Abstract
Aims: To investigate the optimal periprocedural antithrombotic strategy in patients on long-term oral anticoagulation (OAC) who require percutaneous coronary intervention with stenting.
Methods and results: The WOEST study was a randomised controlled trial which recruited 573 patients on long-term OAC who underwent PCI. The periprocedural treatment strategy was left to the operator’s discretion. To assess the safety and feasibility of uninterrupted oral anticoagulation (UAC) and bridging therapy (BT), bleeding complications and MACCE were assessed in patients treated according to UAC (n=241) and BT (n=322) regimen. After 30 days, as well as after one year, there were no significant differences in bleeding complications (HR 1.14, 95% CI: 0.77-1.69, p=0.51, and HR 1.26, 95% CI: 0.94-1.69, p=0.12, respectively) and MACCE. MACCE tended to be less frequent in the UAC group (respectively HR 0.48, 95% CI: 0.15-1.51, p=0.21, and HR 0.72, 95% CI: 0.46-1.14, p=0.16). Additionally, adjustment with a propensity score revealed no significant differences. Periprocedural INR was not associated with bleeding or MACCE.
Conclusions: In the WOEST study, UAC was not associated with an increase of bleeding or MACCE compared to bridging therapy. This is the largest study up to now to support the current guidelines. The WOEST trial is registered with ClinicalTrials.gov, number NCT00769938.
Introduction
Approximately 20-30% of patients with atrial fibrillation (AF) or mechanical heart valves who need oral anticoagulation (OAC) have concomitant ischaemic heart disease which may require percutaneous coronary intervention (PCI) with stenting1,2. The optimal periprocedural anticoagulation treatment during PCI is unclear. There are two options: the first is to continue therapeutic OAC throughout the periprocedural period, and the second is to discontinue OAC prior to PCI. If the second option is chosen and the patient is considered to be at increased risk for thromboembolism, unfractionated heparin (UFH) or low molecular weight heparins (LMWH) are administered as a bridging therapy (BT). In 2010, an expert consensus paper from the working group on thrombosis of the European Society of Cardiology (ESC) recommended the uninterrupted oral anticoagulation (UAC) strategy as the preferred strategy for AF patients at moderate to high risk1. These recommendations are based on circumstantial evidence since there are no randomised trials addressing this challenging issue. The potential advantages of UAC include a minimised risk of atherothrombotic events, as periods with subtherapeutic international normalised ratio (INR) values are avoided, and also a simpler periprocedural treatment regimen. The latter can potentially be cost-saving as patients do not require hospitalisation for warfarin re-initiation. Therefore, we decided to perform a sub-analysis to test the hypothesis that periprocedural UAC would not increase bleeding or thrombotic or thromboembolic complications in patients receiving OAC undergoing PCI in the WOEST trial3,4.
Methods
The What is the Optimal antiplatElet and anticoagulant therapy in patients with oral anticoagulation and coronary StenTing (WOEST) trial was an open-label randomised controlled trial which recruited 573 patients on long-term OAC who underwent PCI. Patients were randomised to receive clopidogrel alone or clopidogrel and aspirin after PCI4. The entry and exclusion criteria were described in the original publication3,4. The periprocedural treatment was left to the discretion of the attending physician with combinations ranging from stopping OAC with no BT to UAC plus LMWH. For the purpose of this sub-analysis, the UAC group (n=241) was defined as the group of patients in whom OAC was continued throughout the hospitalisation. In the BT group (n=322), OAC was interrupted before PCI and the operator decided if heparin or LMWH was administered or not. The 10 patients who were excluded from the intention-to-treat analysis in the original WOEST trial publication were also excluded from this sub-analysis4. All data were collected prospectively and were entered into a central database. Follow-up stopped one year after inclusion or at the time of death. All events requiring medical attention were verified by a blinded events committee. Each bleeding event during one-year follow-up was classified separately according to the Thrombolysis In Myocardial Infarction (TIMI) criteria and the Bleeding Academic Research Consortium (BARC) criteria5,6. Major adverse cardiac and cerebrovascular events (MACCE) consisted of death, myocardial infarction (MI), stroke, target vessel revascularisation, and stent thrombosis (according to the Academic Research Consortium [ARC] criteria)7, and each individual component of the primary and secondary endpoints independently. Myocardial infarction (MI) and periprocedural MI were defined according to the 2007 definitions and were described in the original publication8. The diagnosis of stroke was made by the treating neurologist, and CT or MRI was used to distinguish ischaemic from haemorrhagic strokes. The study was conducted according to the principles of the Declaration of Helsinki. All patients gave written informed consent.
Statistical analysis
Standard statistical hypothesis tests were used for the baseline comparison: chi-square or Fisher’s exact and Student’s t or Mann-Whitney where appropriate. Primary and secondary endpoints based on time to first event were assessed by comparison of Kaplan-Meier-based cumulative incidence rates with the log-rank test. As a measure of strength, we calculated hazard ratios (HRs) and 95% confidence intervals (CIs). When applicable, we used multivariate Cox proportional hazard regression to correct for baseline imbalances.
Propensity scores were used to adjust for potential bias in the comparison between non-randomised UAC and BT groups. The propensity score was calculated as the predicted probability that the patient was treated by UAC as opposed to BT using logistic regression. We subsequently adjusted the aforementioned analyses by means of propensity weighting.
The variables included in the propensity score analysis are listed in Online Appendix A. All calculations were carried out with R software (version 3.0; www.r-project.org).
Results
The baseline characteristics of both groups are detailed in Table 1. In the UAC group, the use of clopidogrel at baseline was higher. The number of smokers and mean ejection fraction at baseline were significantly lower in the UAC group. There was no significant difference in the number of patients randomised to double (clopidogrel plus OAC) or triple therapy (aspirin, clopidogrel and OAC) after PCI (p=0.169).
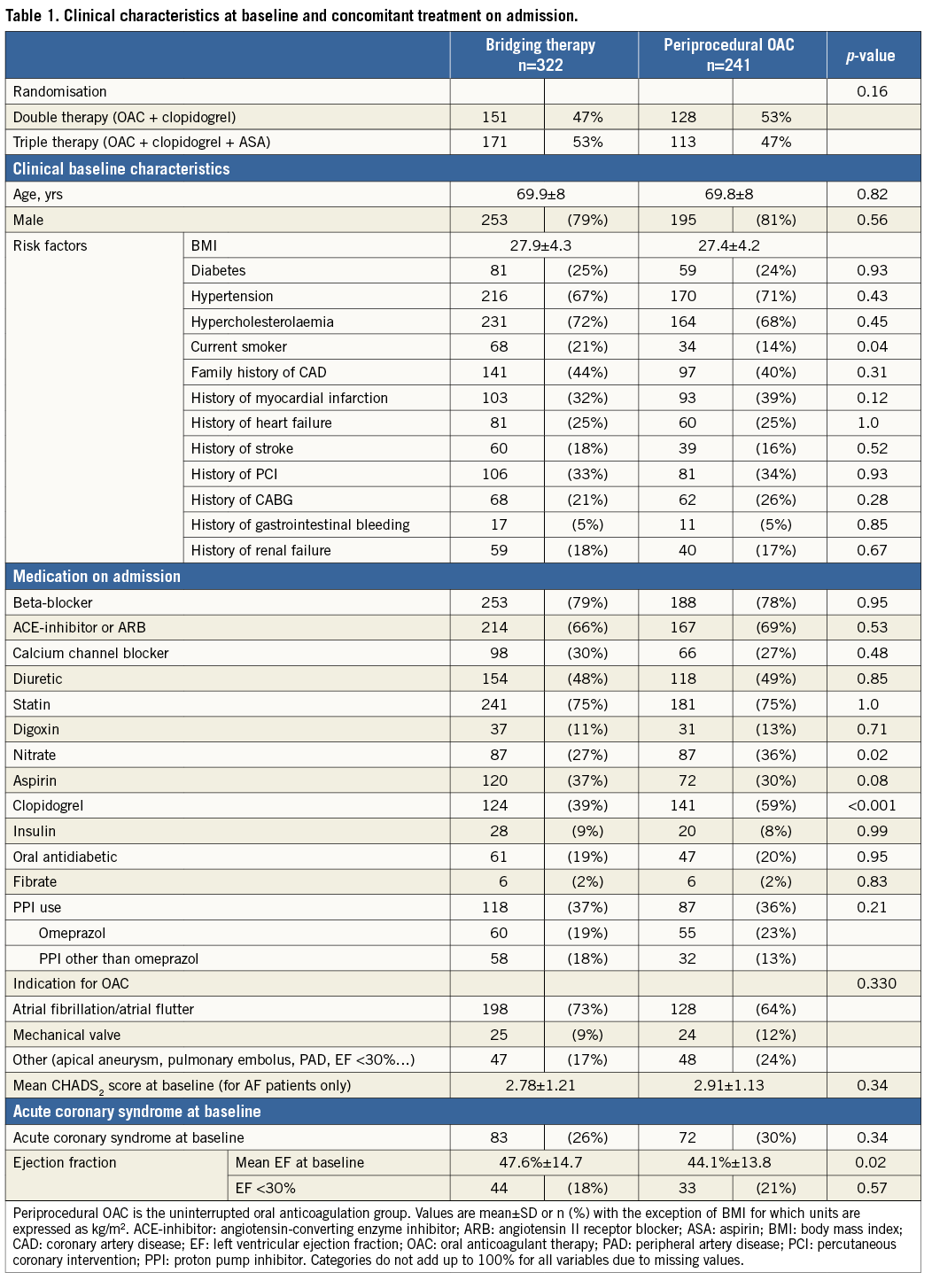
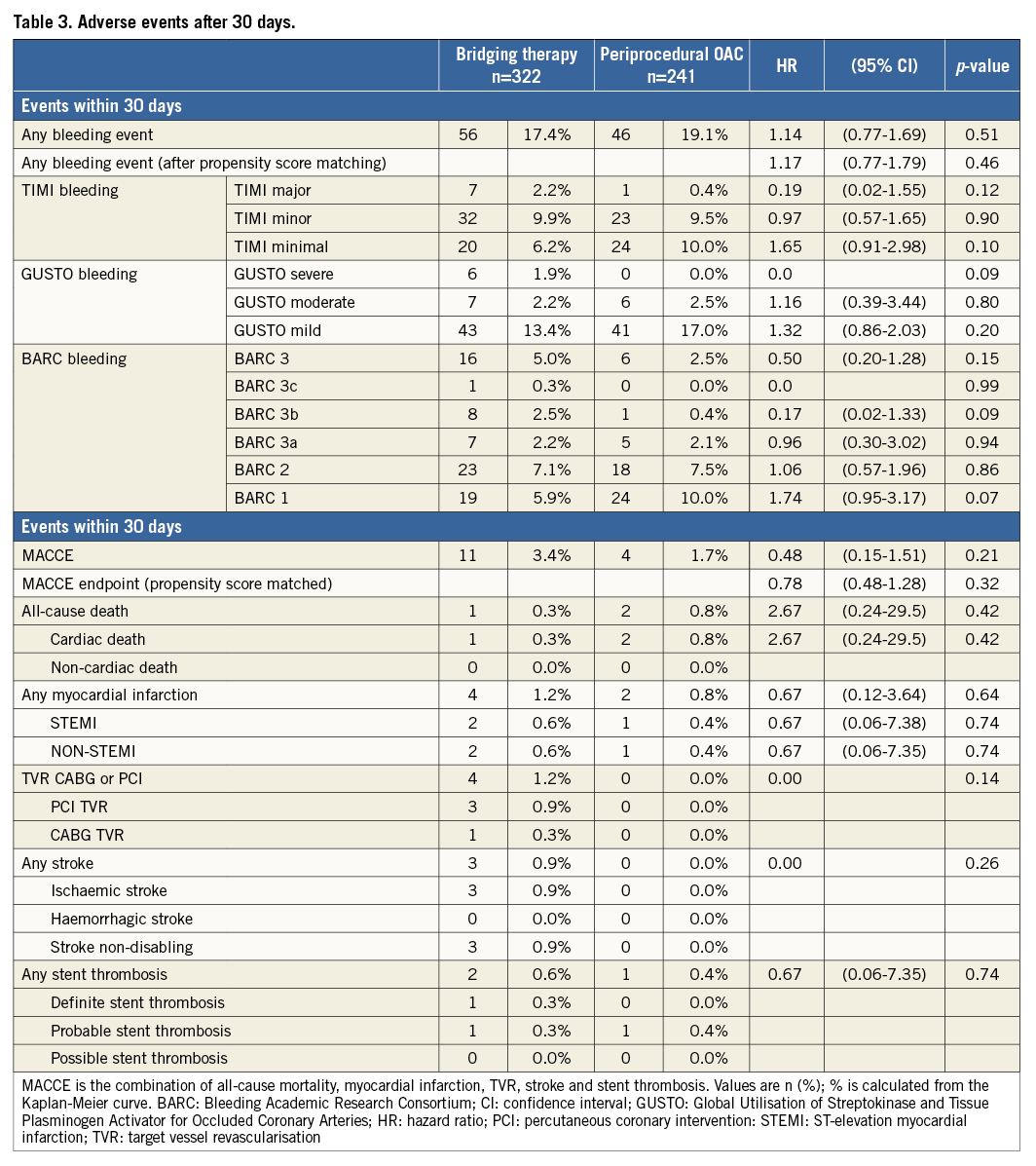
Procedural variables are depicted in Table 2: radial access was more common and DES were used slightly more frequently in the UAC group. Moreover, the periprocedural use of LMWH and GP IIb/IIIa blockers was significantly lower in the UAC group, whereas the UFH bolus was significantly larger. As expected, the periprocedural INR was higher in the UAC group (2.53 vs. 1.48, p<0.001). Bridging in the BT group was performed according to local standards in each participating hospital. The length of hospitalisation after PCI was the same for both groups after elective PCI (median one day), but was significantly longer in ACS patients in the UAC group. The rate of adverse events during the 30-day and one-year follow-up is shown in Figure 1 and Figure 2, Table 3 and Table 4. After 30 days and one year, there were no significant differences in the occurrence of bleeding events (19.1% vs. 17.4%, p=0.51, and 35.6% vs. 29.8%, p=0.12, respectively) or MACCE (1.7% vs. 3.4%, p=0.21, and 12% vs. 16.1%, p=0.15, respectively) between the two groups. However, the incidence of BARC 1 bleeding was significantly higher in the UAC group after one year. The MACCE endpoint occurred less frequently in the UAC group than the BT group after 30 days as well as after one year, but this difference did not reach statistical significance. In fact, the number of all individual MACCE components including death, stroke, myocardial infarction, target vessel revascularisation and stent thrombosis was lower in the UAC group after one year, but these endpoints were not significantly different either. After adjustment with the propensity score, the proportions of bleeding events (HR 1.17, 95% CI: 0.77-1.79, p=0.46, and HR 1.27, 95% CI: 0.93-1.73, p=0.13, respectively) and MACCE (HR 0.78, 95% CI: 0.48-1.28, p=0.32, and HR 0.78, 95% CI: 0.47-1.27, p=0.31, respectively) after 30 days as well as after one year did not reveal any differences between the propensity-matched groups (Table 3, Table 4, Online Table 1 and Online Table 2). After multivariate analysis, periprocedural INR was not associated with bleeding (p=0.09) or MACCE (p=0.21).
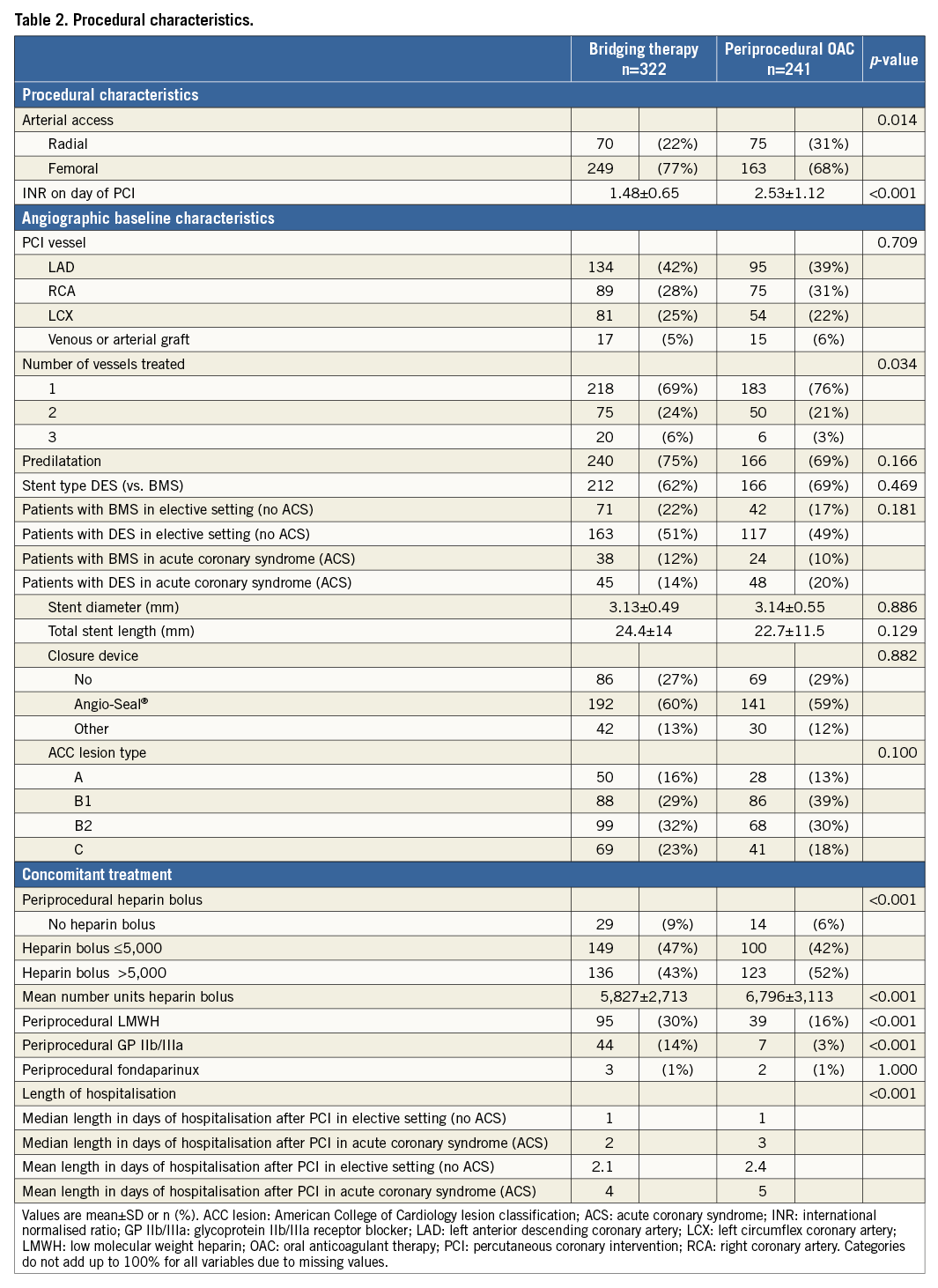
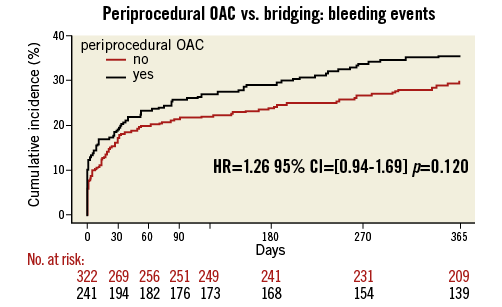
Figure 1. Periprocedural OAC vs. bridging: any bleeding during one-year follow-up. Black line: uninterrupted oral anticoagulation; red line: bridging therapy
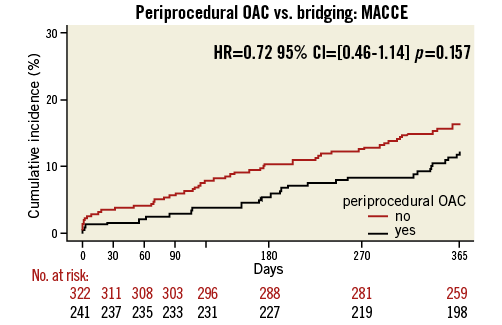
Figure 2. Periprocedural OAC vs. bridging: MACCE (death, myocardial infarction, stroke, target vessel revascularisation and stent thrombosis) during one-year follow-up. Black line: uninterrupted oral anticoagulation; red line: bridging therapy
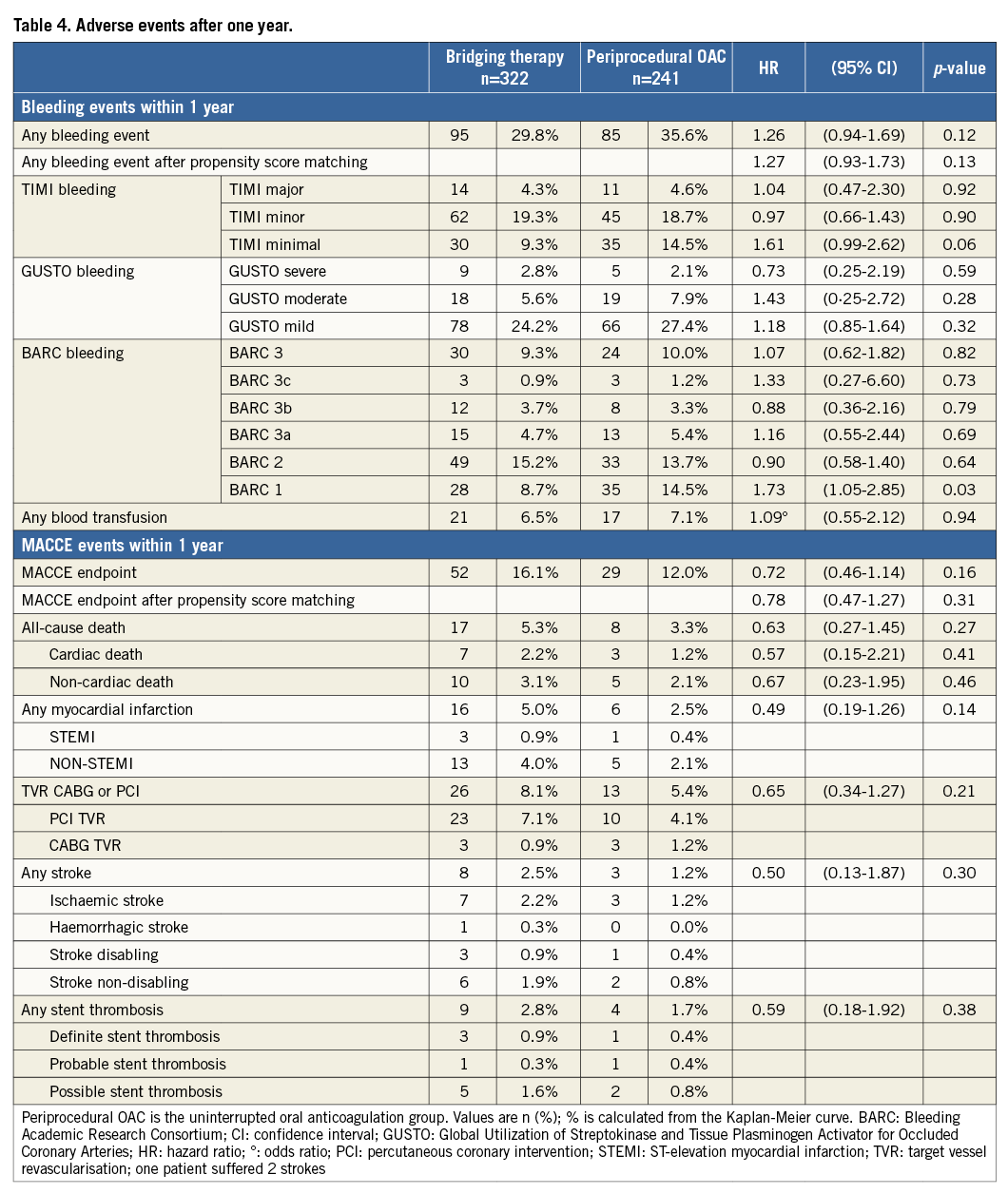
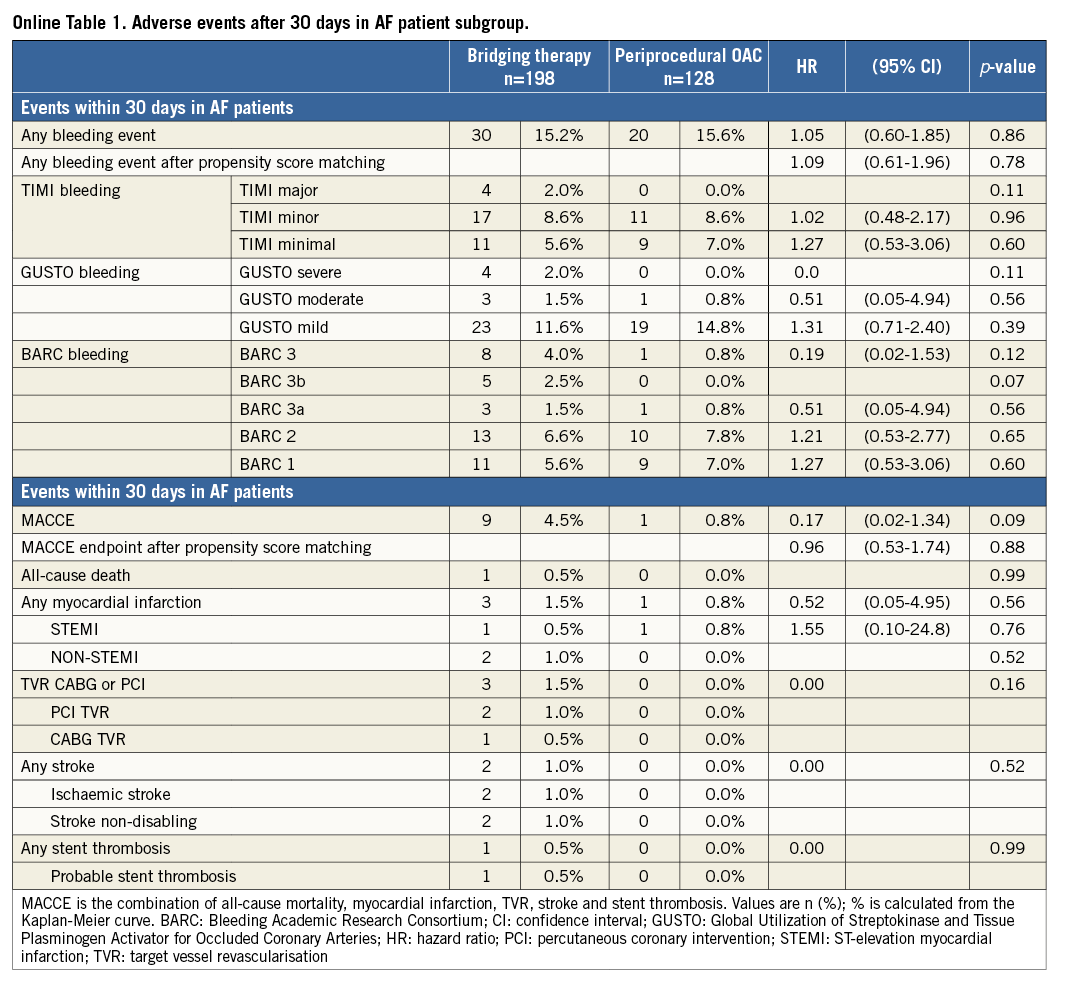
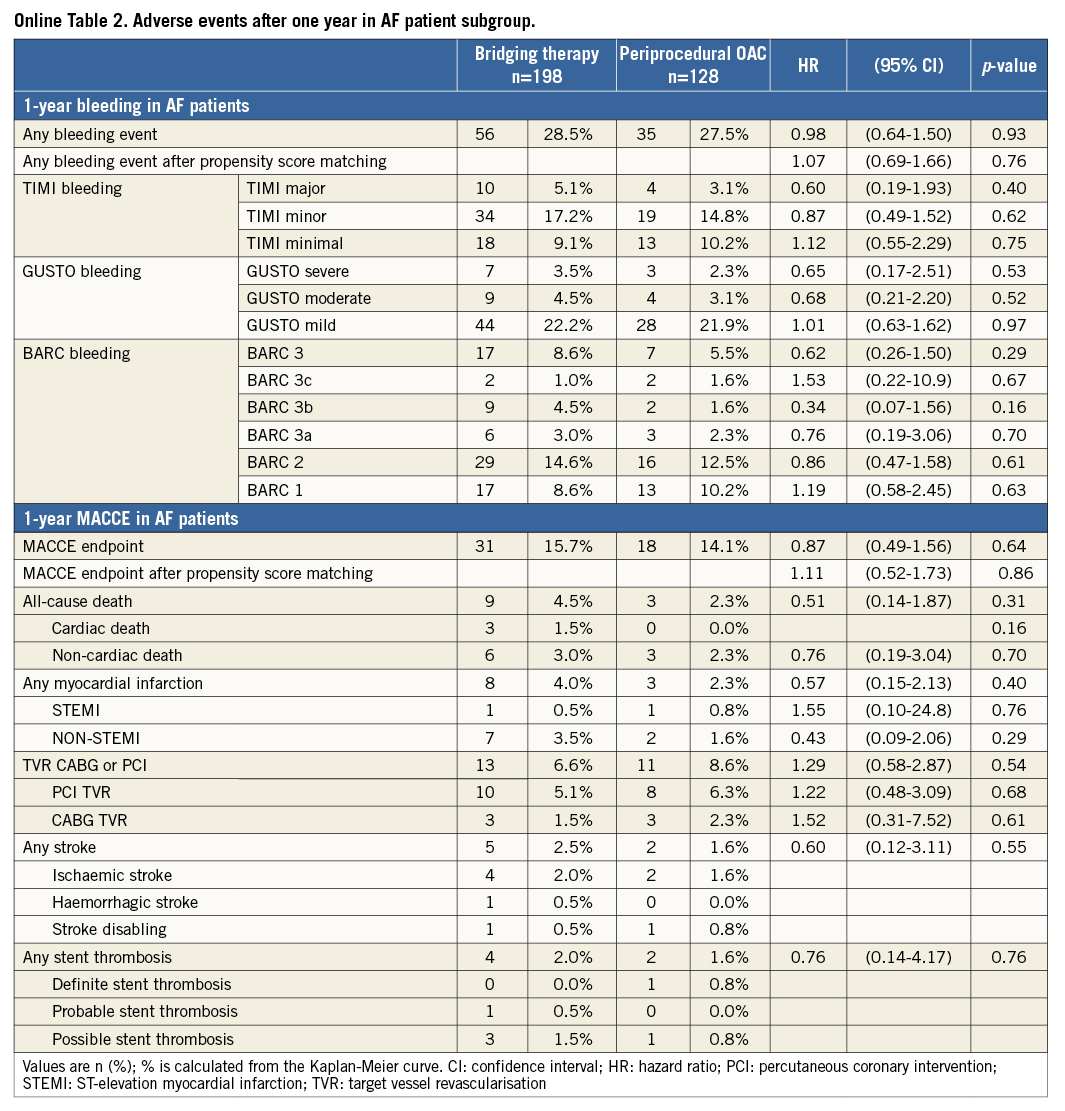
The results for the subset of patients with AF were similar as compared with the total study population (Online Table 1 and Online Table 2, Online Figure 1 and Online Figure 2). In OAC patients with underlying AF requiring PCI, there were no significant differences in overall occurrence of bleeding events or MACCE after 30 days (bleeding: HR=1.05, 95% CI: 0.60-1.85, p=0.86, MACCE: HR=0.17, 95% CI: 0.02-1.34, p=0.09) and one year (bleeding: HR=0.98, 95% CI: 0.64-1.50, p=0.93, MACCE: HR=0.87, 95% CI: 0.49-1.56, p=0.64) (Online Table 1 and Online Table 2, Online Figure 1 and Online Figure 2).
Discussion
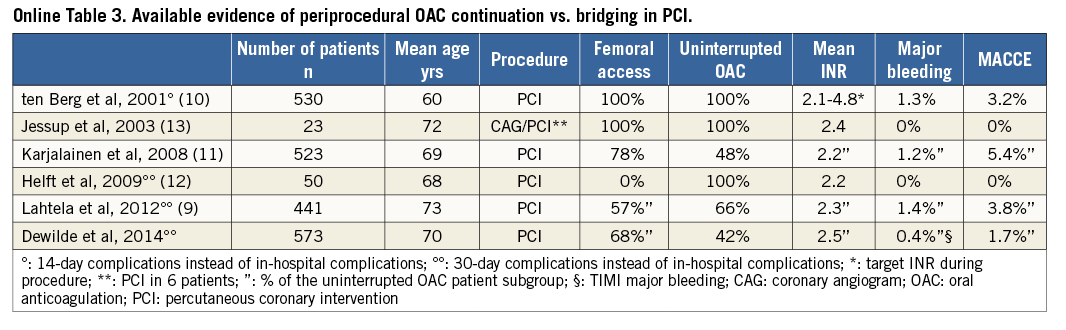
This report is the sixth large observational study on UAC or BT in patients undergoing PCI in atrial fibrillation9-13 (Online Table 3). Our main finding is that, in patients treated with OAC who require PCI, a periprocedural strategy of UAC is not associated with more bleeding or ischaemic complications as compared to a BT strategy at 30 days or one year. These findings were consistent in the subset of patients with AF. Furthermore, periprocedural INR was not associated with the occurrence of bleeding events or MACCE. The incidence of bleeding events was similar for both groups and the incidence of MACCE was slightly, but not significantly, lower in the UAC group. However, despite the fact that the difference in rates of MACCE did not reach statistical significance, the number needed to harm with BT strategy at one-year follow-up was 24, which we believe to be clinically relevant, especially since the alternative strategy of UAC is simpler. Patients were randomised to either double or triple therapy, but this had no impact on the present sub-analysis, since there was no significant difference in the number of patients on double and triple therapy within the investigated subgroups (47% vs. 53%, p=0.169) (Table 1). The adjustment with a propensity score revealed no significant differences in bleeding endpoint or MACCE after both 30 days and one-year follow-up.
Recently, the safety and efficacy of BT have been evaluated in patients undergoing PCI and also in patients undergoing coronary angiography, pacemaker or defibrillator implantation and pulmonary vein ablation9-16. BT offered no advantages in any of these studies and possibly even increased bleeding events. Moreover, in the Management of patients with Atrial Fibrillation undergoing Coronary Artery Stenting (AFCAS) trial, the number of access-site bleedings after PCI was higher in the BT group9. BT was also associated with prolonged hospitalisation and caused delay for an eventual invasive strategy in OAC patients with ACS17,18. Therefore, a BT strategy seems to offer no advantages over UAC, while it does carry disadvantages.
In contrast to coronary angiography or device implantation, PCI procedures also require procedural anticoagulation to avoid thrombotic complications, such as acute stent thrombosis, during the intervention1. Theoretically, warfarin could replace LMWH and UFH, which are traditionally used as periprocedural anticoagulants, since warfarin is known to increase activated clotting time in a predictable fashion19. Three other advantages of PCI with a UAC strategy are: i) avoidance of potential thromboembolic complications such as stroke, which are associated with periods of subtherapeutic anticoagulation; ii) elimination of a period of transient prothrombotic state due to protein C and S suppression after warfarin re-initiation1; and iii) avoidance of a time frame of excess bleeding risk when patients are given a short period of quadruple therapy (OAC, clopidogrel, aspirin and heparin) after the intervention until a therapeutic INR is reached. Finally, the UAC strategy offers a potential economic benefit by reducing hospitalisation by a few days, which are normally necessary for INR to return to therapeutic levels9,18. In the present study, mean hospitalisation time after PCI did not differ significantly in patients after elective stenting. However, hospitalisation was slightly longer in patients treated with UAC strategy who underwent PCI for ACS. This is contrary to what one would expect, but it could be a consequence of the severity of the disease rather than the time needed to re-initiate warfarin therapy.
In this study, UFH was administered periprocedurally in most patients undergoing PCI, whereas theoretically one could consider performing PCI without additional heparin in patients with therapeutic warfarin anticoagulation. Treating physicians did not avoid additional heparin bolus during PCI in most patients included in the present study, probably because of the fear of periprocedural thromboembolic complications such as stent thrombosis. On the one hand, this additional heparin bolus could be a possible explanation for the high bleeding rate observed in both the UAC and the BT groups and is also a possible explanation for the higher than expected bleeding rates in the original WOEST trial4. On the other hand, it was recently shown that, in patients receiving OAC who underwent transradial coronary angiography, the rate of radial artery occlusion was higher when these patients did not receive an additional standard intravenous UFH bolus20. For the time being, the question as to whether a heparin bolus has to be given in patients with therapeutic INR requiring PCI remains unanswered. If a heparin bolus is necessary, it is unclear what the optimal dosage would be.
In the absence of randomised controlled trials comparing these two treatment strategies, the only available evidence comes from a few non-randomised studies addressing this subject (Online Table 3). In earlier reports, such as the AFCAS registry and the randomised prospective Balloon Angioplasty and Anticoagulation (BAAS) study, the simple UAC strategy proved at least as safe as the more complex BT strategy9,10. In the AFCAS registry which was designed to study AF patients undergoing PCI, 290 patients were treated with the UAC strategy and 161 with the BT strategy. The conclusion was that UAC did not increase perioperative bleeding nor thrombotic complications during PCI and that UAC was a simple and cost-effective alternative to BT9. In the BAAS study, therapeutic INR levels (2.1-4.8) did not lead to a higher MACCE or bleeding rate in 530 patients12. Also, three other studies including PCI patients confirm these findings and support the view that UAC is a safe and cost-efficient strategy in this patient subset and should therefore be the preferred strategy11-13.
Published guidelines on this subject are confusing, since they sometimes contain recommendations with opposing regimens and others even completely ignore this clinical challenge. Before 2010, there was a consensus that BT was to be used preferably with a periprocedural INR <2.0 or even below 1.521-23. In the 2005 European and American PCI guidelines, no recommendations were made concerning this issue24,25. Only recently, the 2010 European Society of Cardiology Working Group on Thrombosis guideline was the first clearly to recommend uninterrupted OAC as the preferred strategy in patients with AF undergoing PCI. Furthermore, this guideline recommended the radial approach as the first choice during therapeutic anticoagulation, because of lower rates of bleeding and possibly even mortality, especially in STEMI patients1,26.
Limitations
This study has several limitations. This is a non-randomised study with its inherent bias. Since patients were not randomised to a UAC or BT strategy, the decisions made were always a result of risk-weighing in an individual patient by the patient’s treating physician. In addition to the differences in periprocedural use of OAC, other differences in patient management during the one-year follow-up may account for modification of the final results. Even though propensity score analysis did not reveal any differences in the results, we can never be sure to have corrected for all baseline, procedural and other differences that may influence outcome. Second, there is no universal definition of bridging therapy in this study, since every participating hospital had its own bridging protocol. Third, the number of patients included is relatively low and there was no power calculation for this sub-analysis. Nevertheless, this patient sub-analysis is the largest patient cohort up to now in which the question of periprocedural UAC vs. BT has been addressed. Fourth, we do not have information on how many patients were in the therapeutic range before PCI, because control of the INR was left to the specialised thrombosis service, which operates independently from hospitals in The Netherlands. We do know from the RELY trial, however, that the quality of OAC control by this service is good, with a mean of 70% of patients in the therapeutic range at any given time27. Fifth, since the study was designed in 2008, the definition of periprocedural MI is based on the (second) universal definition and not the most recent one from 20128. Also, this study was designed before the HAS-BLED score and the CHA2DS2-VASc score were established, and therefore they could not be used to estimate bleeding risk and make decisions about the use of oral anticoagulants1. Finally, some data are lacking, such as the use of vitamin K to reverse anticoagulation, simply because these data were not collected.
Conclusion
In conclusion, performing PCI with a UAC strategy was not associated with an increase in the number of bleeding events or MACCE in this study. Furthermore, bleeding or MACCE was not related to INR levels. This is the largest study up to now to support the recommendations of the 2010 consensus of the European Society of Cardiology Working Group on Thrombosis to adopt a periprocedural strategy of continuing OAC in a therapeutic window during PCI in patients with long-term OAC indication.
| Impact on daily practice In patients with long-term OAC indication who undergo PCI, a periprocedural strategy of continuing OAC in a therapeutic window during PCI is safe and effective and could reduce hospitalisation time. Most importantly, one can avoid a time frame of excess bleeding risk in high-risk patients when these patients are given a short period of quadruple therapy (OAC, clopidogrel, aspirin and [low molecular weight] heparin) after the intervention until a therapeutic INR is reached. This is the largest study to support the 2010 recommendations of the European Society of Cardiology Working Group on Thrombosis to continue OAC during PCI. |
Acknowledgements
We want to thank H.W.M. Plokker and M.A. Bosschaert, St Antonius Hospital, Nieuwegein, The Netherlands, for helping to make the WOEST trial possible. We also want to thank the blinded committee which adjudicated all events: B.E. Schölzel and B.J. Van Den Branden, Amphia Ziekenhuis, Breda, The Netherlands; H.W.M. Plokker, St Antonius Hospital, Nieuwegein, The Netherlands, and F.W.A. Verheugt, Onze Lieve Vrouwe Gasthuis (OLVG), Amsterdam, The Netherlands. These individuals did not receive compensation in association with their work on this article.
Funding
Antonius Ziekenhuis Foundation, Strect Foundation.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Online Appendix A. Variables used in the propensity score analysis.
SECTION STATISTICAL ANALYSIS
The following variables were included in the propensity score analysis: age, gender, BMI, current smoker, history of MI, aspirin use at baseline, OAC use at baseline, clopidogrel use at baseline, ECG rhythm at baseline, ECG ST-T-segment changes, radial or femoral access, omeprazol use at baseline, number of vessels treated, predilatation, stent type, stent length, maximum balloon pressure stent placement, post-dilatation, visible thrombus pre-PCI, calcified lesion, ACC/AHA lesion type, TIMI flow post procedure, periprocedural UFH bolus, periprocedural LMWH use, periprocedural glycoprotein IIb/IIIa (GP IIb/IIIa) blocker use, and acute coronary syndrome at baseline.
Online Appendix B. WOEST study investigators.
The Netherlands: Medisch Centrum Alkmaar, Alkmaar: A.A.C.M. Heestermans; Academisch Medisch Centrum Amsterdam, Amsterdam: M.M Vis; Onze Lieve Vrouwe Gasthuis, Amsterdam: J.P. Herrman, T. Slagboom; Amphia Ziekenhuis, Breda: J. Vos; Catharina Ziekenhuis, Eindhoven: B.R.G. Brueren; UMCG (Universitair Medisch Centrum Groningen), Groningen: B.J.G.L. De Smet; Sint Antonius Ziekenhuis, Nieuwegein: W.J.M. Dewilde, N.J. Breet, J.M. ten Berg, T. Oirbans; Maasstad Ziekenhuis, Rotterdam: K. Sheikjoesoef; Twee Steden Ziekenhuis, Tilburg: W. Aarnoudse, W. Dewilde; Isala klinieken, Zwolle: S. Rasoul, A.W. van ’t Hof.
Belgium: OLV Aalst (Onze Lieve Vrouw Ziekenhuis), Aalst: C. Van Mieghem; UZ (Universitair Ziekenhuis Antwerpen), Antwerpen: T. Vandendriessche; ZOL (Ziekenhuizen Oost Limburg), Genk: M. Vrolix; Maria Middelares, Gent: K. Cornelis; UZ KUL (Universitair Ziekenhuis Katholieke Universiteit Leuven), Leuven: T. Adriaenssens.
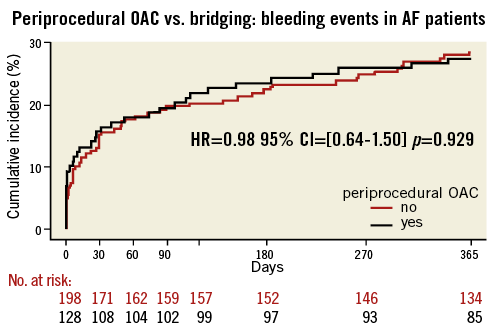
Online Figure 1. Periprocedural OAC vs. bridging: any bleeding during one-year follow-up in AF patient subgroup. Black line: uninterrupted oral anticoagulation; bleeding endpoint: any bleeding during one-year follow-up; red line: bridging therapy.
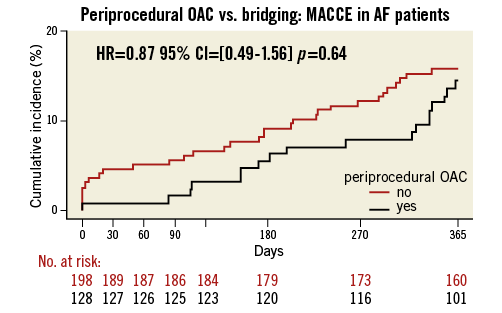
Online Figure 2. Periprocedural OAC vs. bridging in AF patient subgroup: MACCE (death, myocardial infarction, stroke, target vessel revascularisation and stent thrombosis) during one-year follow-up. Black line: uninterrupted oral anticoagulation; red line: bridging therapy.