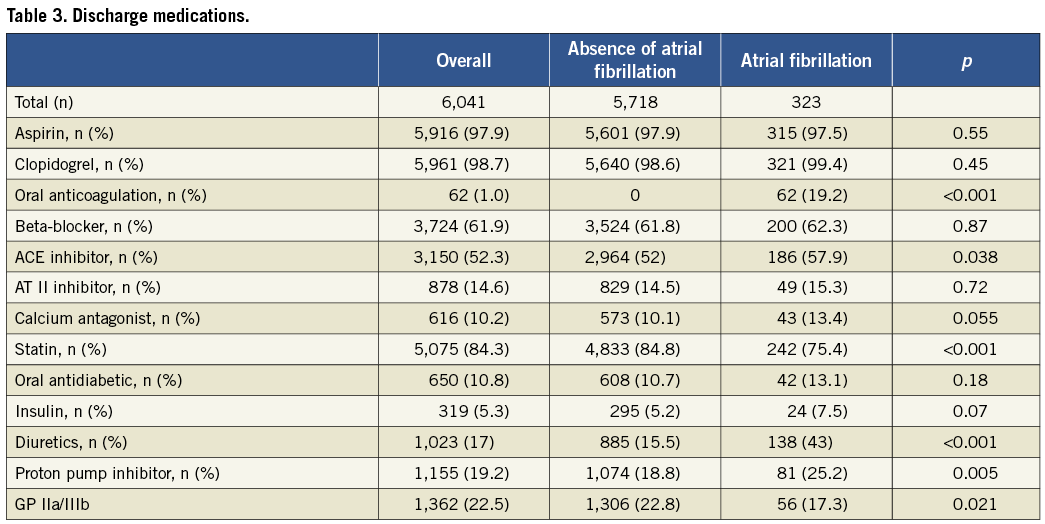
AF was documented in 323 patients (5.3%). Two patients had undergone radiofrequency ablation prior to inclusion into the registry and one patient had undergone left atrial appendage occlusion prior to inclusion. Among patients with AF, 26 patients (8.0%) had an INR >2 at the time of PCI, and a radial access was chosen in a single patient only, due to chronic occlusion of the iliac arteries bilaterally. Baseline characteristics of all patients undergoing PCI with or without AF are summarised in Table 1. Patients with AF were older (72±9 years vs. 63±12 years, p<0.001), had a higher prevalence of hypertension (66% vs. 57%, p=0.002), renal failure (5% vs. 3%, p=0.03), and reduced left ventricular ejection fraction (50±13% vs. 54±12%, p<0.001). Conversely, the prevalence of dyslipidaemia (48% vs. 53%, p=0.03), smoking (43% versus 52%, p=0.001) and a family history of CAD (22% vs. 28%, p=0.01) was lower in patients with AF. Procedural characteristics and medications at the time of discharge are shown in Table 2 and Table 3. Periprocedural administration of glycoprotein IIb/IIIa inhibitors was less frequent among patients with AF as compared to those without (17% vs. 23%, p=0.02). A CHADS2 score ≥2 was recorded among 203 AF patients (62%). 315 patients (98%) were discharged on aspirin and 321 patients (99%) on clopidogrel, and 62 patients (19.2%) were discharged on oral anticoagulation; in addition, 69 patients (21%) were recommended to reinstall VKA within three months after the procedure. Amiodarone and digoxin were prescribed to 56 (17.3%) and 22 (5.6%) patients with AF, respectively. At the end of follow-up, 40.5% and 3.8% of the patients with and without AF were on oral anticoagulation (p<0.001), respectively. Adherence to aspirin amounted to 90.7% among patients with AF, and to 67.4% among patients without AF p<0.0001); no difference with regard to clopidogrel intake was observed at the end of follow-up (22.4% without AF vs. 20.5% with AF, p=0.50).
CLINICAL OUTCOME
Among patients with AF, 19 patients underwent radiofrequency ablation and two patients underwent percutaneous left atrial appendage occlusion during the study period. Patients with AF had a higher risk of the primary composite endpoint after initial (HR 2.08, 95% CI 1.66-2.61, p<0.0001) and full adjustment (HR 1.59, 95% CI 1.26-2.00, p<0.0001) throughout four years of follow-up with a similar hazard during the first (HR 1.67, 95% CI 1.20-2.32, p=0.003) and subsequent years (HR 1.68, 95% CI 1.19-2.37, p=0.003) (Table 4, Figure 1). Patients with new-onset AF were observed to have a higher risk of adverse outcome as compared to patients without AF (HR 2.04, 95% CI 1.38-3.00, p<0.0001), and a strong trend towards adverse outcome was noted among patients with pre-existing AF (HR 1.43, 95% CI 0.99-2.08, p=0.06) (Figure 2).
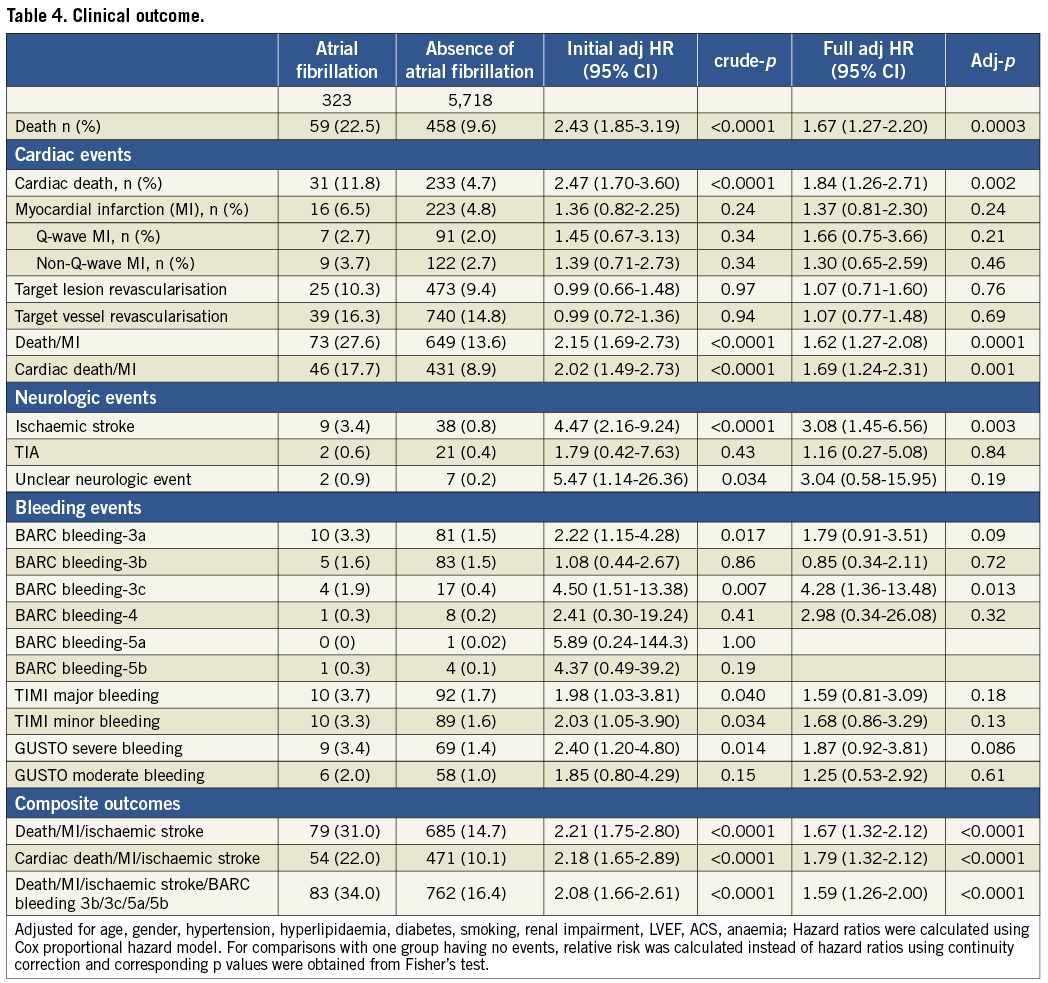
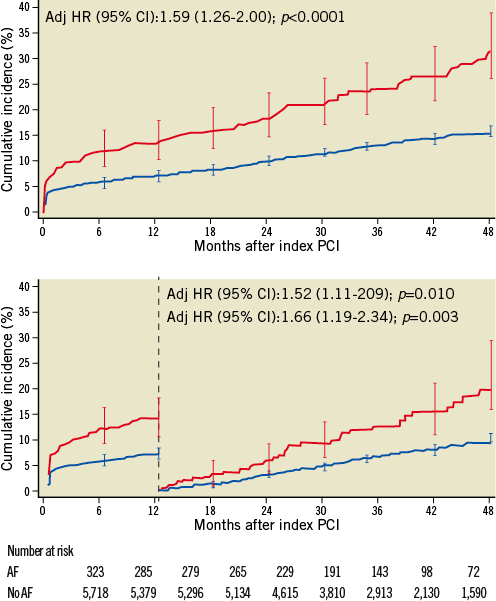
Figure 1. Primary composite outcome of all-cause mortality, myocardial infarction, stroke, and BARC bleeding type 3b, 3c, 5a, and 5b through 4 years of follow-up and landmark analysis with the landmark set at 1 year. The red line indicates patients with and the blue line patients without atrial fibrillation at baseline.
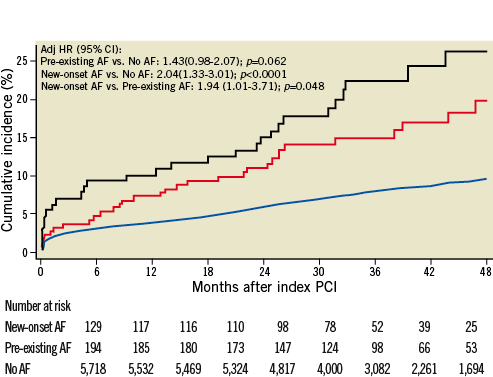
Figure 2. Primary composite outcome of all-cause mortality, myocardial infarction, stroke, and BARC bleeding type 3b, 3c, 5a, and 5b through four years of follow-up according to pre-existing atrial fibrillation or new onset of atrial fibrillation. The black line represents patients with new-onset atrial fibrillation during the course of the index hospitalisation. The red line represents patients with pre-existing atrial fibrillation prior to hospital admission. The blue line indicates patients without atrial fibrillation.
Patients with AF had a higher risk of all-cause death after initial (HR 2.43, 95% CI 1.85-3.19, p<0.001), and full adjustment (HR 1.67, 95% CI 1.27-2.20, p<0.003) (Table 4, Figure 3) up to four years. In a landmark analysis with cut-off set at one year, the risk of all-cause mortality increased steadily during the first (HR 1.51, 95% CI 1.01-2.27, p=0.047) and subsequent years (HR 1.85, 95% CI 1.27-2.69, p=0.001). AF was associated with an increased risk of ischaemic stroke (HR 3.08, 95% CI 1.45-6.56, p=0.003) and intracranial bleeding (HR 4.28, 95% CI 1.36-13.48, p=0.013) after initial and full adjustment (Table 4). There was no difference between patients with and without AF in terms of ARC-defined ST (Table 5).
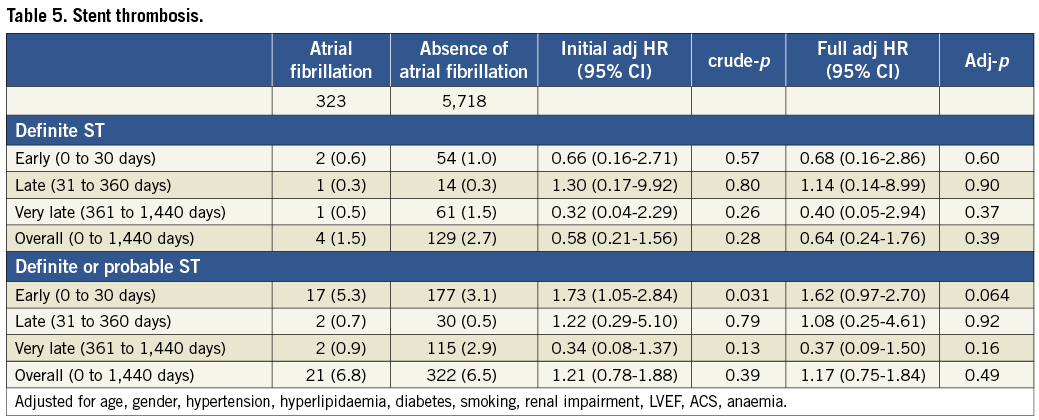
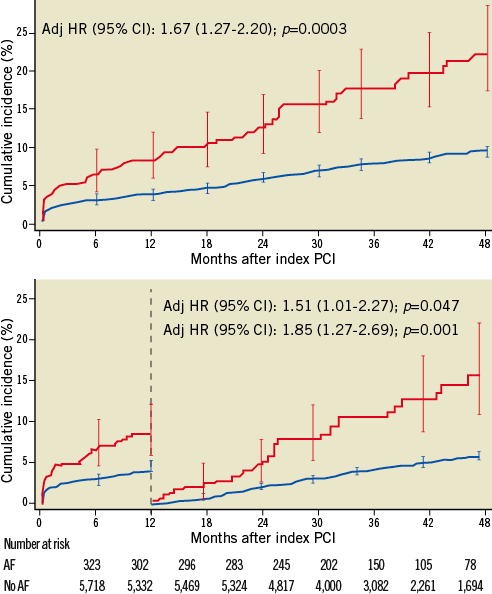
Figure 3. All-cause mortality up to four years of follow-up and landmark analysis at one year. The red line indicates patients with and the blue line patients without atrial fibrillation at baseline.
A graduated increase in the incidence rate (IR) of the primary endpoint was observed with increasing CHA2DS2-VASc scores (adjusted p for trend <0.0001) (Figure 4). At the same time, the risk of adverse clinical outcome tended to increase with increasing mOBRI score (Table 6).
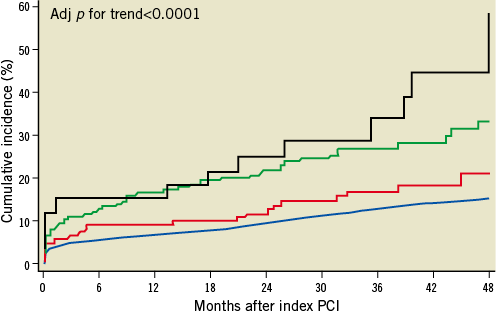
Figure 4. Primary outcome up to four years of follow-up according to severity of atrial fibrillation based on CHA2DS2-VASc score at baseline. The black line shows the Kaplan-Meier curve for patients with a CHA2DS2-VASc score >5. The green line represents patients with a CHA2DS2-VASc score of 4-5. The red line represents patients with a CHA2DS2-VASc score of 1-3. The blue line indicates patients without atrial fibrillation.
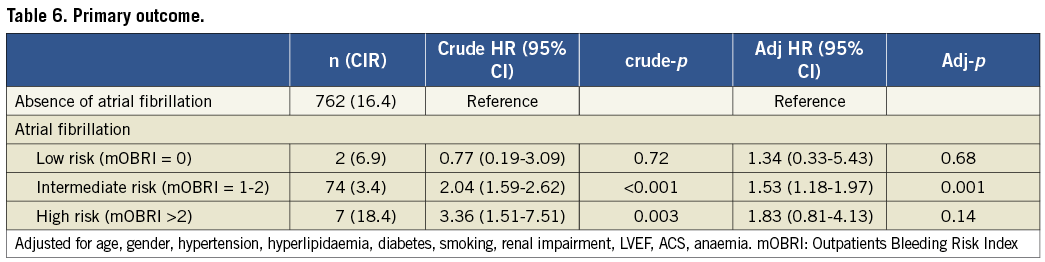
A stratified analysis of the primary composite endpoint across major subgroups showed a fourfold increased risk among patients with AF and renal failure (HR 3.80, 95% CI 1.84-7.85), while patients with AF but without renal failure showed only a 47% increase in relative risk (HR 1.47, 95% CI 1.14-1.88). The difference was marginally significant (pinteraction=0.05) (Figure 5). Outcome among patients with AF treated with or without oral anticoagulation in addition to dual antiplatelet therapy is summarised in Figure 6.
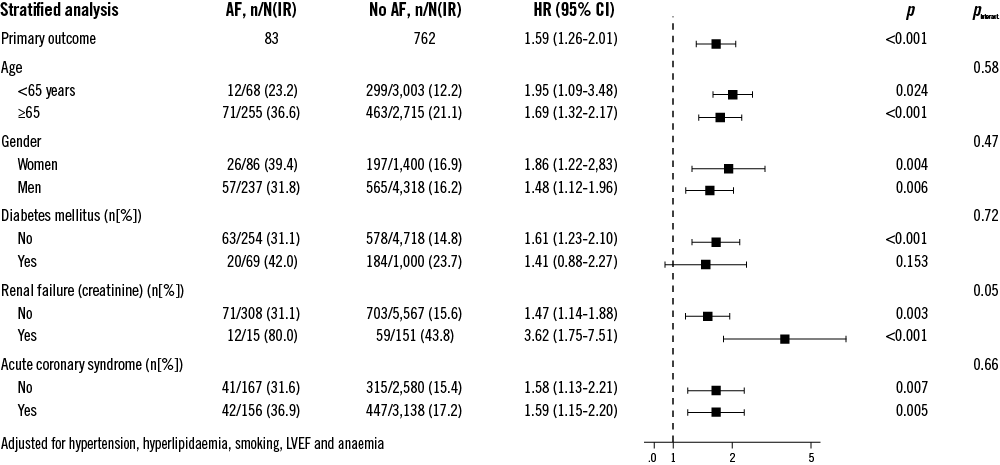
Figure 5. Stratified analysis of the primary composite outcome across major subgroups.
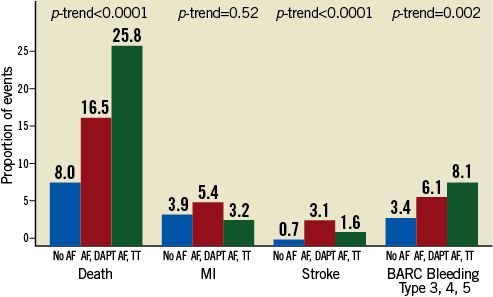
Figure 6. Stratified analysis according to atrial fibrillation treated with or without oral anticoagulation at discharge: single components of the primary outcome in patients with no atrial fibrillation (blue bars), patients with atrial fibrillation on dual antiplatelet therapy without oral anticoagulation (red bars), and patients with atrial fibrillation on triple antithrombotic therapy (green bars).
Discussion
The principal findings of the present analysis can be summarised as follows: 1) a diagnosis of AF was documented in 5.3% of unselected patients undergoing PCI; 2) AF was associated with an increased risk of the primary composite endpoint of all-cause mortality, non-fatal MI, ischaemic stroke, and types 3b, 3c, 5a, 5b BARC bleeding through four years; 3) AF was also associated with an increased risk of the individual endpoints of all-cause mortality, ischaemic stroke, and intracranial bleeding; 4) the increased risk was directly related to increasing CHA2DS2-VASc scores; 5) AF in combination with renal failure portended a particularly poor long-term outcome.
The prevalence of AF in this unselected patient population undergoing PCI with the unrestricted use of DES amounted to 5.3%, which is consistent with previous reports of patients with stable CAD and acute MI2-7. In line with established risk factors for AF8,9, patients with AF were older and had a higher prevalence of hypertension and compromised left ventricular ejection fraction. Of note, only a minority of patients were treated with triple antiplatelet therapy, while most patients were discharged on DAPT.
Patients with AF had a higher risk of the primary composite outcome as well as the individual endpoints including all-cause mortality, ischaemic stroke, and intracranial bleeding compared to patients without AF, whereas there were no differences in terms of non-fatal MI, ST, or repeat revascularisation procedures. Landmark analyses for the primary composite endpoint as well as all-cause mortality demonstrated a steady risk during the entire duration of follow-up with no differences between the first year and subsequent years. The association of AF with all-cause mortality corroborated the findings of previous studies8-10,14, persisted even after full adjustment of baseline characteristics, and was not accompanied by differences in the incidence of non-fatal MI or ST, suggesting a potential relationship with the occurrence of ischaemic and haemorrhagic strokes. The CHA2DS2-VASc score directly correlated with the risk of the primary composite endpoint.
We observed a particularly increased risk of adverse outcome among patients with new-onset AF. This observation is in line with a community-based study, which showed an excess mortality within the first four months after newly diagnosed AF (HR 9.62, 95% CI 8.93-10.32) as compared to beyond the first four months (HR 1.66, 95% CI 1.59-1.73). Similarly, patients with acute MI complicated by AF were found to have an increased risk of all-cause mortality (HR 1.82, 95% CI 1.39-2.39, p<0.001) and stroke (HR 2.29, 95% CI 1.43-3.68, p<0.001) up to three years of follow-up in the OPTIMAAL experience10.
Patients with AF were at increased risk of both ischaemic stroke and intracranial bleeding. In addition, a trend towards a higher incidence of BARC bleeding type 3a was observed in patients with AF. Oral anticoagulation was installed at discharge in 19% of patients - in a majority of patients in combination with DAPT - whereas a recommendation to recommence oral anticoagulation within three months of DES implantation (after discontinuation of either aspirin or clopidogrel) was made in another 22% of patients. Treatment recommendations from the ESC Working Group on Thrombosis support uninterrupted VKA treatment as an alternative to heparin bridging therapy in the periprocedural phase, endorse triple therapy post-interventionally instead of temporary replacement of VKA by dual antiplatelet drug therapy, and recommend the avoidance of DES in patients with very high bleeding risk14. A North American consensus document recommends a radial access in order to reduce periprocedural bleeding, generally prefers BMS over DES, and suggests considering proton pump inhibitors (e.g., pantoprazole) for patients with triple therapy15. Antithrombotic strategies following coronary artery stenting differ between European and North American consensus documents. In elective cases, European experts recommend triple therapy for the duration of three months for patients treated with limus analogues and six months for patients treated with paclitaxel-eluting stents, and for the duration of six months in patients undergoing PCI for acute coronary syndromes irrespective of the stent. In contrast, North American guidelines recommend triple therapy for at least six months in patients with low risk of stent thrombosis and bleeding, and for 12 months in patients with high risk of stent thrombosis and low risk of bleeding. Both consensus documents were published after recruitment of our study population and did not reflect the standard of care at our institution during the inclusion phase of our registry.
In the largest randomised trial to date comparing 573 patients undergoing PCI, patients receiving dual therapy with oral anticoagulants and clopidogrel were found to have significantly fewer TIMI bleeding events compared with patients receiving triple therapy including aspirin, whereas numbers of stent thrombosis, myocardial infarction, and stroke were comparable (Dewilde W, What is the Optimal Antiplatelet and Anticoagulant Therapy in Patients with Oral Anticoagulation and Coronary Stenting (WOEST) trial. Oral presentation, ESC, Munich 2012). Among AF patients followed in a large integrated healthcare system in Northern California, oral anticoagulation reduced the risk of ischaemic stroke and all-cause mortality, while increasing the risk of intracranial haemorrhage as compared to patients without oral anticoagulation26. Patients with both a diagnosis of CAD requiring revascularisation by PCI as well as AF represent a high-risk population at either side of the risk scale (ischaemia and bleeding). Thus, the presence of CAD has been associated with a strong trend towards an increased risk of thromboembolic events21 among patients with AF. At the same time, triple antithrombotic therapy in patients undergoing PCI with AF has been associated with a higher incidence of major bleeding events compared with other regimens27. Of note, risk scores for bleeding or stroke cannot be easily generalised to patients with AF undergoing PCI with DES as they do not take into consideration the risk of stent thrombosis21,28 and the duration of antithrombotic therapy. However, we did not observe a difference in the incidence of ST between patients with and without AF in the present study. Although we can only speculate how event rates in terms of ischaemic risk reduction and bleeding risk increase would have been with a more aggressive regimen of triple antithrombotic therapy, the adverse outcome of patients with AF undergoing PCI with DES requires more research as to the optimal intensity and duration of antithrombotic therapy.
In a stratified analysis of the primary composite outcome, we observed an excessive risk for patients with both AF and renal failure, corroborating the results of previous reports showing an increased risk of ischaemic stroke and all-cause mortality in such patients29,30. The increased thromboembolic risk among patients with AF and renal failure may be related to elevations in inflammatory markers and a procoagulant milieu31. Moreover, renal failure has been identified as an independent predictor of major haemorrhage in a cohort of outpatients treated with oral anticoagulants32, potentially related to platelet dysfunction in patients with renal failure33,34.
Limitations
This study has several limitations. Among patients with AF, different antithrombotic regimens were applied according to the discretion of the treating physician, not following a prospectively defined regimen; even though the recommended antithrombotic regimen at discharge and during follow-up was recorded, information in terms of the intensity of anticoagulation and compliance was not available. However, patients taking oral anticoagulation for indications other than AF were excluded from the present study cohort. The current study reports on antithrombotic strategies including the use of aspirin, clopidogrel and VKA. The impact on outcome of combinations with recently introduced antiplatelet agents such as prasugrel and ticagrelor, as well as with novel antithrombotic agents such as dabigatran, rivaroxaban, and apixaban, are not reflected by the present analysis. Bleeding data were recorded as evidenced by significant changes in haemoglobin values, adverse events leading to hospitalisation and patient self-reported episodes. Due to recollection bias, minor bleeding events may have been underreported as compared with major bleeding events. We cannot differentiate whether atrial fibrillation is a risk marker or a risk factor although we adjusted for known risk factors associated with atrial fibrillation. In order to address this issue we looked into patients with new-onset atrial fibrillation and observed an increased risk, suggesting that atrial fibrillation is a risk factor for adverse outcome rather than a marker.
Conclusions
Among patients with CAD undergoing revascularisation with the unrestricted use of DES, AF confers an increased risk of all-cause mortality, ischaemic stroke, and intracranial bleeding. The risk increase correlates with the CHA2DS2-VASc score. The current study provides the basis for future research investigating optimal intensity and duration of antithrombotic treatment among patients with CAD and AF.
Conflict of interest statement
The authors have no conflicts of interest to declare.

