Abstract
Aims: Surgery after drug-eluting stent (DES) implantation may be associated with increased risk for perioperative stent thrombosis (ST).
Methods and results: We evaluated the outcomes of 67 patients who underwent non-cardiac (n=51) or cardiac (n=16) surgery after DES implantation at our institution between 2008 and 2010 and who underwent preoperative “bridging” with a glycoprotein IIb/IIIa inhibitor. Surgery occurred after a mean time of 13.9±1.7 and 8.7±2 months post stenting for non-cardiac (NCS) and cardiac surgery, respectively. Glycoprotein IIb/IIIa inhibitors were administered preoperatively for a mean of 7.1±0.4 and 7.8±0.7 days, respectively, then discontinued four to six hours before surgery. Most patients received aspirin through the perioperative period (33 NCS patients and 15 cardiac surgery patients). Clopidogrel was restarted as early as possible in the postoperative period. In the non-cardiac surgery group, two patients (3.9%, 95% confidence intervals 0.5% to 13.5%) suffered acute ST in the immediate postoperative period and four patients suffered major bleeding by the Global Utilisation of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries (GUSTO) criteria. One cardiac surgery patient had probable ST one hour postoperatively.
Conclusions: In spite of preoperative “bridging” with a glycoprotein IIb/IIIa inhibitor, postoperative stent thrombosis can still occur in patients with prior DES undergoing surgery requiring antiplatelet medication interruption.
Introduction
Patients with drug-eluting stents (DES) who subsequently require surgery may be at increased risk for perioperative stent thrombosis, a catastrophic complication with high morbidity and mortality1,2. A recent systematic literature review reported a perioperative stent thrombosis rate of 2.3% if non-cardiac surgery was performed within six months, 1.7% if performed between six and 12 months, and 0.9% if performed more than 12 months from DES implantation3. Thus, delaying surgery for at least 12 months after DES implantation is currently recommended4,5. However, in some patients surgery cannot be delayed and the surgical team requires antiplatelet therapy interruption. Moreover, perioperative DES thrombosis has been reported even several years after DES implantation6. Although the optimal perioperative management of such patients remains controversial, administration of a short-acting anticoagulant or antiplatelet regimen has been proposed to minimise the risk for perioperative stent thrombosis7-11. We describe the outcomes of 67 patients who required non-cardiac or cardiac surgery post DES implantation in whom thienopyridine administration was interrupted preoperatively and glycoprotein IIb/IIIa inhibitor infusion was used as a “bridge” to surgery.
Materials and methods
PATIENTS
We retrospectively reviewed the medical records of 67 consecutive patients who required surgery (51 patients underwent non-cardiac and 16 patients underwent cardiac surgery) after DES implantation at our institution between 2008 and 2010 with thienopyridine administration interruption and who underwent “bridging” with a glycoprotein IIb/IIIa inhibitor. Mean age was 65±1.2 years and all patients were men. During the study period all patients who were considered to have an elevated risk for perioperative stent thrombosis (patients who underwent stent implantation within 12 months prior to surgery, patients who had undergone stenting of the left main coronary artery or proximal left anterior descending artery, patients with diabetes, bifurcation stenting, or a history of stent thrombosis) underwent “bridging” with a glycoprotein IIb/IIIa inhibitor. The study was approved by our institutional review board.
BRIDGING PROTOCOL
Bridging was performed using the following protocol: clopidogrel was discontinued for five to seven days prior to surgery. On the day following clopidogrel discontinuation, patients were admitted to the hospital and were started on intravenous glycoprotein IIb/IIIa inhibitor infusion. The type of glycoprotein IIb/IIIa inhibitor used was at the discretion of the treating physician. Eptifibatide was administered as an intravenous bolus of 180 µg/kg followed by a continuous infusion of 2.0 µg/kg/min. In patients with creatinine clearance <50 ml/min the continuous infusion was lowered to 1.0 µg/kg/min. Tirofiban was administered at a dose of 0.4 μg/kg/min over 30 minutes, followed by 0.1 μg/kg/min in patients with normal renal function. In patients with creatinine clearance <30 ml/min, the tirofiban dose was reduced by 50%. The glycoprotein IIb/IIIa inhibitor infusion was discontinued four to six hours prior to surgery. Postoperatively, a 600 mg loading dose of clopidogrel was given as early as possible after surgery (as soon as cleared by the surgical team) and then continued at a once-daily dose of 75 mg. If clopidogrel could not be resumed, a recommendation was made to restart the glycoprotein IIb/IIIa infusion two hours after the end of surgery and to continue it for up to six hours after the resumption of clopidogrel treatment.
PATIENT MONITORING
During and after surgery, patients were placed on continuous cardiac and haemodynamic monitoring. Electrocardiograms were obtained in patients who had clinical suspicion for cardiac events and cardiac biomarkers were measured at 12-hour intervals, or earlier if clinically indicated. Stent thrombosis was defined using the Academic Research Consortium criteria12, and significant bleeding was defined using the Global Utilisation of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries (GUSTO) criteria13.
STATISTICAL ANALYSIS
Continuous variables were reported as mean ± SD or median (interquartile range) and discrete variables were reported as frequencies. Analyses were performed using JMP 8 (SAS Institute, Cary, NC, USA) and SPSS (SPSS Inc., Chicago, IL, USA).
Results
PATIENT CHARACTERISTICS
The clinical characteristics of the study patients are presented in Table 1. All patients were men with a high prevalence of atherosclerosis risk factors. The indication for DES implantation for most patients was an acute coronary syndrome. Most of the implanted DES were sirolimus-eluting, but all DES types were included in the study.
SURGERY AND PREOPERATIVE MANAGEMENT
The mean and median times between DES implantation and NCS were 13.9±1.7 and 11 (interquartile range 6-18) months, respectively. The mean and median times between DES implantation and cardiac surgery were 8.7±2.0 and 7.5 (interquartile range 4-10.25) months, respectively (Table 1). For non-cardiac surgery, two patients (4%) were considered high risk, 32 (63%) were intermediate and 17 (33%) were low risk. Most non-cardiac surgical procedures were abdominal, head and neck and orthopaedic surgeries (Table 2). Aspirin was continued during the perioperative periods in most study patients (65% of those with non-cardiac vs. 94% of those with cardiac surgery, Table 2). All cardiac surgery patients underwent coronary artery bypass grafting (CABG), except one patient who underwent combined mitral valve repair and CABG.
PERIOPERATIVE OUTCOMES IN THE NON-CARDIAC SURGERY GROUP
In the non-cardiac surgery group, two patients (3.9%, 95% confidence intervals 0.5% to 13.5%) had angiographically confirmed acute stent thrombosis in the immediate postoperative period compared to none in the cardiac surgery group (Table 2).
The first patient was a 64-year-old man who had received a sirolimus-eluting stent in the distal right coronary artery to treat restenosis of a bare metal stent 19 months prior to pancreatectomy and splenectomy for pancreatic adenocarcinoma. Both aspirin and clopidogrel were stopped and he received eptifibatide for 19 days until six hours before surgery. The long duration of eptifibatide infusion was required because the patient initially underwent endoscopic-guided pancreatic biopsy that delayed performance of open surgery. Six hours after surgery, he developed inferior ST-segment elevation acute myocardial infarction (STEMI). Emergency coronary angiography revealed thrombosis of the distal right coronary artery stent and this was managed with placement of a bare metal stent (Figure 1).
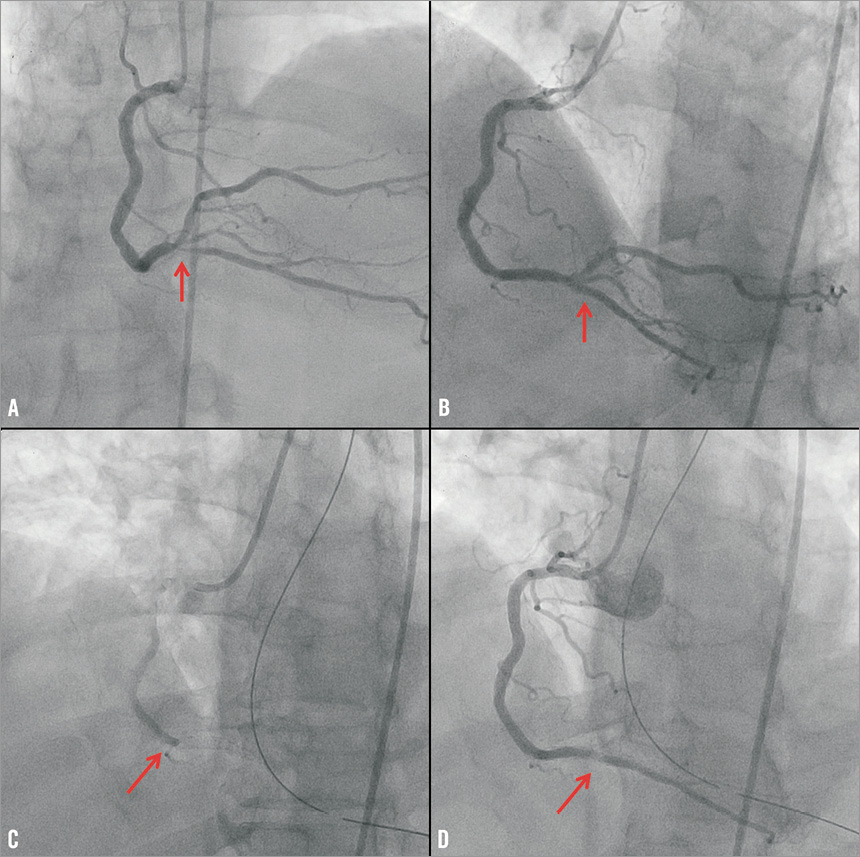
Figure 1. Coronary angiography demonstrating an in-stent restenotic lesion of the distal right coronary artery (arrow, panel A), that was successfully treated with implantation of a sirolimus-eluting stent (arrow, panel B). Acute thrombosis of the distal right coronary artery stent (arrow, panel C), that was successfully treated using a bare metal stent (arrow, panel D).
The second patient was a 62-year-old man who had received a zotarolimus-eluting stent in the proximal right coronary artery to treat restenosis of a sirolimus-eluting stent 13 months prior to transurethral prostate resection.The administration of both aspirin and clopidogrel was suspended and he was started on eptifibatide four days prior to surgery. Eptifibatide was discontinued six hours before surgery. Two hours after surgery he developed inferior STEMI. Emergency coronary angiography showed acute thrombosis of the proximal right coronary artery stent that was treated with placement of a bare metal stent (Figure 2).
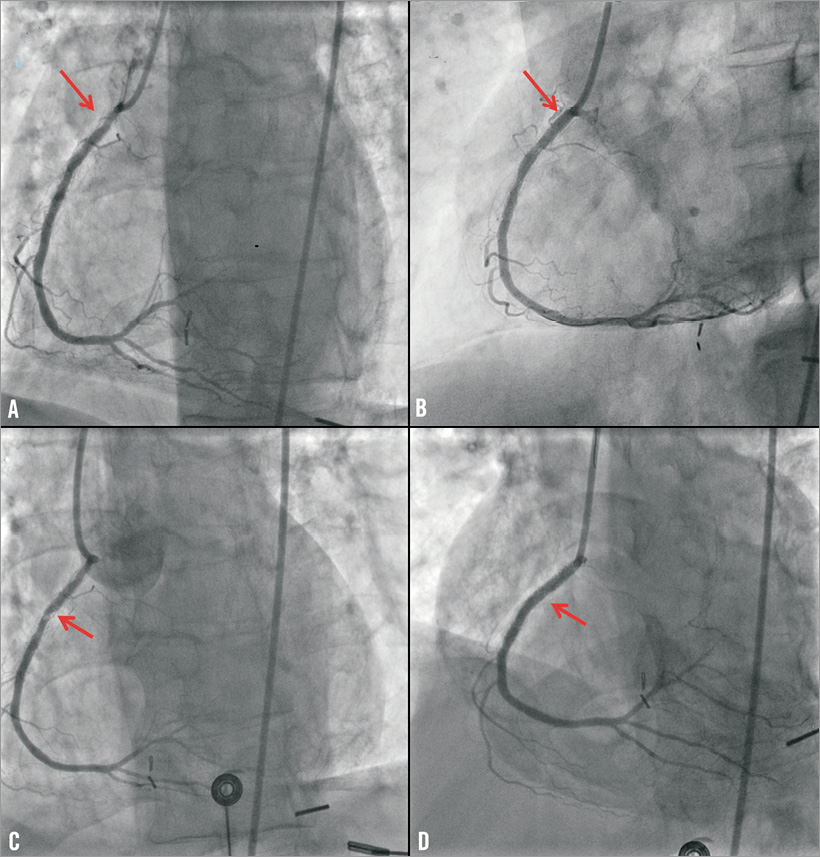
Figure 2. Coronary angiography demonstrating an in-stent restenotic lesion of the proximal right coronary artery (arrow, panel A), that was successfully treated with implantation of a zotarolimus-eluting stent (arrow, panel B). Acute thrombosis of the proximal right coronary artery stent (arrow, panel C), that was successfully treated using a bare metal stent (arrow, panel D).
Four patients (7.8%, 95% confidence intervals 2.2% to 18.8%) had bleeding complications (two moderate and two severe according to the GUSTO criteria). Two patients had epistaxis, one before lung biopsy (did not require transfusion) and one following nasopharyngeal mass excision (required two units of red blood cells). Epistaxis was successfully treated with cautery in both patients. The third patient had haemoperitoneum following liver biopsy and was treated conservatively with transfusion of three units of red blood cells; he expired after a prolonged hospital course secondary to sepsis. The fourth patient had gastrointestinal bleeding following sigmoidectomy and was treated with transfusion of three units of red blood cells.
PERIOPERATIVE OUTCOMES IN THE CARDIAC SURGERY GROUP
One patient developed STEMI in the postoperative period. He was a 63-year-old man who had undergone sirolimus-eluting stent implantation in the proximal right coronary artery due to non-ST-segment elevation acute myocardial infarction. He underwent CABG and mitral valve repair 24 months after stenting. Aspirin and clopidogrel were suspended for 17 days preoperatively (he was bridged with eptifibatide for five days but then developed thrombocytopenia and eptifibatide was stopped). He developed inferior STEMI one hour after surgery (although he had received a saphenous vein graft to the right posterior descending artery) that was medically managed (the patient did not undergo coronary angiography because of thrombocytopenia).
A second patient received a drug-eluting stent of unknown type to the mid left circumflex coronary artery after presenting with a STEMI at a different hospital, and was subsequently referred to our institution for CABG due to multivessel disease. Aspirin was continued during the perioperative period. He received eptifibatide for four days prior to surgery and died 12 hours after surgery. Pathology revealed that the patient had extension of his prior myocardial infarction but did not have stent thrombosis.
A third patient developed thrombocytopenia during eptifibatide infusion. Four months prior to surgery he presented with non-ST-segment acute myocardial infarction and underwent unprotected left main stenting with an everolimus-eluting stent. He subsequently developed recurrent non-ST-segment acute myocardial infarction and was referred for CABG. Eptifibatide was started but the patient developed thrombocytopenia. The eptifibatide infusion was stopped and he did not receive any antiplatelet agent for 13 days until surgery, which was uneventful.
A fourth patient had haematuria while on eptifibatide and required transfusion of one unit of red blood cells.
Discussion
The main findings of our study are that, in patients undergoing major surgery after DES implantation requiring interruption of thienopyridine administration, preoperative administration of a glycoprotein IIb/IIIa inhibitor (a) may not prevent postoperative stent thrombosis, and (b) may be associated with high risk for thrombocytopenia and bleeding.
Patients who undergo DES implantation frequently require subsequent surgery14-21. At our institution the incidence of major or minor non-cardiac surgery was 7% at one year, 18% at two years, and 22% at three years post DES implantation3. Although continuation of aspirin and a thienopyridine may minimise the risk for stent thrombosis, thienopyridine discontinuation is frequently requested by the surgeon to prevent severe perioperative bleeding.
In DES patients requiring thienopyridine interruption prior to surgery, a short-acting antiplatelet therapy has been proposed as a bridge to surgery. Eptifibatide and tirofiban have a short half-life and are theoretically attractive for this application. Tirofiban achieves maximal platelet inhibition after five minutes of infusion and platelet function normalises three to eight hours after discontinuation. Similarly, eptifibatide has a half-life of two to three hours and reverses completely after four hours. No perioperative stent thrombosis occurred in two series of three8 and four7 DES patients bridged with tirofiban to surgery. In the largest published study to date, 30 patients underwent tirofiban “bridging” before non-cardiac surgery: no patient developed stent thrombosis or other adverse cardiac events during the index hospitalisation11. The Maintenance of platelet inhiBition with cangreloR after dIscontinuation of thienopyriDines in patients undergoing surGEry (BRIDGE) trial randomised 210 patients with acute coronary syndrome or percutaneous coronary intervention on thienopyridine undergoing CABG to cangrelor or placebo for at least 48 hours and up to seven days. This was discontinued one to six hours prior to CABG (cangrelor was not administered in the postoperative period)22. There was no difference in the incidence of ischaemic or bleeding events during the perioperative period between the two study groups22.
In our study two of 51 patients undergoing non-cardiac surgery after glycoprotein IIb/IIIa administration developed angiographically documented stent thrombosis (Figure 1 and Figure 2). In both cases the stent thrombosis occurred only hours after surgery. Moreover, one patient developed probable stent thrombosis two hours after CABG. In all patients DES implantation had occurred >12 months (13, 19, and 24 months) prior to surgery.
The current guidelines for the perioperative management of patients who need surgery post DES implantation recommend postponing surgery for at least 12 months post stenting, if feasible. The 12-month cut-off is based on expert opinion and on the currently recommended duration for dual antiplatelet therapy post DES implantation, which is based on the duration of therapy used in DES approval clinical trials4,5. Our cases demonstrate that postponing surgery for >12 months post stenting is not necessarily safe, as all stent thrombosis cases in our series occurred with surgery performed >12 months post stenting, in spite of using preoperative glycoprotein IIb/IIIa “bridging”. Patients with prior DES implantation should therefore undergo surgery at centres able to perform primary percutaneous coronary intervention, in order to treat perioperative stent thrombosis promptly, should it occur1,23. Repeat stent implantation should in general be avoided if a good angiographic result can be obtained with balloon angioplasty alone; however, if repeat stenting is needed to treat the thrombosed stent, bare metal stent implantation is preferable to repeat drug-eluting stent implantation to minimise the risk for recurrent stent thrombosis, as was done in two patients in our series.
The three stent thrombosis cases in our series highlight the main limitation of preoperative bridging with any short-acting agent, namely the lack of platelet inhibition postoperatively, when the stent thrombosis risk is highest due to activation of the sympathetic nervous system, the platelets and the coagulation system1,3. Occasionally patient care needs dictate delay of the surgery for >5-7 days from discontinuation of oral antiplatelet therapy, resulting in the need for prolonged glycoprotein IIb/IIIa inhibitor administration, which carries high cost and risk for thrombocytopenia (occurred in 3% of patients in our cohort) and bleeding. However, a recent report by Rassi et al24 suggested that there was no increased risk of bleeding in patients who underwent eptifibatide bridging before cardiac surgery, utilising bleeding events and transfusion rates without standard bleeding score. Only intravenous short-acting antiplatelet agents are currently available (glycoprotein IIb/IIIa inhibitors), and their administration requires prolonged hospitalisation, is costly and exposes the patients to additional risks, such as nosocomial infections.
Given the limitations of glycoprotein IIb/IIIa administration for prevention of perioperative stent thrombosis, what other strategies are available? Continuation of both aspirin and a thienopyridine (or aspirin alone if dual therapy is not considered safe) during the perioperative period may significantly reduce the risk for stent thrombosis, but may also increase the risk of bleeding. If continuation is decided, clopidogrel should be utilised rather than more potent oral platelet ADP P2Y12 receptor antagonists, such as prasugrel and ticagrelor. Although ticagrelor is shorter-acting than clopidogrel, three to five days are still required for its antiplatelet effect to dissipate; therefore, it would not be a good alternative to clopidogrel and would also be subject to the same limitations of glycoprotein IIb/IIIa administration, i.e., lack of antiplatelet effect during the immediate postoperative period when the stent thrombosis risk is highest. Additional strategies are needed to allow better prediction and treatment of perioperative stent thrombosis. For minor surgical procedures, the risk of bleeding is low and continuing dual antiplatelet therapy is preferred25. However, this is not a good option for high-risk surgical procedures, such as neurosurgery. An ongoing study, the “Bridging Therapy in Patients at High Risk for Stent Thrombosis Undergoing Surgery” (NCT00653601) is evaluating preoperative “bridging” with a glycoprotein IIb/IIIa inhibitor. Small molecule glycoprotein IIb/IIIa inhibitors cannot be administered to patients with severe renal failure or on dialysis; unfractionated heparin could provide some protection against stent thrombosis in such patients, although stent thrombosis is a platelet-mediated event and antiplatelet therapies may be preferable1. The same considerations apply when low molecular weight heparin is utilised to prevent stent thrombosis26.
Limitations
Our study has several limitations. The most important limitation is that it does not include a control group, hence it cannot address whether preoperative “bridging” with a glycoprotein IIb/IIIa inhibitor can prevent perioperative stent thrombosis compared to a strategy of antiplatelet therapy discontinuation without “bridging”. It included a relatively small number of patients, although it is the largest study of its kind reported to date. As is common among the veteran population, all study patients were men and our results may not be extrapolated to women, although it is unlikely that gender-based differences exist in the risk of perioperative stent thrombosis.
In summary, patients undergoing surgery post DES implantation have a high risk for postoperative stent thrombosis that may occur even after preoperative administration of a glycoprotein IIb/IIIa inhibitor. Novel strategies for predicting, preventing and treating perioperative DES thrombosis are needed.
Funding
Funding was provided by the Department of Veterans Affairs , United States.
Conflict of interest statement
S. Banerjee has received research grants from Gilead and the Medicines Company; has received consultant/speaker honoraria from Covidien and Medtronic; and has ownership in MDCARE Global (spouse) and intellectual property in HygeiaTel. E. Brilakis has received speaker honoraria from St. Jude Medical, Terumo, and Bridgepoint Medical and a research grant from Guerbet; his spouse is an employee of Medtronic. The other authors have no conflicts of interest to declare.

