Introduction
Complex percutaneous coronary intervention (PCI) patients have a greater risk of periprocedural complications, which could possibly be associated with poor short- and long-term prognoses.
Cangrelor, an intravenous P2Y12 receptor inhibitor with rapid onset and offset of platelet inhibition, reduces the risk of thrombotic complications compared with clopidogrel in patients undergoing elective or emergent PCI. Pharmacodynamic (PD) investigations have been conducted in various clinical settings, with various assays of platelet reactivity assessment and with some contrasting data regarding the degree of platelet inhibition and rates of high residual platelet reactivity (HRPR), particularly in patients with ST-segment elevation myocardial infarction (STEMI)12. Cangrelor use is currently approved in patients naïve to oral P2Y12 inhibitors with chronic (CCS) or acute coronary syndrome (ACS) undergoing PCI with a European guidelines class IIb recommendation. Yet, the process of switching from cangrelor to an oral P2Y12 inhibitor remains a topic of ongoing investigation (ClinicalTrials.gov: NCT04634162 and NCT04668144)3.
Methods
The PharmacOdynaMic effects of cangrelor in PatiEnts wIth acute or chronIc coronary syndrome undergoing percutaneous coronary intervention (POMPEII) registry (ClincalTrials.gov: NCT04790032) is a prospective study conducted at Federico II University of Naples enrolling all patients undergoing PCI with cangrelor.
PD assessments were performed with 3 assays: 1) the gold standard for platelet function assessment, light transmission aggregometry (LTA; following 20 and 5 μM adenosine diphosphate [ADP] stimuli); 2) the VerifyNow P2Y12 test; and 3) the multiplate electrode aggregometry (MEA) ADP-test.
Among 70 patients enrolled from March 2021 to May 2022, 41 underwent elective complex PCI4. All patients were P2Y12 inhibitor-naïve and received aspirin, unfractionated heparin, and cangrelor (30 μg/kg bolus followed by 4 μg/kg/min infusion for 2 hours) prior to the start of PCI per the standard of care. Blood samples for PD assessments were collected at baseline (before cangrelor bolus administration) and at 30 minutes, 3 hours (1 hr after cangrelor infusion cessation) and 4-6 hours (2-4 hrs after cangrelor infusion cessation) after cangrelor initiation. All PD tests were performed within 30 minutes from blood collection by experienced laboratory personnel. HRPR standard definitions were used15.
Clinical events were adjudicated by an independent clinical events committee composed of two cardiologists not involved in the patients’ recruitment or management.
The study complied with the Declaration of Helsinki, was approved by the internal ethics committee, and all patients gave their written informed consent.
Results
The mean age of the study population was 69.3±8.8 years, and 24% were female. The transition from cangrelor to oral P2Y12 inhibitors was left to the operator’s discretion: 36 patients received 600 mg clopidogrel at 2 hrs (upon cangrelor infusion cessation), 1 received 60 mg prasugrel at 2 hrs (upon cangrelor infusion cessation) and 4 received 180 mg ticagrelor at the end of PCI (a mean of 68 minutes after cangrelor bolus). The mean duration of PCI was 99.4±40.7 minutes.
Thirty minutes after the start of the cangrelor initiation, all patients showed low P2Y12 reactivity with a mean inhibition of platelet aggregation (IPA) of 55.7±18.0% as measured by LTA with 20 μM ADP (34% with IPA ≤50%, 19.5% with IPA ≥70%, 4.9% with IPA ≥80% and none with IPA ≥90%) (Figure 1A, Figure 1B). HRPR occurred in 1 case (2.4%), as shown by LTA (following both 20 and 5 μM ADP stimuli), but not confirmed by VerifyNow or MEA (Figure 1B-Figure 1E); however, this patient also showed HRPR at 3 hrs and 4-6 hrs after clopidogrel administration with all assays. Only 1 (2.4%) bailout case with the use of the glycoprotein IIb/IIIa inhibitor (GPI) tirofiban was needed due to an intraprocedural thrombotic complication; however, this patient showed an appropriate response to cangrelor. HRPR occurred in 55% at 3 hrs and 22.5% at 4-6 hrs as measured by LTA with 20 μM ADP and was consistent with the other assays (Figure 1E). All patients with HRPR after cangrelor cessation had received clopidogrel as the switching drug, while no HRPR occurred in the ticagrelor-switched patients (Figure 1F).
No deaths, no ischaemic events and 6 (15%) minor bleedings occurred within 48 hrs.
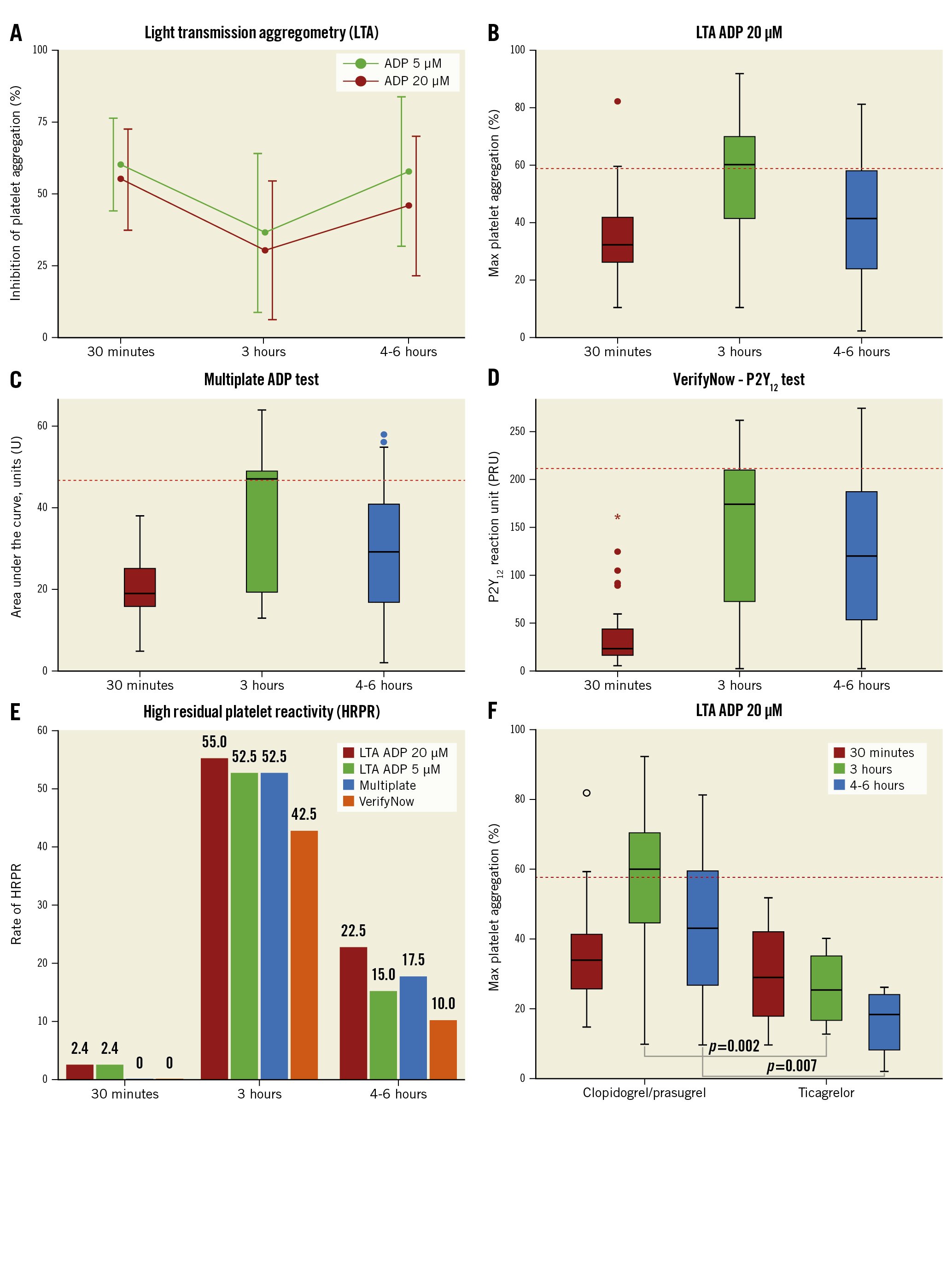
Figure 1. Pharmacodynamic data at 30 minutes (during cangrelor infusion), 3 hours (1 hr after cangrelor infusion cessation) and 4-6 hours (2-4 hrs after cangrelor infusion cessation) from cangrelor administration. A) Inhibition of platelet aggregation (IPA, %) as measured by LTA with 20 μM and 5 μM ADP stimuli. B) Box plot of maximal platelet aggregation as measured by LTA with 20 μM ADP stimulation. C) Box plot of units of area under the curve (AUC) as measured by the MEA Multiplate ADP test. D) Box plot of P2Y12 reaction unit (PRU) as measured by the VerifyNow P2Y12 test. E) Rates of HRPR at 30 minutes, 3 hours and 4-6 hours with all tests defined as: 20 μM ADP-induced maximal aggregation >59%, 5 μM ADP-induced maximal aggregation >46%, AUC >46U, and PRU >208, respectively. F) Box plot of maximal platelet aggregation as measured by LTA with 20 μM ADP stimulation stratified by switch with ticagrelor. Red dashed lines in panels B-D and F represent the cut-off for HRPR definition. ADP: adenosine diphosphate; LTA: light transmission aggregometry; MEA: multiplate electrode aggregometry; U: units
Discussion
The main findings are as follows:
- Cangrelor was shown to be effective and safe in patients undergoing complex PCI at high thrombotic risk.
- The switch from cangrelor to clopidogrel could expose patients to a variable period of on-treatment HRPR with inadequate platelet inhibition, while the use of ticagrelor soon after starting the cangrelor infusion might be ideal to cover the gap. This might be of relevance in such a delicate clinical setting. To date, there are differences between the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) recommendations on transitioning from cangrelor to ticagrelor, with the first recommending ticagrelor be given together with the start of the cangrelor infusion, as was tested in the CANTIC Study2, and our data, although limited in numbers, support such a strategy.
Limitations
This is a small observational study underpowered for clinical events. The clinical implications of these findings warrant investigation in an adequately powered clinical trial.
Conclusions
Pharmacodynamic data show that cangrelor might be a useful option for a fast platelet inhibition during complex PCI, but caution should be paid to a potential rebound effect at the end of its infusion while the oral P2Y12 effect is not yet achieved.
Funding
This study was internally supported by the Azienda Ospedaliera Universitaria Federico II. No direct or indirect external funding was received.
Conflict of interest statement
G. Gargiulo reports personal fees from Daiichi Sankyo, outside the submitted work. M. Valgimigli received an institutional grant from Terumo and consulting fees from AstraZeneca, Terumo, Alvimedica/CID, Abbott Vascular, Daiichi Sankyo, Bayer, CoreFlow, Idorsia Pharmaceuticals, Vifor, Bristol-Myers Squibb, Biotronik, Boston Scientific, Medtronic, Vesalio, Novartis, Chiesi, PhaseBio, and ECRI, outside the submitted work. The other authors have no conflicts of interest to declare

