Abstract
Background: Although P2Y12 inhibitor monotherapy has emerged as a promising alternative for dual antiplatelet therapy (DAPT), there remains concern regarding the safety of clopidogrel monotherapy.
Aims: We sought to investigate clinical outcomes of clopidogrel monotherapy in patients with and without on-treatment high platelet reactivity (HPR).
Methods: In the SMART-CHOICE study, three-month DAPT followed by P2Y12 inhibitor monotherapy was compared with 12-month DAPT in patients undergoing percutaneous coronary intervention. A platelet function test was performed for 833 patients with clopidogrel-based therapy. The primary endpoint was major adverse cardiovascular and cerebrovascular events (MACCE: a composite of all-cause death, myocardial infarction, or stroke) at 12 months.
Results: Overall, 108 (13.0%) patients had HPR on clopidogrel. Patients with HPR had a significantly higher rate of MACCE than patients without HPR (8.7% vs 1.5%, adjusted HR 3.036, 95% CI: 1.060-8.693, p=0.038). The treatment effect of clopidogrel monotherapy for the 12-month MACCE was not significantly different compared with DAPT among patients with HPR (8.0% vs 9.4%, adjusted HR 0.718, 95% CI: 0.189-2.737, p=0.628) and without HPR (2.2% vs 0.9%, adjusted HR 2.587, 95% CI: 0.684-9.779, p=0.161; adjusted p for interaction=0.170).
Conclusions: Clopidogrel monotherapy showed treatment effects comparable to DAPT for MACCE in patients with or without HPR. However, HPR was significantly associated with an increased risk of MACCE in clopidogrel-treated patients regardless of maintenance of aspirin. Clinical Trial Registration: Comparison Between P2Y12 Antagonist Monotherapy and Dual Antiplatelet Therapy After DES (SMART-CHOICE) (ClinicalTrials.gov: NCT02079194).
Introduction
The cornerstone of treatment for patients undergoing percutaneous coronary intervention (PCI) is antiplatelet therapy1,2. Previous studies have consistently reported that prolonged dual antiplatelet therapy (DAPT) can reduce myocardial infarction and stent thrombosis. However, it also increases the risk of bleeding compared to DAPT for a short or standard duration followed by aspirin monotherapy3,4,5. The optimal duration of DAPT has not yet been determined, although numerous trials have been conducted on this issue. In this regard, P2Y12 inhibitor monotherapy after a short duration of DAPT has emerged as a promising novel alternative treatment strategy6.
Several randomised trials have consistently reported that a short duration of DAPT followed by P2Y12 inhibitor monotherapy and conventional DAPT have comparable protective effects against recurrent ischaemic events, leading to reduced risk of bleeding in patients undergoing PCI7,8,9,10,11. The Smart Angioplasty Research Team: Comparison Between P2Y12 Antagonist Monotherapy vs Dual Antiplatelet Therapy in Patients Undergoing Implantation of Coronary Drug-Eluting Stents (SMART-CHOICE) trial has demonstrated that three-month DAPT followed by P2Y12 inhibitor monotherapy is non-inferior to 12-month DAPT for the composite of all-cause death, myocardial infarction, and stroke in patients receiving contemporary drug-eluting stents (DES)8.
Clopidogrel was predominantly used as a P2Y12 inhibitor for DAPT in the SMART-CHOICE trial. However, there remain concerns about clopidogrel monotherapy among patients with on-treatment high platelet reactivity (HPR). It is well known that patients with HPR show an increased risk of ischaemic events12. Although a routine platelet function test (PFT) has not been recommended in contemporary practice, a PFT was assessed in some of the patients enrolled in the SMART-CHOICE trial. In this context, this study sought to investigate whether the effects of clopidogrel monotherapy would be similar to clopidogrel-based DAPT for patients with or without HPR.
Methods
STUDY DESIGN AND POPULATION
The study design and main results of the SMART-CHOICE trial have been reported previously8,13. Briefly, SMART-CHOICE was a multicentre, randomised clinical trial that demonstrated the non-inferiority of P2Y12 inhibitor monotherapy after three-month DAPT to 12-month DAPT for the composite of ischaemic events in patients receiving current-generation DES (ClinicalTrials.gov: NCT02079194). Detailed enrolment criteria are available in a previous report8. The institutional review board at each participating centre approved the trial protocol. All participants provided written informed consent.
RANDOMISATION, PROCEDURE, AND MEDICAL TREATMENT
Patients were randomised to the P2Y12 inhibitor monotherapy group (aspirin plus a P2Y12 inhibitor for three months and a P2Y12 inhibitor alone thereafter) or the long-term DAPT group (aspirin plus a P2Y12 inhibitor for at least 12 months) in a 1:1 ratio at the index procedure or at the follow-up visit within three months after the index procedure. Coronary angiography and PCI were performed according to standard guidelines14. The diameter and length of the stent were not restricted, and the stents were limited to second-generation stents which allowed short-term DAPT8. Antithrombotic treatment related to PCI was also performed according to standard guidelines2. All patients received 300 mg of aspirin and 300-600 mg of clopidogrel as a loading dose orally before PCI, unless they had previously received these antiplatelet agents. When patients presented with an acute coronary syndrome, 60 mg of prasugrel, 180 mg of ticagrelor, or clopidogrel loading dose were used. After the procedure, all patients received DAPT with aspirin 100 mg once daily plus clopidogrel 75 mg once daily or prasugrel 10 mg once daily or ticagrelor 90 mg twice daily for three months. Administration of aspirin was stopped at three months after the index procedure in the P2Y12 inhibitor monotherapy group but was continued indefinitely in the DAPT group. Administration of the P2Y12 inhibitor was continued in both groups. Other medications, including beta-blockers, renin-angiotensin system blockade and statins, were prescribed according to guidelines, if indicated2.
SELECTION OF P2Y12 INHIBITOR AND ON-TREATMENT PLATELET FUNCTION TEST
In the SMART-CHOICE trial, three kinds of P2Y12 inhibitor (clopidogrel, ticagrelor, and prasugrel) were allowed. The selection of the P2Y12 inhibitor was left to the discretion of treating physicians. PFT was performed using a VerifyNow P2Y12 assay (Accumetrics Inc., San Diego, CA, USA) at 2-4 weeks after randomisation. The decision to perform a PFT was entirely at the discretion of the attending physician. VerifiyNow tests were performed by an experienced laboratory at each participating centre blinded to clinical data following the instructions of the device company. Regardless of the results of the PFT, patients were assigned to randomised arms until clinical events occurred.
For this post hoc analysis, HPR on clopidogrel was defined as a platelet reactivity unit (PRU) level of more than 275, based on previous studies for the same regional and racial population15,16. The cut-off value of HPR was re-evaluated within the study population. Sensitivity analysis with different cut-off values of HPR (PRU >208) based on the latest expert consensus document12 was also performed. Clinical outcomes between P2Y12 inhibitor monotherapy after three-month DAPT and 12-month DAPT were compared among patients with or without HPR. We also compared outcomes between patients receiving clopidogrel and those receiving a potent P2Y12 inhibitor monotherapy (Figure 1).
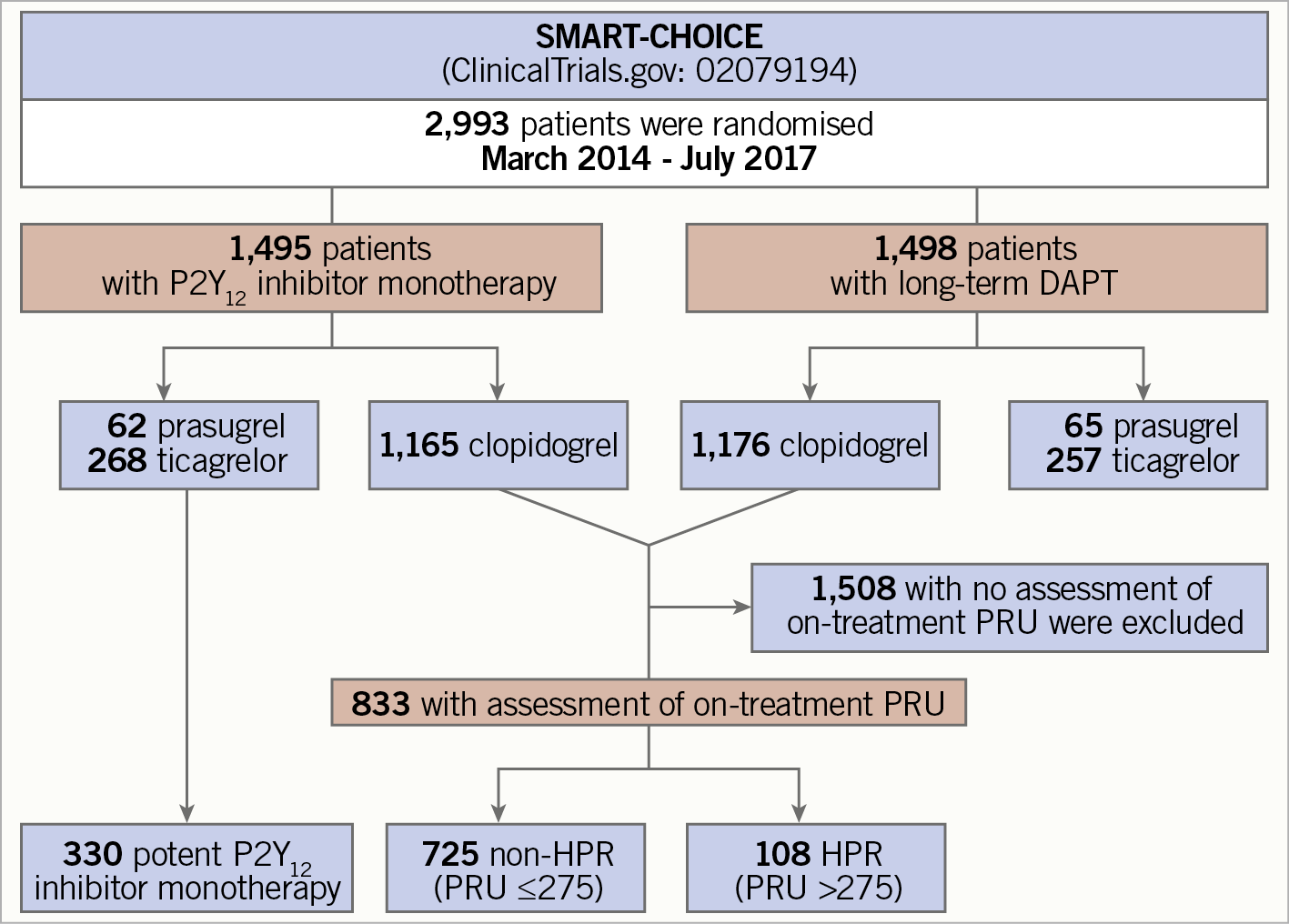
Figure 1. Study flow. DAPT: dual antiplatelet therapy; HPR: high platelet reactivity; PRU: platelet reactivity unit
STUDY ENDPOINTS AND DEFINITION
The primary endpoint was major adverse cardiovascular and cerebrovascular events (MACCE), defined as a composite of all-cause death, myocardial infarction, and stroke at 12 months. Secondary endpoints included each component of MACCE, cardiac death, stent thrombosis, and bleeding events at 12 months after the index procedure. All clinical outcomes were defined according to the Academic Research Consortium, including the addendum to the definition of myocardial infarction17. All deaths were considered cardiac unless an undisputed non-cardiac cause was present. Periprocedural cardiac enzyme level within 48 hours after the index procedure without concomitant ischaemic symptoms or electrocardiographic findings indicative of ischaemia was not counted as a clinical event. Stroke was defined as any non-convulsive focal or global neurologic deficit of abrupt onset lasting for more than 24 hours or leading to death, which was caused by ischaemia or haemorrhage within the brain. Stent thrombosis was defined as definite or probable stent thrombosis according to the Academic Research Consortium classification. Bleeding events were adjudicated and classified according to the Bleeding Academic Research Consortium classification18. Major bleeding was defined as Bleeding Academic Research Consortium type 3, 4, or 5 bleeding.
STATISTICAL ANALYSIS
All categorical variables are presented as numbers and relative frequencies (percent). Continuous variables are presented as means and standard deviations or medians with first and third quartiles, according to their distribution, which was checked by the Kolmogorov-Smirnov test and visual inspection of Q-Q plots. Discrete or categorical variables were analysed using the chi-square or Fisher’s exact test. Continuous variables were analysed using the Mantel-Haenszel statistic or analysis of variance to test differences according to their distribution. Post hoc analyses were not performed. Cumulative event rates were estimated with the Kaplan-Meier method and compared using the log-rank test or the Breslow test. We censored patients who were lost to follow-up at the time of the last known contact. The optimal cut-off value of on-treatment PRU for predicting 12-month MACCE after the index procedure was determined to maximise sensitivity and specificity using receiver operating characteristic analysis. The derived cut-off value was validated using the maximally selected log-rank statistics as a sensitivity analysis. A Cox proportional hazards regression model was used to calculate the hazard ratio (HR) and 95% confidence intervals (CI). The assumption of proportionality was assessed graphically with a log-minus-log plot and tested by Schoenfeld residuals. Cox proportional hazards models for all clinical outcomes satisfied the proportional hazards assumption. Multivariable analysis was performed to evaluate the impact of HPR on 12-month MACCE according to clinical characteristics (Supplementary Table 1), and the final model included variables of age, sex, diabetes mellitus, smoking, previous stroke, chronic kidney disease, and left ventricular ejection fraction (LVEF). Multivariable analysis for evaluating the impact of treatment strategy was performed according to clinical characteristics (Table 1), and the final model included variables of age and sex.
All analyses were two-tailed, and clinical significance was defined at p<0.05. All statistical analyses were performed using SPSS for Windows, Version 22 (IBM Corp., Armonk, NY, USA) and R version 3.6.0 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Between March 2014 and July 2017, a total of 2,993 patients were enrolled. Of these, 1,495 were randomly assigned to receive P2Y12 inhibitor monotherapy and 1,498 were randomly assigned to receive 12-month DAPT (Figure 1). Clopidogrel was used as a P2Y12 inhibitor in 2,341 (78.2%) patients, and prasugrel or ticagrelor as a potent P2Y12 inhibitor was used in 652 (21.8%) patients.
CUT-OFF VALUE FOR HPR AMONG PATIENTS RECEIVING CLOPIDOGREL
A PFT was performed for 833 (35.6%) patients receiving clopidogrel at a mean of 27 days after the index procedure. The optimal cut-off value of HPR on clopidogrel for predicting 12-month MACCE was more than 275 PRU in this population (Supplementary Figure 1), confirming that the cut-off value of HPR suggested previously was an appropriate determinant for predicting 12-month MACCE. The baseline characteristics of the patients divided according to the derived cut-off value are summarised in Supplementary Table 1.
BASELINE CHARACTERISTICS OF THE STUDY POPULATION
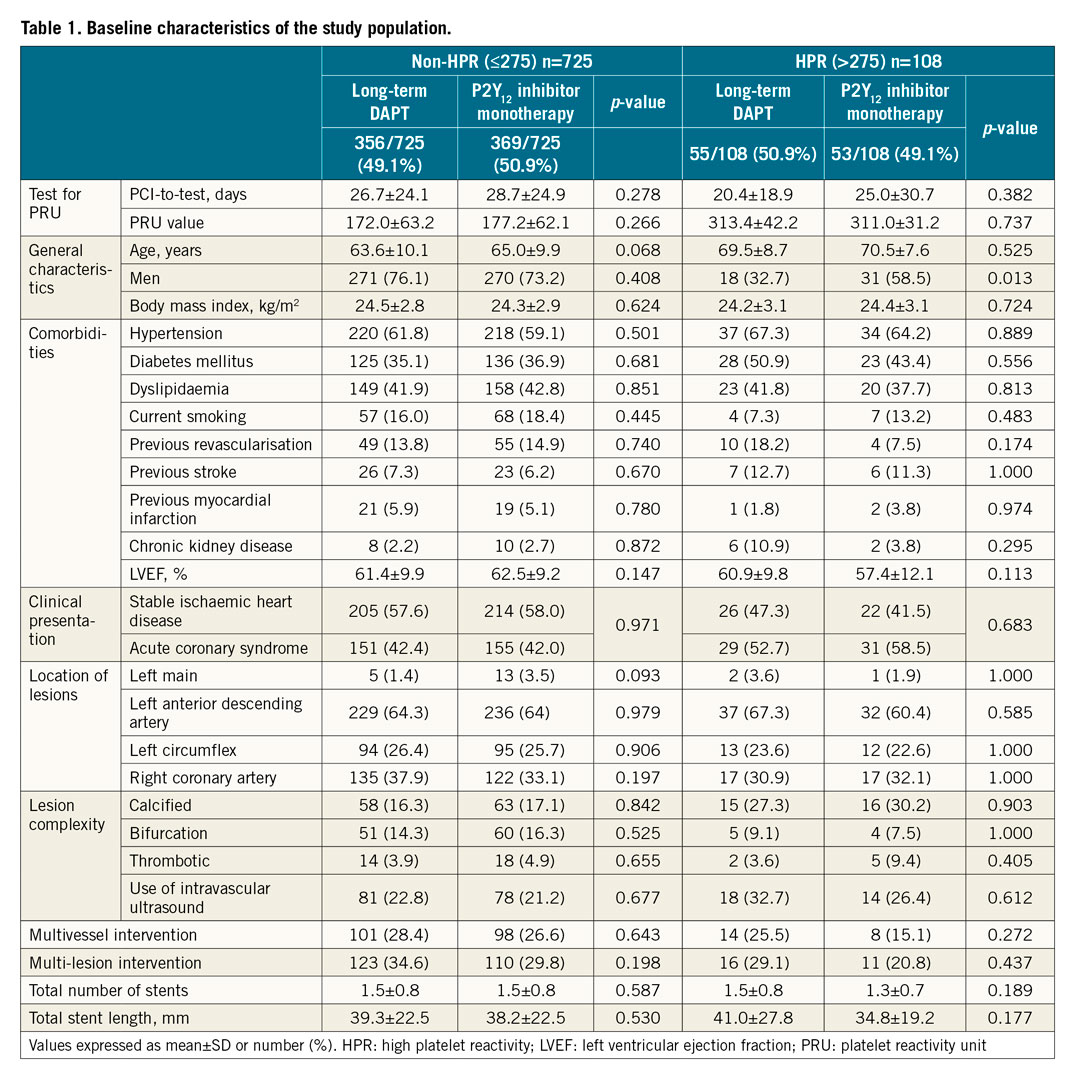
Table 1 summarises the baseline characteristics of the study population according to the treatment strategy (short-term DAPT followed by P2Y12 inhibitor monotherapy vs long-term DAPT) and on-treatment PRU on clopidogrel. Of 833 patients receiving clopidogrel, 108 (13.0%) patients had HPR, including 53 patients (49.1%) in the P2Y12 inhibitor monotherapy group and 55 (50.9%) in the long-term DAPT group. There was no significant difference in the on-treatment PRU level according to the treatment strategy (DAPT 313.4±42.2 vs P2Y12 inhibitor monotherapy 311.0±31.2, p=0.737) in patients with HPR. Among patients with HPR on clopidogrel, the proportion of men was higher in the clopidogrel-based monotherapy than in the DAPT group (58.5% vs 32.7%, p=0.013). There was no significant difference in other baseline characteristics according to the treatment strategy among patients without HPR on clopidogrel.
CLINICAL OUTCOMES ACCORDING TO HPR ON CLOPIDOGREL AND DAPT DURATION
The median follow-up duration of the study population was 365 days. HPR was related to an increased risk of MACCE compared to non-HPR (adjusted HR 3.036, 95% CI: 1.060-8.693, p=0.038) (Central illustration, Supplementary Table 2). The effect of clopidogrel monotherapy was not significantly different from that of clopidogrel-based long-term DAPT for MACCE among patients with HPR (8.0% vs 9.4%, adjusted HR 0.718, 95% CI: 0.189-2.737, p=0.628) or without HPR on clopidogrel (2.2% vs 0.9%, adjusted HR 2.587, 95% CI: 0.684-9.779, p=0.161) (Table 2, Figure 2). In the landmark analysis for the three-month landmark point, results also showed that the clopidogrel monotherapy was comparable to long-term DAPT (Supplementary Figure 2). For bleeding events (Figure 3), long-term DAPT showed an increased risk of events compared to clopidogrel monotherapy for patients in the non-HPR group. However, the interaction term was not significant (adjusted p for interaction=0.416). Results of subgroup analysis (Supplementary Figure 3) for 12-month MACCE rates between clopidogrel monotherapy and DAPT were generally consistent across multiple subgroups. Furthermore, when we defined HPR with a different cut-off value of 20812, the risk of 12-month MACCE with clopidogrel monotherapy was also similar to that with DAPT regardless of HPR (Supplementary Figure 4).
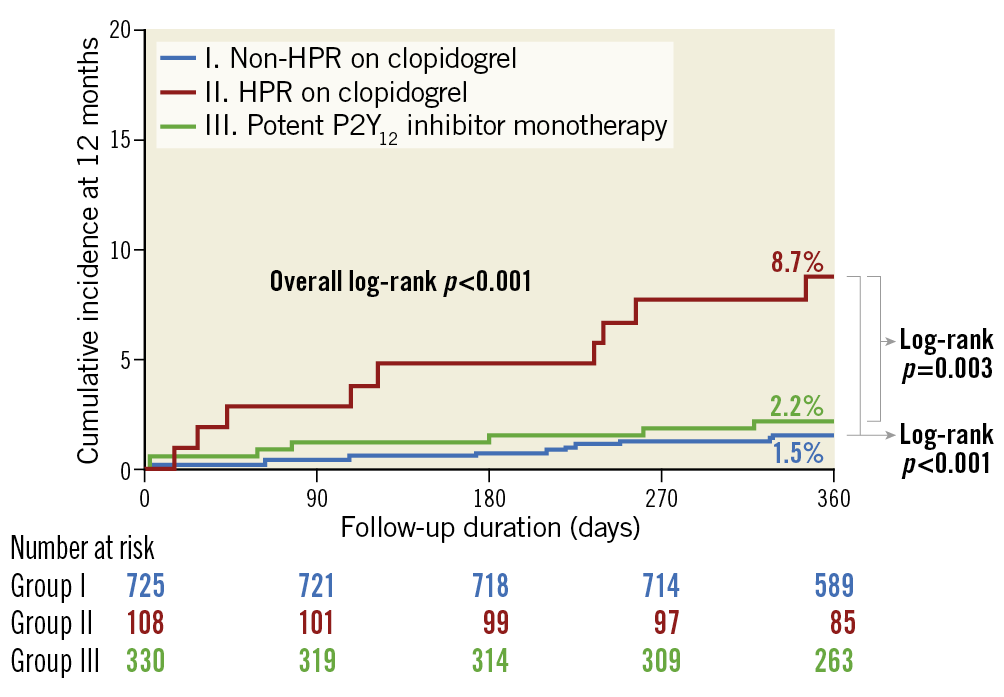
Central illustration. Comparison of 12-month MACCE rate according to on-treatment PRU level and type of P2Y12 inhibitor. The cumulative incidence of MACCE at 12 months was compared according to HPR among patients receiving clopidogrel. It was also compared to those who received potent P2Y12 inhibitor monotherapy. The incidence of 12-month MACCE was significantly higher in patients with HPR than in other groups. HPR: high platelet reactivity; MACCE: major adverse cardiovascular and cerebrovascular events; PRU: platelet reactivity unit
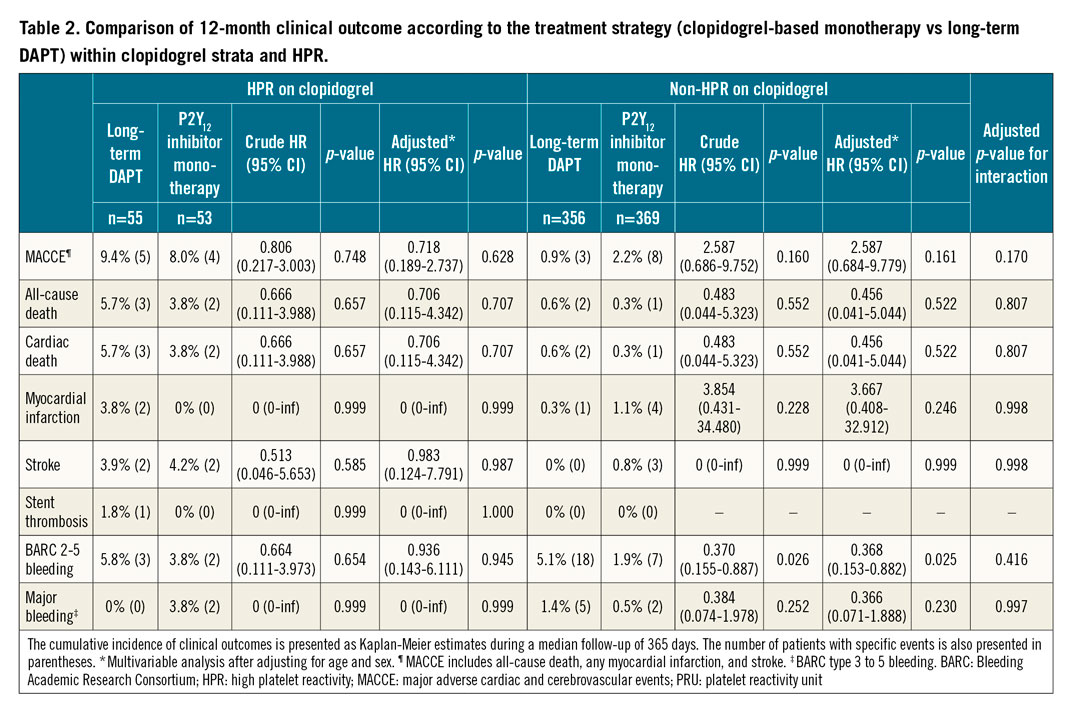
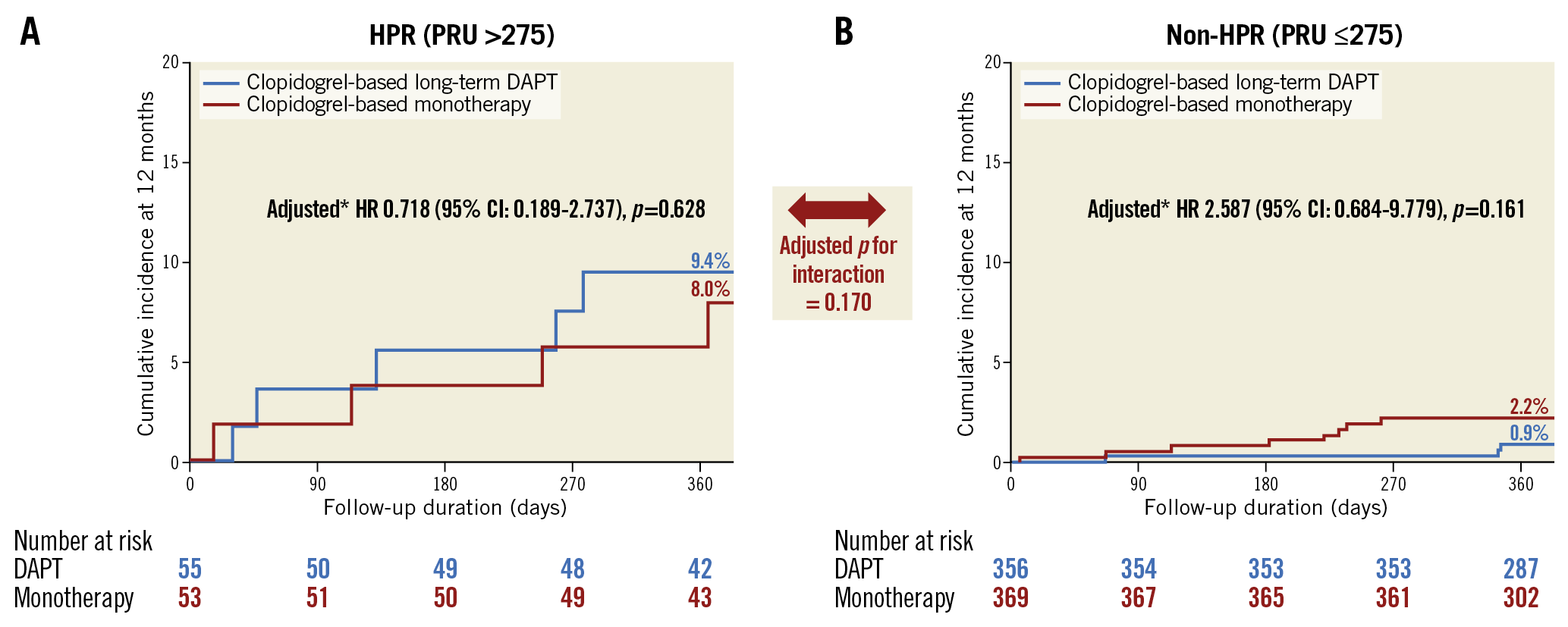
Figure 2. Comparison of 12-month MACCE rate according to HPR on clopidogrel. The cumulative incidence of MACCE at 12 months was compared between the long-term DAPT and monotherapy groups for those (A) with HPR (>275) or (B) without HPR (≤275) among patients on clopidogrel. * Multivariable analysis after adjusting for age and sex. CI: confidence interval; DAPT: dual antiplatelet therapy; HPR: high platelet reactivity; HR: hazard ratio; PRU: platelet reactivity unit
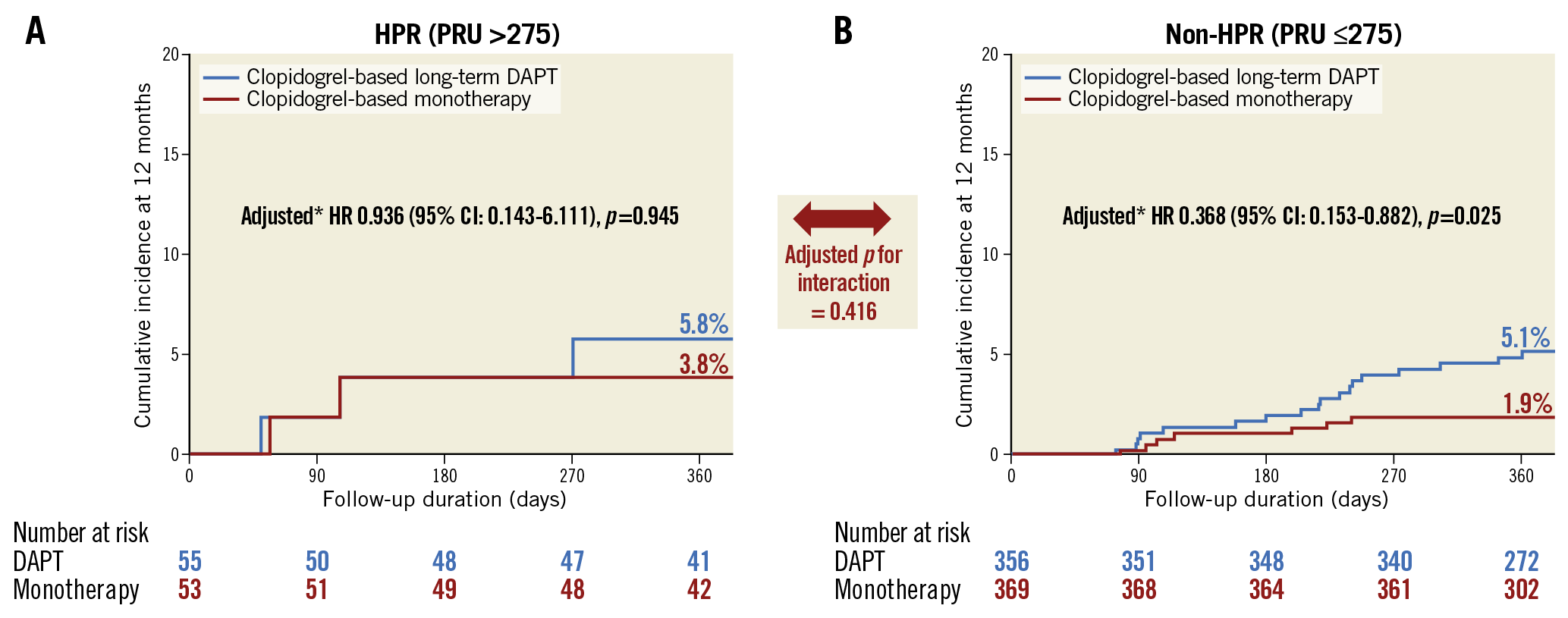
Figure 3. Comparison of 12-month BARC 2-5 bleeding rate according to HPR on clopidogrel. The cumulative incidence of BARC 2-5 bleeding at 12 months was compared between the long-term DAPT and monotherapy groups for those (A) with HPR (>275) or (B) without HPR (≤275) among patients on clopidogrel. * Multivariable analysis after adjusting for age and sex. BARC: Bleeding Academic Research Consortium; DAPT: dual antiplatelet therapy; HPR: high platelet reactivity; HR: hazard ratio; PRU: platelet reactivity unit
COMPARISON OF OUTCOMES WITH PATIENTS RECEIVING POTENT P2Y12 INHIBITOR MONOTHERAPY
Baseline characteristics of the 330 patients receiving potent P2Y12 inhibitor monotherapy are summarised in Supplementary Table 3. The rate of MACCE in patients receiving short-term DAPT followed by monotherapy using a potent P2Y12 inhibitor was 2.2%, significantly lower than that in those with HPR on clopidogrel (2.2% vs 8.7%, HR 0.250, 95% CI: 0.093-0.671, p=0.006) (Central illustration). When we compared the effects of potent P2Y12 inhibitor monotherapy to those with a clopidogrel-based strategy, a consistently lower rate of MACCE occurred in the group receiving clopidogrel, regardless of clopidogrel monotherapy or long-term DAPT with clopidogrel (HR vs clopidogrel monotherapy 0.281, 95% CI: 0.082-0.961, p=0.043 and HR vs clopidogrel with aspirin 0.225, 95% CI: 0.071-0.708, p=0.011) (Supplementary Figure 5).
Discussion
The present study evaluated clinical outcomes of patients receiving clopidogrel-based antiplatelet therapy with or without HPR using data from the SMART-CHOICE trial. Overall, approximately 13% of patients with clopidogrel had HPR. They had a higher risk of 12-month MACCE than those with non-HPR on clopidogrel (Central illustration). Compared with 12-month DAPT, clopidogrel monotherapy had comparable MACCE regardless of HPR on clopidogrel. Meanwhile, potent P2Y12 inhibitor monotherapy was found to be associated with a reduced risk of MACCE compared with clopidogrel-based antiplatelet therapy among patients with HPR on clopidogrel.
Clopidogrel is a prodrug that requires metabolism to inhibit the P2Y12 receptor19. Response to clopidogrel is variable. In a substantial proportion of patients, the response to clopidogrel is inadequate12,20. As a result, concerns about clopidogrel monotherapy have been raised, especially in patients with HPR on clopidogrel. The use of potent P2Y12 inhibitors may be an alternative for these patients. However, ticagrelor and prasugrel are indicated only in patients with acute coronary syndrome and clopidogrel is the most widely used P2Y12 inhibitor in real-world practice21. Therefore, to investigate the effect of clopidogrel monotherapy according to on-treatment HPR is of great clinical importance. Although one-month DAPT followed by clopidogrel monotherapy reduced a composite of cardiovascular and bleeding events in the Short and Optimal Duration of Dual Antiplatelet Therapy After Everolimus-Eluting Cobalt-Chromium Stent (STOPDAPT)-2 trial10, no data on the safety of clopidogrel monotherapy in patients with HPR on clopidogrel are available. Therefore, we performed a post hoc study of the SMART-CHOICE trial to compare clopidogrel monotherapy with clopidogrel plus aspirin among patients with or without HPR on clopidogrel.
In this study, patients with HPR on clopidogrel had a significantly higher risk of MACCE than those without HPR on clopidogrel. This result is in line with previous studies showing that HPR on clopidogrel is independently associated with stent thrombosis and MI22. However, continuation of aspirin was not associated with favourable outcomes in patients with HPR on clopidogrel or in those with non-HPR on clopidogrel. There are several explanations for these results. First, besides PRU level, patients with HPR on clopidogrel have a higher risk profile than those with non-HPR on clopidogrel. Therefore, maintenance of aspirin might not adequately improve clinical outcomes of patients with HPR on clopidogrel. In these patients, the use of potent P2Y12 inhibitors instead of clopidogrel might be more rational than extending the duration of aspirin treatment. In the present analysis, patients receiving potent P2Y12 inhibitor monotherapy had comparable outcomes to those with non-HPR on clopidogrel. They showed better outcomes than those with HPR on clopidogrel regardless of the maintenance of aspirin. Recently, the Ticagrelor with Aspirin or Alone in High-Risk Patients after Coronary Intervention (TWILIGHT) trial demonstrated that, among high-risk patients who underwent PCI and completed three-month DAPT, ticagrelor monotherapy was associated with a lower incidence of clinically relevant bleeding than ticagrelor plus aspirin, without showing a higher risk of death, MI, or stroke9. Second, in the SMART-CHOICE trial, patients exclusively received second-generation DES, which reduced stent thrombosis and MI significantly compared to first-generation DES. After three months of PCI with second-generation DES, uncovered struts were rare in an optical coherence tomography study23. In the SMART-CHOICE trial, three-month DAPT before clopidogrel monotherapy might have resulted in consistent P2Y12 inhibitor monotherapy effects on MACCE regardless of the on-treatment platelet reactivity on clopidogrel.
The proportion of patients with HPR on clopidogrel seemed to be low in this study compared to that in previous studies15,16. It is difficult to know the exact causes; the timing of PRU measurements might explain such results. While on-treatment PFT was evaluated at approximately four weeks after the index procedure in the present analysis, previous studies reported PRU levels immediately or shortly after the index procedure24,25,26,27. Response to clopidogrel varied significantly over time, being higher at baseline than at one month after PCI28. Although the optimal timing of PRU assessment remains controversial, in our opinion it is rational to allow sufficient time before measuring on-treatment platelet reactivity.
Limitations
This study has several limitations. First, the number of patients with HPR on clopidogrel and their rates of adverse events at 12 months were relatively small to have adequate power to confirm our findings. Second, PFTs were not available at all centres and were performed based on clinicians’ discretion. As a result, not all patients underwent a PFT; 35.6% of patients receiving clopidogrel were assessed with a PFT. There is no doubt that there might be a selection bias. Patients at high risk who might benefit from conventional DAPT might have been excluded. Additionally, the study protocol did not define the exact time for blood collection according to the last clopidogrel administration. Furthermore, although CYP2C19 genotyping might be used as an optional tool for guiding antiplatelet therapy12,29, it was not available for this study. Third, the attending physicians selected the type of P2Y12 inhibitor. Ticagrelor or prasugrel might have been prescribed instead of clopidogrel in patients whose clopidogrel monotherapy might be inadequate to prevent adverse events. Although the selection of P2Y12 inhibitors was made at the time of randomisation and before measuring on-treatment PRU on clopidogrel, there might potentially be selection bias in this analysis. Fourth, although the SMART-CHOICE trial was a randomised study, this was a post hoc study. Randomisation was not stratified by on-treatment platelet reactivity on clopidogrel. Although baseline characteristics were mostly well balanced between the groups, unmeasured factors might have affected study outcomes. Fifth, the cut-off value of HPR remains controversial and the previous expert consensus document has recommended PRU >20812,25,26. However, it should be noted that this cut-off value was based on a Western population study30. For East Asians, previous studies have reported that the cut-off value was to be higher than for Westerners. Additionally, when we analysed patients with a cut-off for HPR of more than 208, results consistently showed no significant difference in the treatment effect of clopidogrel monotherapy regardless of HPR. Sixth, information on the use of aspirin or a P2Y12 inhibitor was assessed at each follow-up. In the SMART-CHOICE trial, the overall adherence to the study protocol was 79.3% in the P2Y12 inhibitor monotherapy group and 95.2% in the DAPT group. In the main paper, intention-to-treat and per-protocol analyses showed similar conclusions, suggesting that potential biases caused by differential adherence and treatment crossover are likely to be small. However, in the present study, it was hard to analyse exact drug adherence. Thus, these results were not free from non-adherence issues.
Conclusions
Although P2Y12 inhibitor monotherapy after short DAPT has emerged as a novel promising antiplatelet strategy after PCI, HPR on clopidogrel is one of the major concerns with clopidogrel monotherapy. Our results indicated that clopidogrel monotherapy and clopidogrel plus aspirin showed comparable treatment effects for MACCE among patients with or without HPR. However, HPR on clopidogrel was significantly associated with an increased risk of MACCE in clopidogrel-treated patients regardless of maintenance of aspirin. A potent P2Y12 inhibitor rather than prolonged clopidogrel-based DAPT can be considered for patients with HPR on clopidogrel. To validate this escalating strategy of P2Y12 inhibitors according to the on-treatment PRU, large-scale and long-term clinical trials are needed.
|
Impact on daily practice This substudy of SMART-CHOICE tested the clinical impact of high platelet reactivity (HPR) on clopidogrel of those who were treated with clopidogrel-based antiplatelet therapy after PCI. Clopidogrel monotherapy and clopidogrel plus aspirin showed comparable treatment effects on major adverse cardiovascular and cerebrovascular events (MACCE) among patients with or without HPR. However, HPR was significantly associated with an increased risk of ischaemic events. Dual antiplatelet therapy (DAPT) had no additional benefit in reducing ischaemic events. Potent P2Y12 inhibitor monotherapy rather than prolonged clopidogrel-based DAPT might be a rational antiplatelet strategy in patients with HPR on clopidogrel. However, this strategy requires confirmation in a large clinical trial. |
Acknowledgements
The authors would like to acknowledge the SMART-CHOICE Investigators: Hyeon-Cheol Gwon (principal investigator), Joo-Yong Hahn, Young Bin Song, Taek Kyu Park, Joo Myung Lee, Jeong Hoon Yang, Jin-Ho Choi, Sang Hoon Lee (Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Republic of Korea); Dong-Bin Kim (Catholic University St. Paul’s Hospital, Seoul, Republic of Korea); Byung Ryul Cho (Kangwon National University Hospital, Chuncheon, Republic of Korea); Woong Gil Choi (Konkuk University Chungju Hospital, Chungju, Republic of Korea); Hyuck Jun Yoon (Keimyung University Dongsan Medical Center, Daegu, Republic of Korea); Seung-Woon Rha (Republic of Korea University Guro Hospital, Seoul, Republic of Korea); Deok-Kyu Cho (Myongji Hospital, Goyang, Republic of Korea); Seung Uk Lee (Kwangju Christian Hospital, Gwangju, Republic of Korea); Sang Cheol Cho, Sun-Ho Hwang (Gwangju Veterans Hospital, Gwangju, Republic of Korea); DongWoon Jeon (National Health Insurance Service Ilsan Hospital, Goyang, Republic of Korea); JaeWoong Choi (Eulji General Hospital, Seoul, Republic of Korea); Jae Kean Ryu (Daegu Catholic University Medical Center, Daegu, Republic of Korea); Eul-Soon Im (Dongsuwon General Hospital, Suwon, Republic of Korea); Moo-Hyun Kim (Dong-A University Hospital, Busan, Republic of Korea); In-Ho Chae (Seoul National University Bundang Hospital, Seongnam, Republic of Korea); Ju-Hyeon Oh, Woo Jung Chun, Yong Hwan Park, Woo Jin Jang (Samsung Changwon Hospital, Sungkyunkwan University School of Medicine, Changwon, Republic of Korea); Sang-Hyun Kim, Hack-Lyoung Kim (Seoul National University Boramae Medical Center, Seoul, Republic of Korea); Jang Hyun Cho (St Carollo Hospital, Suncheon, Republic of Korea); Dong Kyu Jin (Soonchunhyang University Cheonan Hospital, Cheonan, Republic of Korea); Il Woo Suh (SAM Medical Center, Anyang, Republic of Korea); Jong Seon Park (Yeungnam University Hospital, Daegu, Republic of Korea); Eun-Seok Shin, Shin-Jae Kim (Ulsan University Hospital, Ulsan, Republic of Korea); Sang-Sig Cheong (Gangneung Asan Hospital, Gangneung, Republic of Korea); Seok Kyu Oh, Kyeong Ho Yun (Wonkwang University Hospital, Iksan, Republic of Korea); Sung Yun Lee (Inje University Ilsan Paik Hospital, Goyang, Republic of Korea); Jong-Young Lee (Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Republic of Korea); Jei Keon Chae (Chonbuk National University Hospital, Jeonju, Republic of Korea); Young Youp Koh (Chosun University Hospital, Gwangju, Republic of Korea);Wang Soo Lee (Chung-Ang University Hospital, Seoul, Republic of Korea); Yong Mo Yang (Cheongju Saint Mary's Hospital, Cheongju, Republic of Korea); Jin-Ok Jeong (Chungnam National University Hospital, Daejeon, Republic of Korea); Jang-Whan Bae (Chungbuk National University Hospital, Cheongju, Republic of Korea); and Joon-Hyouk Choi (Jeju National University Hospital, Jeju, Republic of Korea).
Funding
This study was supported by unrestricted grants from the Korean Society of Interventional Cardiology (grant number 2013-3), Abbott Vascular, Biotronik, and Boston Scientific.
Conflict of Interest statement
J.Y. Hahn has received grants from Abbott Vascular, Biotronik, Boston Scientific, Daiichi Sankyo, and Medtronic, and speaker’s fees from AstraZeneca, Daiichi Sankyo, and Sanofi-Aventis. H.C. Gwon has received research grants from Abbott Vascular, Boston Scientific, and Medtronic, and speaker’s fees from Abbott Vascular, Boston Scientific, and Medtronic. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

