Abstract
Aims: Clopidogrel discontinuation after percutaneous coronary intervention (PCI) with drug-eluting stent (DES) implantation has been reported to correlate with stent thrombosis. Whether these events are a consequence of the rebound phenomenon or a lack of protection in unhealed vessels is unclear. This study aimed to determine the link between clopidogrel cessation and cardiovascular events after PCI with DES.
Methods and results: The population included 1,903 patients who underwent PCI with DES implantation from 2003 to 2007. We compared patients who stopped their clopidogrel within the first month (group 1, n=97), from one to six months (group 2, n=344), from six to 12 months (group 3, n=468), and after 12 months (group 4, n=994) following the PCI. In each group, the composite of death, myocardial infarction and stent thrombosis at 30 days and between 31 and 60 days after clopidogrel cessation was indexed. Baseline characteristics were similar among groups. The event rate observed in the 0-30 day interval following cessation was higher only in group 1 (5.2%) compared to all other groups: 1.2% (group 2), 0.9% (group 3) and 0.6% (group 4) (p=0.004). The event rates from 31 to 60 days following cessation were low and similar among the four groups. When the elapsed time between the index PCI and the clopidogrel cessation was analysed as a continuous variable, the probability of events occurring within the first 30 days became similar to that observed in the 31-60 day interval following cessation after a minimum of 10.2 months.
Conclusions: Cardiac events seen immediately after clopidogrel cessation are not related to a rebound phenomenon, but are more likely influenced by the lack of healing at the time of cessation, which decreases over time. This increased risk related to the lack of healing seems to disappear after 10.2 months.
Introduction
The efficacy of antiplatelet therapy, especially thienopyridines, in the reduction of cardiac events that occur early after percutaneous coronary intervention (PCI) has been well documented1-5. Current guidelines recommend dual antiplatelet therapy with aspirin and clopidogrel for one year following PCI with drug-eluting stent (DES) implantation6,7.
Clopidogrel discontinuation within the first six months after PCI with DES implantation is associated with an increased risk of early adverse cardiovascular events, mainly stent thrombosis (ST)8-10. However, the impact of clopidogrel cessation after six months on short-term adverse cardiovascular events remains debated in the literature8-12. Moreover, it is unknown whether these early events that occur after clopidogrel cessation are related to mechanical factors only (stent related mechanism) or to a rebound effect (platelet aggregation mechanism). The presence of a rebound phenomenon after clopidogrel discontinuation, defined as a transient hyperthrombotic state, must be clearly distinguished from events that occur early after the clopidogrel cessation, but are related to mechanical factors, such as the lack of strut endothelialisation or residual dissection, for example. Indeed, the presence of a rebound effect would suggest that clopidogrel discontinuation is always associated with an increased risk of early events no matter the delay between the cessation and the initial PCI. More importantly, the presence of such an effect would also suggest the need for a progressive decrease in the clopidogrel dose rather than an abrupt cessation. Some clinical situations, such as the need for surgery or the occurrence of bleeding, require temporary or definitive clopidogrel discontinuation. In consequence, the need for clopidogrel discontinuation remains a critical decision for the physician.
The aim of this study was to assess the safety of clopidogrel cessation over time following PCI with DES implantation with respect to the events that occur within the immediate 30 days following cessation in reference to those events that occur subsequently. In addition, we sought to assess the possible presence of a rebound effect immediately post cessation of the drug.
Methods
Study population and design
From January 2003 to December 2007, a cohort of 1,903 patients who underwent PCI with DES implantation at our centre was entered into this analysis. Only patients known to have discontinued their clopidogrel therapy were included. We compared patients who stopped their clopidogrel within the first month (group 1, n=97), between one and six months (group 2, n=344), between six and 12 months (group 3, n=468), and after 12 months (group 4, n=994) following the PCI with ≥1 DES. In each group, we indexed the primary composite endpoint of death, myocardial infarction, and definite stent thrombosis (ST), at 30 days and between 31 and 60 days after the clopidogrel cessation.
Percutaneous coronary intervention procedures
PCI was performed according to the guidelines current at the time of the procedure7. In all cases, the interventional strategy was at the discretion of the responsible physician. Intra-procedural anticoagulation was ensured using bivalirudin or unfractionated heparin to achieve an activated clotting time of >250 seconds in all patients. Glycoprotein IIb/IIIa inhibitors were used at the operators’ discretion and according to guidelines. All patients received an aspirin loading dose of 325 mg before the procedure and continued this regimen indefinitely. After a clopidogrel loading dose of 300 to 600 mg, additional antiplatelet therapy with a 75-mg clopidogrel maintenance dose was instituted in all patients until the clopidogrel cessation.
Follow-up and endpoint definitions
The collection of clopidogrel cessation dates and clinical follow-up was conducted via telephone contact or office visits by independent research personnel who were unaware of the study objectives. In case of hospitalisation, data were obtained by a systematic review of the discharge summary and all clinical events were adjudicated by independent physicians via source documentation. During the 60-day follow-up after cessation of clopidogrel, the primary composite endpoint of death, myocardial infarction, and ST was systematically indexed.
“Angiographic success” was defined as a post procedure stenosis of ≤30% with a Thrombolysis in Myocardial Infarction flow grade 3 on individual lesion targeted. All events were adjudicated by independent physicians from source documents using the following definitions. “Major adverse cardiac events” were defined as the composite criteria death-myocardial infarction-ST. “Death” was defined as all-cause mortality. “Myocardial infarction” was defined as all new Q-wave myocardial infarctions (QWMI) defined as the association of a total creatinine kinase (CK) elevation ≥3 times the upper limit of normal and/or a total CK-MB elevation ≥2 times the upper limit of normal plus pathologic Q-waves in at least two contiguous leads on electrocardiogram13. “Any ST” was defined as definite ST according to the Academic Research Consortium definition14.
Statistical analysis
A dedicated data-coordinating centre (Data Center, Cardiovascular Research Institute, Washington, DC, USA) performed all data management and analyses. Continuous variables were expressed as mean ± standard deviation, except for the elapsed time from PCI procedure to cessation of clopidogrel, which was expressed as median (25th-75th). Categorical variables were expressed as absolute numbers and percentages. Baseline characteristics among the groups were compared by using the chi-square test or the Fisher exact test for categorical variables and by ANOVA for continuous variables as appropriate. In order to assess the association between time intervals after clopidogrel cessation and risk of composite events, we estimated the probability of the composite event within each of the post-cessation time windows (0-30 days and 31-60 days post-cessation) by logistic regression analysis and plotted these 2 probabilities, including their 95% confidence intervals, against the days to cessation from the PCI with DES implantation procedure. All statistical analyses were performed using SAS version 9.1® (SAS Institute, Cary, NC, USA). Statistical significance was assumed at p value <0.05.
Results
Patient characteristics
Baseline clinical characteristics of the four groups are summarised in Table 1. There was no statistical difference in terms of baseline clinical characteristics among patients who stopped clopidogrel within the first month (group 1), between one and six months (group 2), between six and 12 months (group 3), and after 12 months (group 4) following the initial PCI with DES. The mean age was nearly 66 years in the four groups, 2/3 of the patients were male, nearly 30% of them had a history of diabetes and 12% had history of renal failure (clearance <60 mL/min) on average. The mean left ventricular ejection fraction was around 50% at baseline. Clinical presentation at the time of the initial PCI was similar in the four groups. Nearly 11% of the patients presented as a ST-segment elevation myocardial infarction.
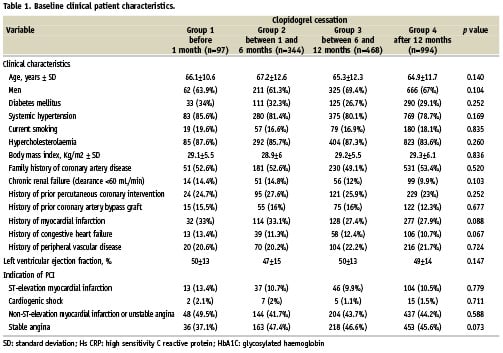
Baseline angiographic and procedural characteristics are summarised in Table 2. There was no statistical difference in terms of baseline angiographic and procedural characteristics among the four groups. Bivalirudin and glycoprotein IIb/IIIa inhibitors were used in 75% and 10% of the cases, respectively. The angiographic success rate was high and similar among the four groups at around 97%.
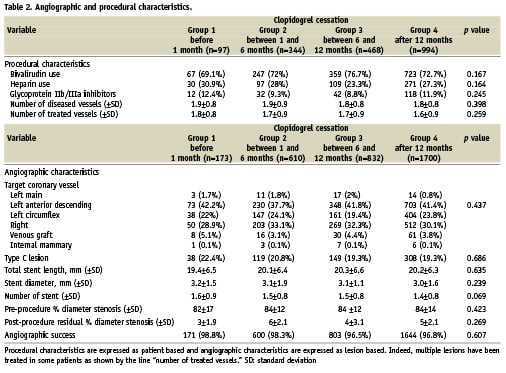
The reasons for clopidogrel cessation are detailed in Table 3. The reasons for clopidogrel cessation in patients who stopped the drug within the first six months after PCI included the need for surgery, the occurrence of bleeding, the development of an allergy, or the lack of compliance. By contrast, physician decision was most likely the reason for clopidogrel cessation in patients who stopped the drug after six months.
Outcomes
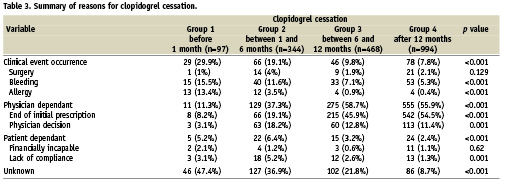
Altogether, 26 events (1.4%) occurred after clopidogrel discontinuation during the 60-day follow-up period. In group 1, the rates of the composite endpoint of death, myocardial infarction, and ST were 5.2% at 30 days and 1.1% between 31 and 60 days after clopidogrel cessation. Similarly, the composite endpoint rates were 1.2% and 0.6% in group 2; 0.9% and 0.2% in group 3; and 0.6% and 0.3% in group 4 for the time intervals of 0-30 days and 31-60 days, respectively, after cessation (Table 4). As shown in Table 4, there were only two events that clearly were not related to a stent complication. The composite endpoint rate in the 0-30 day interval was higher only in group 1 compared to all other groups, p=0.004 (Figure 1). The median time to event was five (5-7) days in group 1. There was no statistical difference noted among groups 2, 3 and 4 for this 0-30 day period. In addition, there was no statistical difference among the four groups for the rate observed within the 31-60 day interval following clopidogrel cessation.
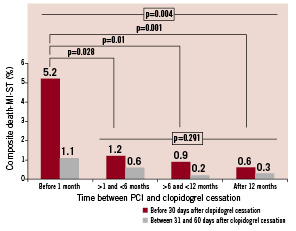
Figure 1. Composite endpoint rates (p values assessed by repeated measures ANOVA using Bonferroni’s adjustment).
When the elapsed time between the index PCI and clopidogrel cessation was analysed as a continuous variable, (Figure 2) the probability of the composite endpoint occurring within the first 30 days was similar to the probability of the event occurring within 31-60 days once a minimum of 10.2 months had elapsed. This interval, however, may be as long as 25 months. Figure 2 also demonstrates that the probability of death, myocardial infarction and ST within the first 30 days following clopidogrel cessation decreased over time and appeared to become asymptotic to a very low probability without any plateau. The probability of death, myocardial infarction, and ST within 31-60 days following cessation appears to be horizontal to the x-axis, suggesting that there is no increased risk of the composite endpoint during this period as a function of time from DES implantation. These data also suggest the lack of any biological rebound phenomenon immediately following the cessation of clopidogrel.
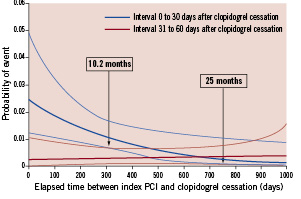
Figure 2. Probability of composite events following clopidogrel cessation.
Discussion
This study suggests that cardiac events seen immediately after clopidogrel cessation are not related to a biological rebound phenomenon associated with clopidogrel, but are more likely influenced by the lack of healing at the time of cessation, which decreases over time. Only the composite event rate observed in the 0-30 day interval was significantly higher as compared to all other groups. Accordingly, a recent randomised trial has reported that the course of platelet aggregation after clopidogrel cessation (assessed with light transmission aggregometry and multiple electrode aggregometry) provides no evidence for the existence of any biological rebound phenomenon15.
In addition, as suggested by probability plots in Figure 2, our results suggest also that the minimum duration of clopidogrel therapy following PCI with DES should be 10.2 months, but may be as long as 25 months before the probability of the composite endpoint event within the first 30 days becomes similar to that of the subsequent 30 days.
Previous published data have highlighted the high prognostic value of an early clopidogrel cessation after PCI (within the first few months)8-10. The current European and North American guidelines recommend a 4-week dual antiplatelet therapy regimen after PCI even in the case of bare metal stent implantation6,7. The role of early antiplatelet therapy discontinuation after PCI in terms of ST is well recognised and is not questioned. In fact, according to the literature, it is the strongest predictor of early events and especially early ST10. However, regarding the risk of bleeding and the need for surgery, it must still be determined when clopidogrel cessation after PCI is safe. In addition, the need for clopidogrel discontinuation after the first month is not a rare situation in clinical practice and is still a critical decision for the physician, especially after DES implantation16. Eisenstein et al recently reported that continued clopidogrel use at six months in a PCI population treated with DES is protective against death and MI and demonstrated lower rates as compared to patients who had discontinued clopidogrel by six months.8 Several studies seem to have identified cessation of clopidogrel as a predictor of late ST at one year in case of DES use, reporting a relative risk of ST between 24.79 and 57.13.10,17 Stone et al have reported a trend for fewer very late ST episodes in those patients remaining on thienopyridine therapy as compared to those who discontinued thienopyridines at one year12. However, other studies have reported contradictory results and did not find any link between discontinuation of antiplatelet therapy and incidence of late or very late events18,19. In a large European multicentre study, thienopyridine discontinuation after six months in patients with DES was not a risk factor for subsequent ST17. Consequently, the impact of clopidogrel cessation after the first month following PCI on short-term adverse cardiovascular events remains largely debated in the literature8-12,17-20. In addition, even if no data is currently available, some authors suggest that clopidogrel might also prevent events not related to stent complications, but instead are related to other plaque evolution in such a population of patients with significant coronary artery disease.
In the current study, our results suggest that the risk of events in both intervals – 0 to 30 – and 31 to 60 days after cessation of clopidogrel – became similar after a minimum of 10.2 months, but could be as long as 25 months following DES implantation. It can be postulated from these data that following DES implantation, if patients are prescribed clopidogrel as the thienopyridine of choice, they should remain on clopidogrel therapy for a minimum of 10.2 months as to attenuate the increased risk of death, myocardial infarction, or ST associated with the lack of healing coupled with DES implantation. In addition, the rate of events not related to a stent complication after clopidogrel discontinuation was very low as only two such events were indexed. The present study was not designed to answer the question of the benefit/risk ratio of a prolonged or definitive clopidogrel discontinuation after PCI with DES, but does support the current American College of Cardiology/American Heart Association guideline recommendations of a minimum of 12 months of thienopyridine therapy following PCI with DES implantation.
Study limitations
This analysis was performed on a retrospective cohort of patients treated at a single centre. Although the aim of the study was to assess the safety of clopidogrel cessation over time following PCI with DES implantation with respect to the events that occur within the immediate 30 days following cessation compared to those events that occur subsequently, the data were not prospectively collected to address this issue. Some patients with hard events, such as cardiac or unexplained death, could have inadvertently been excluded from the final analysis. However, these data yield an important observation in correlation with the American College of Cardiology/American Heart Association guideline recommendations for the duration of thienopyridine therapy post PCI with DES implantation. Additionally, this analysis did not compare event rates following cessation to those rates in a population who continued clopidogrel.
Conclusion
Cardiac events seen immediately after clopidogrel cessation seem to not be related to a biological rebound phenomenon but are more likely influenced by the lack of healing at the time of cessation, which decreases over time. According to our results, this increased risk related to the lack of healing seems to disappear after 10.2 months after DES implantation. However, these data should be confirmed by further prospective and randomised clinical trials before extending such conclusions to clinical practice.

