
Introduction
Oral antiplatelet therapy may require temporary disruption soon after percutaneous coronary intervention (PCI) or acute coronary syndrome, incurring potential thrombotic risks. Glycoprotein IIb/IIIa inhibitors have been used for “bridging”, but limited safety data are available1. The BRIDGE (Maintenance of Platelet Inhibition with Cangrelor After Discontinuation of Thienopyridines in Patients Undergoing Surgery) trial demonstrated that cangrelor, a fast-acting intravenous P2Y12 inhibitor, could be safely used to maintain platelet inhibition prior to cardiac surgery2. Although parenteral antiplatelet bridging was highlighted in recent expert consensus documents3,4, guidelines do not provide clear recommendations, and limited comparative data are available between agents5.
Methods
We identified patients receiving antiplatelet bridging from October 2015 to June 2017 in a large, tertiary care centre. Parenteral agents used specifically for bridging or when extended beyond the duration indicated for PCI (4 hours for cangrelor and 24 hours for eptifibatide) were included. Primary bridge indications were identified via review of clinical records. On-therapy stent thrombosis, coronary reocclusion, Global Use of Strategies to Open Occluded Coronary Arteries (GUSTO)-defined or Bleeding Academic Research Consortium (BARC)-defined bleeding6, and deaths were collected and adjudicated by two investigators. Appropriate bridge dosing for cangrelor was based on the BRIDGE trial’s dosing (0.75 μg/kg/min), and appropriate eptifibatide was determined by the package insert (2 μg/kg/min, dose reduced to 1 μg/kg/min for creatinine clearance ≤50 mL/min).
Results
Thirty-one patients (9 eptifibatide, 20 cangrelor, and 2 both) were included. Two patients received eptifibatide and then were transitioned to cangrelor due to its lower perceived bleeding risk. All but one patient remained on maintenance aspirin therapy during the bridge period. Median age was 63 years and 73% were men (Supplementary Table 1). On admission, 54% presented in cardiogenic shock and 26% with cardiac arrest. During hospitalisation, 71% required mechanical ventilation and 49% required mechanical circulatory support. Eighty-seven percent had a prior coronary stent; median time from last stent was 17 (2-64) days. All unstented patients (13%) were being evaluated for unplanned coronary artery bypass grafting (CABG). Primary bridge therapy indications included surgery (68%), limited enteral absorption/access (16%), and high perceived bleed risk (16%) (Figure 1). Median duration of bridge therapy was 61 (25th-75th percentile 20-100) hours in the cangrelor group and 83 (25th-75th percentile 19-98) hours in the eptifibatide group.
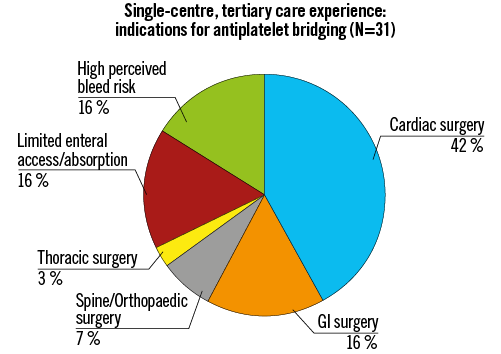
Figure 1. Primary indications for antiplatelet bridging. GI: gastrointestinal
GUSTO severe/life-threatening bleeding and thrombotic events were not observed. GUSTO mild/moderate bleeding occurred in 30% of cangrelor-treated patients and 27% of eptifibatide-treated patients. Of the nine patients who underwent CABG, none experienced BARC type 4 bleeding. No patients required an invasive procedure for bleeding events. Death during index hospitalisation occurred in five (16%) patients. Initial supratherapeutic dosing occurred in five cangrelor-treated patients related to use of doses indicated for PCI (4 μg/kg/min) and there was one case of contraindicated eptifibatide use in end-stage renal disease. Initial subtherapeutic dosing due to renal function occurred in one eptifibatide-treated patient (1 mcg/kg/min). Mild/moderate bleeding occurred in three of five patients with dosing errors during cangrelor treatment.
Discussion
Parenteral antiplatelet bridging with cangrelor or eptifibatide was being employed for ~3 days in this single-centre experience. The most frequent indication was unplanned surgery following PCI (typically within 30 days). Despite high-risk presentations with >15% in-hospital mortality, efficacy profiles were reassuring with no identified stent thrombosis. Differences between groups, including the numerically higher risk of moderate bleeding observed with cangrelor use (compared with eptifibatide), should be cautiously interpreted, given variation in indications for use, concurrent antithrombotic therapies, small sample sizes, and dosing errors.
Antiplatelet bridging is associated with excess bleeding and cost, regardless of strategy, and should be reserved only for select, high-risk cases. When required, therapeutic practices need to be standardised and clinicians should become familiar with indications, bridging doses, and necessary renal adjustments. To mitigate dosing errors, we recommend that all bridge dosing regimens be determined collaboratively by a cardiologist and a pharmacist; this may be crucial when drugs are used “off label” at a dose that differs from standard doses. Patients with renal impairment or unclear timing of surgery may benefit from cangrelor’s rapid, non-renal clearance.
Limitations
This was a small, single-centre, retrospective study intended to inform initial patterns of use of parenteral antiplatelet therapies. Larger, prospective, multicentre studies are required regarding risks of stent thrombosis with oral antiplatelet disruption, approaches to attenuate on-therapy bleeding, and the optimal strategy during this high-risk window.
Conclusion
At this juncture, antiplatelet bridging should only be used in well-selected patients at the appropriate dose for the minimal necessary treatment duration.
| Impact on daily practice We present the first real-world experience of cangrelor use for antiplatelet bridging at a tertiary care centre. Although stent thrombosis was not observed during bridging, bleeding and dosing errors were commonly encountered. |
Conflict of interest statement
A. Qamar and M. Vaduganathan are supported by the NHLBI T32 postdoctoral training grant (T32HL007604). D.L. Bhatt discloses the following relationships - Advisory Board: Cardax, Elsevier Practice Update Cardiology, Medscape Cardiology, Regado Biosciences; Board of Directors: Boston VA Research Institute, Society of Cardiovascular Patient Care; Chair: AHA Quality Oversight Committee; Data Monitoring Committees: Cleveland Clinic, DCRI, HCRI, Mayo Clinic, Mount Sinai School of Medicine, PHRI; Honoraria: ACC (Senior Associate Editor, Clinical Trials and News, ACC.org), Belvoir Publications (Editor in Chief, Harvard Heart Letter), DCRI (clinical trial steering committees), HCRI (clinical trial steering committee), HMP Communications (Editor in Chief, Journal of Invasive Cardiology), JACC (Guest Editor; Associate Editor), PHRI (clinical trial steering committee), Slack Publications (Chief Medical Editor, Cardiology Today’s Intervention), Society of Cardiovascular Patient Care (Secretary/Treasurer), WebMD (CME steering committees); Other: Clinical Cardiology (Deputy Editor), NCDR-ACTION Registry Steering Committee (Chair), VA CART Research and Publications Committee (Chair); Research Funding: Abbott, Amarin, Amgen, AstraZeneca, Bristol-Myers Squibb, Chiesi, Eisai, Ethicon, Forest Laboratories, Ironwood, Ischemix, Lilly, Medtronic, Pfizer, Roche, Sanofi Aventis, The Medicines Company; Royalties: Elsevier (Editor, Cardiovascular Intervention: A Companion to Braunwald’s Heart Disease); Site Co-Investigator: Biotronik, Boston Scientific, Abbott; Trustee: ACC; Unfunded Research: FlowCo, Merck, PLx Pharma, Takeda. The other authors have no conflicts of interest to declare.
Supplementary data
Supplementary Table 1. Patient profiles, bridging indications, and on-treatment clinical events in antiplatelet bridging recipients.
To read the full content of this article, please download the PDF.

