
Abstract
Aims: The HEAT-PPCI trial compared bivalirudin and unfractionated heparin in patients undergoing primary percutaneous coronary intervention (PPCI). The aim of this study was to report pre-specified, secondary analyses comparing the effects of P2Y12 inhibiting agents on platelet reactivity and clinical events.
Methods and results: All patients received preprocedural oral antiplatelet therapy. During the early stages of the trial, the P2Y12 inhibitor of choice was prasugrel with some use of clopidogrel. Later, routine therapy switched to ticagrelor. For cases performed during working hours, multiple electrode aggregometry (MEA) was used to assess ADP-induced platelet aggregation at the end of the index procedure. The effect of P2Y12 inhibitors on the primary efficacy (major adverse cardiac events [MACE]) and safety (major bleeding) outcomes was assessed in all patients. Multiple logistic regression was used to adjust for differences in baseline characteristics. With MEA data from 469 patients, prasugrel therapy resulted in significantly greater suppression of ADP-induced platelet aggregation at 40 U (23, 78) (median; interquartile range [IQR]) when compared against ticagrelor 75 U (41, 100.75); p<0.001 or clopidogrel 79 U (56, 96); p<0.001. In the entire study population (N=1,803), prasugrel therapy was associated with significantly fewer MACE (26/497; 5.2%) in comparison to ticagrelor (83/1,123; 7.4%) or clopidogrel (18/183; 9.8%); odds ratio (OR) 0.64, confidence interval (CI): 0.41-0.99, p=0.045. For major bleeding, there were no significant differences among the three groups - clopidogrel (3/183; 1.6%), prasugrel (13/497; 2.6%) and ticagrelor (43/1,123; 3.8%); OR 0.73, CI: 0.39-1.35, p=0.31. Patients treated with clopidogrel had more high-risk features and clopidogrel use was more common as an alternative to prasugrel. After adjustment, there were no significant differences in the rates of MACE (OR 0.70, CI: 0.41-1.21, p=0.20) or major bleeding (OR 0.80, CI: 0.41-1.60, p=0.53).
Conclusions: In HEAT-PPCI, patients who received prasugrel (rather than clopidogrel or ticagrelor) had significantly greater suppression of ADP-induced platelet aggregation at the end of the procedure. After adjustment for differences in baseline characteristics, there were no significant differences in ischaemic or bleeding outcomes among the antiplatelet therapies.
Abbreviations
ADP: adenosine diphosphate
AST: acute stent thrombosis
CI: confidence interval
CVA: cerebrovascular accident
CVD: cardiovascular disease
DAPT: dual antiplatelet therapy
EOP: end of procedure
GP: glycoprotein
HPR: high platelet reactivity
IQR: interquartile range
LD: loading dose
MACE: major adverse cardiac events
MEA: multiple electrode aggregometry
MI: myocardial infarction
MLR: multiple logistic regression
OR: odds ratio
PCI: percutaneous coronary intervention
PFT: platelet function testing
PPCI: primary percutaneous coronary intervention
RCT: randomised controlled trial
ST: stent thrombosis
STEMI: ST-segment elevation myocardial infarction
TLR: target lesion revascularisation
UFH: unfractionated heparin
Introduction
In the management of ST-elevation myocardial infarction (STEMI), current guidelines recommend administration of dual antiplatelet therapy as early as possible after diagnosis1,2. Newer agents, ticagrelor and prasugrel, have been shown to have a faster, more potent and more consistent antiplatelet action in comparison to clopidogrel. In clinical trials recruiting mixed acute coronary syndrome populations, the newer agents have demonstrated superior efficacy to clopidogrel and are now the preferred agents3-5, although advantage in primary percutaneous coronary intervention (PPCI) subgroups is more difficult to establish.
Studies have shown that the therapeutic response to P2Y12 inhibitors may be slower in STEMI patients than in other populations6-9. Specifically, in a substantial proportion of patients, even the newer P2Y12 inhibitors may fail to achieve adequate suppression of adenosine diphosphate (ADP)-induced platelet aggregation at two hours following a loading dose (LD)10,11. Previous randomised trials comparing measures of platelet inhibition with prasugrel and ticagrelor in a STEMI population have shown comparable results10-12.
The HEAT-PPCI (How Effective are Antithrombotic Therapies in Primary Percutaneous Coronary Intervention Study) trial compared unfractionated heparin (UFH) and bivalirudin in the setting of PPCI13. The aim of this study was to report pre-specified, secondary analyses from the HEAT-PPCI trial comparing the effects of P2Y12 inhibitors on platelet reactivity and clinical outcomes.
Methods
STUDY POPULATION AND DESIGN
The design and results of HEAT-PPCI have been described previously13. In brief, HEAT-PPCI was a single-centre, open-label, randomised controlled trial comparing UFH and bivalirudin in a PPCI population. The study recruited 100% of all eligible patients presenting to the host institution during the recruitment period, thus creating a true “real-world” population. Exclusion criteria were kept to the minimum and comprised the following: age ≤18 years; known intolerance, hypersensitivity or contraindication to any trial medication; active bleeding at presentation; artificial ventilation, reduced conscious level or other factors precluding the administration of oral antiplatelet therapy; physician refusal to administer antiplatelet loading (uncertain diagnosis/risk of bleeding), and previous enrolment in this trial.
This substudy reports results from two distinct denominator groups: a) clinical outcomes were assessed on all patients recruited in the HEAT-PPCI trial; b) tests to assess suppression of ADP-induced platelet aggregation were performed on patients recruited during the normal working hours of the research laboratory for sample analysis (09h00-17h00, Monday-Friday). We were unable to perform these analyses outside of normal working hours for logistic reasons due to unavailability of staff and equipment.
The primary efficacy outcome compares the rate of major adverse cardiac events (MACE) at 28 days. This is a composite of all-cause mortality, cerebrovascular accident (CVA), re-infarction or additional unplanned target lesion revascularisation (TLR). The primary safety outcome was the rate of major bleeding at 28 days, classified as type 3-5 according to the Bleeding Academic Research Consortium (BARC) definition.
STUDY MEDICATION
All patients received preprocedural dual antiplatelet therapy with aspirin and a P2Y12 inhibitor. During the early stages of the trial, the P2Y12 inhibitor of choice was prasugrel. Use of clopidogrel was also allowed depending on operator preference but was generally reserved for patients >75 years of age, having a body weight <60 kg or a previous history of CVA. The clinical guidelines at the host institution changed during the course of the trial and ticagrelor became the default P2Y12 inhibitor. The recommended loading dose (LD) was 60 mg for prasugrel, 600 mg for clopidogrel and 180 mg for ticagrelor. For inter-hospital transfers, the antiplatelet LD was administered at the referring hospital prior to transfer. For direct ambulance admissions, the P2Y12 inhibitor loading was performed on arrival at the host institution.
PLATELET FUNCTION TESTING (PFT)
Multiple electrode aggregometry (MEA) was performed using the multiplate analyser (Roche Diagnostics, Mannheim, Germany). This is a whole blood assay based on measures of electrical impedance. ADP-induced platelet aggregation is initiated by the addition of ADP (6.5 µM) to the test cells. The change in impedance resulting from the adhesion and aggregation of platelets on the surface of two silver-coated highly conductive copper electrodes is analysed for each sensor unit for six minutes14. These values are transformed to units and plotted against time. From this, one can measure the area under the curve expressed as arbitrary aggregation units (U). All materials including reagents were obtained from the manufacturer (Roche Diagnostics).
Whole blood for platelet function testing was obtained via the arterial sheath in the cardiac catheterisation lab at the end of the index procedure (EOP). In patients assigned to receive bivalirudin, the infusion was still running at the time of sampling. The first 10 ml were discarded and then blood was drawn into a 3 ml blood collection tube. The tubes were filled to capacity and inverted gently a few times to ensure proper mixing with the anticoagulant in situ.
We also pre-specified the assessment for the presence of high platelet reactivity (HPR) to ADP. This was defined as MEA ADP test >46 U based on previous studies and the latest consensus document of the Working Group for On-Treatment Platelet Reactivity15.
STATISTICAL ANALYSIS
Categorical variables were presented as numerator/denominator (percentage). Comparisons were made using chi-squared or Fisher’s exact tests as appropriate. Non-normally distributed continuous data (normality assessed by means of the Kolmogorov-Smirnov test) were presented as median with interquartile ranges (IQR) and were compared with a two-sided Wilcoxon test and a Wilcoxon matched-pairs signed-rank test as appropriate.
Multivariable logistic regression was used to adjust for differences in baseline characteristics. The number of covariates offered to the model was necessarily limited by the number of outcomes16. Model covariates were therefore selected in three stages: firstly, variables with significant differences at a univariate level; secondly, variables that were deemed clinically significant by the research clinicians; thirdly, variables that emerged as significant predictors of the outcomes when entered into a multivariate model. The following baseline parameters were offered to the model: age, gender, weight, antithrombotic therapy, diabetes, cardiogenic shock, hypertension, hyperlipidaemia, smoking status, previous myocardial infarction (MI), P2Y12 inhibitor treatment, glycoprotein (GP) IIb/IIIa inhibitor use, femoral arterial access, mildly impaired ejection fraction (EF), moderately impaired EF and severely impaired EF. Consequently, MACE was adjusted for left ventricular ejection fraction (LVEF), diabetes, female sex, and antithrombotic therapy. Bleeding outcomes were adjusted for cardiogenic shock, femoral access, and LVEF.
To generate odds ratios (OR) specific to each of the three P2Y12 agents and to consider potential differences in case mix for these three groups, crude and case mix adjusted logistic regression models were produced for MACE and bleeding outcomes.
In all cases a p-value ≤0.05 was considered statistically significant. All statistical analyses were performed with SAS software, version 9.3 (SAS Institute, Cary, NC, USA).
ETHICAL AND REGULATORY APPROVALS
The main study protocol including the platelet substudy was approved by the National Research Ethics Service (NRES, North West) and the UK Medicines and Healthcare Products Regulatory Agency. The main trial was registered at www.clinicaltrials.gov (NCT01519518). The study was partially funded by unrestricted grants from The Medicines Company and AstraZeneca, but these companies had no involvement in any aspect of trial design, conduct or reporting. The first author and the last author (who is also the principal investigator for the main study) accept full responsibility for the accuracy and completeness of the data and all analyses.
Results
CLINICAL OUTCOMES
Of the 1,812 patients recruited in HEAT-PPCI, 1,803 received a loading dose of P2Y12 inhibitors (clopidogrel 183, prasugrel 497 and ticagrelor 1,123) (Figure 1). Follow-up at 28 days was complete in 100% of patients. Baseline characteristics of the population in the three groups are shown in Supplementary Table 1. Patients who received clopidogrel had a significantly less favourable risk profile especially in terms of older age, lower body weight, history of diabetes, previous MI and previous PCI. As can be seen in Figure 2, use of clopidogrel was more prevalent when prasugrel was the alternative agent.
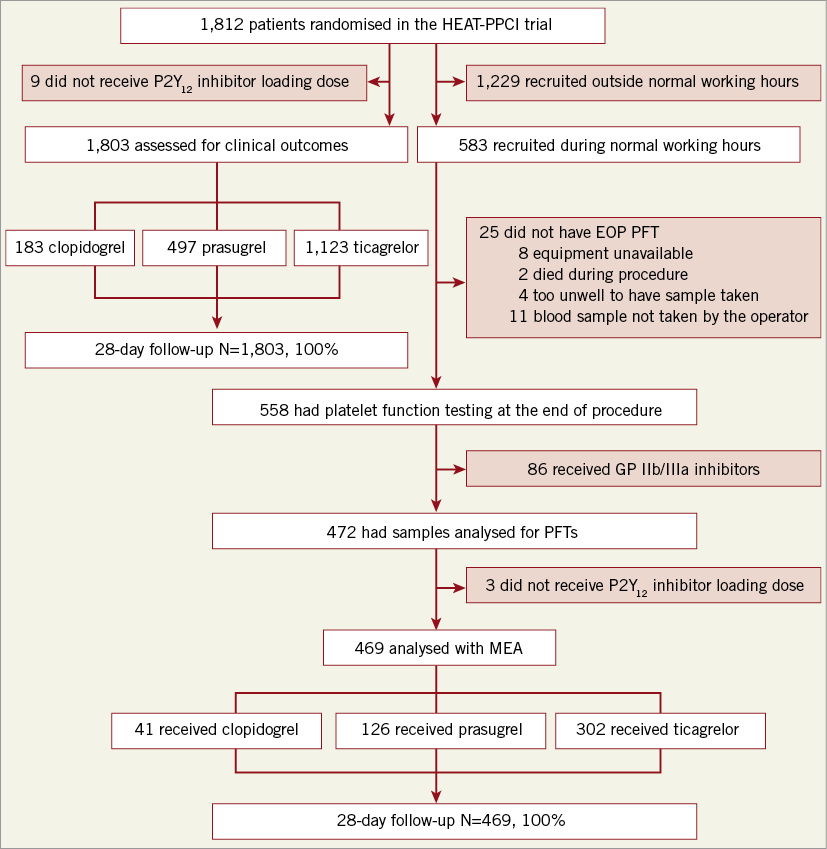
Figure 1. Substudy flow chart.
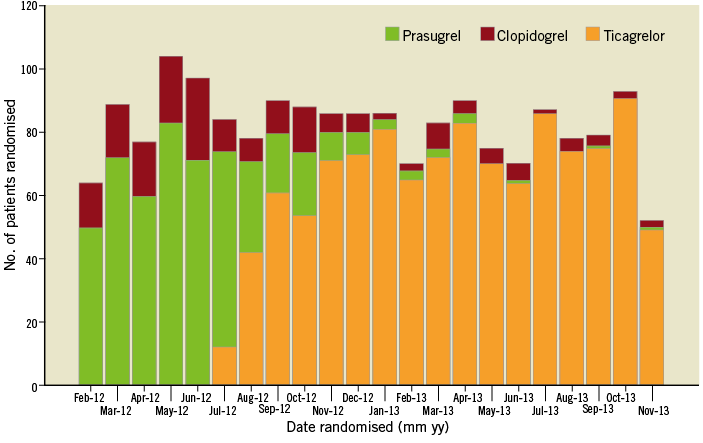
Figure 2. Evolution of P2Y12 inhibitor use over time.
Median duration from the time of administration of P2Y12 inhibitor LD to EOP was 65 min (IQR 42-98). This was significantly higher for clopidogrel (95 min; IQR 60-120) in comparison to prasugrel (70 min; IQR 45-98) or ticagrelor (60 min; IQR 40-92); p=0.001. The median duration from the time the sample was obtained to the analysis was 50 min (IQR 20-70).
Table 1 shows clinical outcomes in the entire study population. Significantly fewer patients had MACE with prasugrel (26/497; 5.2%) in comparison to ticagrelor (83/123; 7.4%) or clopidogrel (18/183; 9.8%); OR 0.64 (confidence interval [CI]: 0.41, 0.99), p-value 0.045. For major bleeding, there were no significant differences among the three groups – clopidogrel (3/183; 1.6%), prasugrel (13/497; 2.6%), and ticagrelor (43/1,123; 3.8%); OR 0.73, CI: 0.39-1.35, p=0.31. After adjustment of the baseline characteristics using multiple logistic regression (MLR), there were no significant differences in the number of patients who had either MACE (OR 0.70, CI: 0.41-1.21, p=0.20) or major bleeding (OR 0.80, CI: 0.41-1.60, p=0.53) (Table 1). Figure 3 illustrates the differences among the three groups for MACE and bleeding outcomes before and after the adjustment.
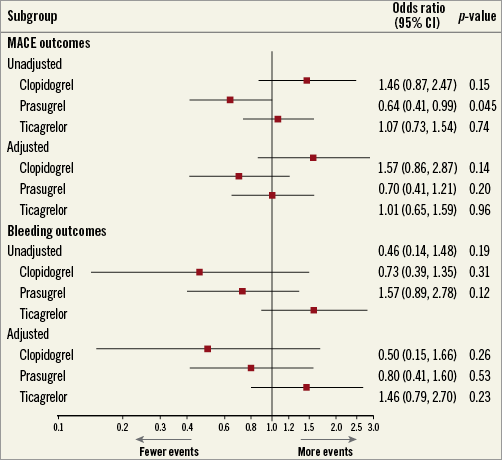
Figure 3. Odds ratio plot for clinical outcomes.
ADP-INDUCED PLATELET AGGREGATION
Figure 1 outlines the flow of patients included in the platelet function substudy. Of the 1,812 patients randomised in the HEAT-PPCI trial, 583 (32.2%) were recruited during normal working hours. Patients who received GP IIb/IIIa inhibitors (15.4%) were excluded from the analysis. The baseline characteristics from the final cohort of 469 patients who underwent analysis are shown in Supplementary Table 2. The risk factor profiles appear more matched in this cohort. Figure 4 illustrates the results. Prasugrel therapy resulted in significantly greater suppression of ADP-induced platelet aggregation at 40 U (23, 78) when compared against ticagrelor 75 U (41, 100.75), p<0.001 or clopidogrel 79 U (56, 96), p<0.001. These results have not been corrected for the time differences from administration of LD to measurement of aggregation for each antiplatelet therapy.
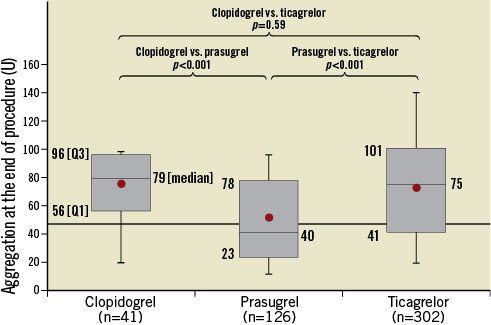
Figure 4. Comparison of suppression of ADP-induced platelet aggregation.
Assessing the HPR values, a significantly higher number of patients achieved adequate suppression of ADP-induced platelet aggregation of <46 U at the end of the procedure in the prasugrel group (70/126; 55.6%) in comparison to the clopidogrel group (7/41; 17.1%), p<0.001 or the ticagrelor group (84/302; 27.8%), p<0.001.
Discussion
The results show a significantly higher level of suppression of ADP-induced aggregation with prasugrel in the acute phase compared to ticagrelor or clopidogrel. Previous trials have suggested that both ticagrelor and prasugrel induce higher levels of platelet inhibition as compared to clopidogrel and decrease the proportion of patients with HPR7,17,18. Comparing prasugrel and ticagrelor against each other in a STEMI setting, the RAPID 1 study showed that a 60 mg prasugrel LD was non-inferior to a 180 mg ticagrelor LD in reducing the primary endpoint of the study of HPR two hours after drug administration10. In the RAPID 2 study, there was no significant difference in platelet inhibition one hour after administration between an increased oral ticagrelor LD of 360 mg when compared with the standard prasugrel LD12. Alexopoulos et al found no differences between the two agents in STEMI: both drugs exhibited an initial delay in the onset of their antiplatelet action11. After six to 12 hours, ticagrelor has been shown to achieve significantly better suppression of ADP-induced aggregation when compared to prasugrel19,20.
Despite the observed higher degree of suppression of ADP-induced aggregation in the prasugrel group compared to clopidogrel or ticagrelor, after adjustment for baseline characteristics by MLR, this did not translate into significantly different rates of MACE or bleeding events. The recently published randomised controlled trial, PRAGUE-18, comparing prasugrel and ticagrelor in a STEMI population was prematurely terminated for futility21. No significant differences were found for primary or key secondary endpoints at 30 days. The post hoc analyses of the PLATO and TRITON studies looking specifically at STEMI populations provide similar indications22,23. In PLATO-STEMI subgroup analysis, the primary endpoint (MI, stroke, or cardiovascular death) with ticagrelor was 9.4% at 12 months23. In TRITON-STEMI, the primary endpoint (cardiovascular death, non-fatal MI, or non-fatal stroke) with prasugrel was achieved in 10.0% patients at 15 months22. However, it is not possible to compare the two cohorts directly due to differences in study design and patient populations.
Due to the emergency nature of the STEMI setting, effective inhibition of ADP-induced platelet aggregation remains a concern as there is insufficient time in the initial phase for antiplatelet agents to work6-9. This overall inadequate level of ADP suppression achieved by antiplatelet agents in the acute phase could explain why prasugrel, even with a relatively higher rate of ADP suppression, failed to impact on the clinical outcomes in the adjusted population. Previously, it has been observed that even the newer agents require at least four hours to reach an optimal biological efficacy following the LD in STEMI patients10,11. With the apparently most efficacious agent prasugrel, only just over half of the patients exhibited adequate ADP suppression at the end of the procedure. The predefined definitions of “adequate” response of antiplatelet agents to ADP-induced aggregation are not biologically relevant15. This could also account for the failure of these agents to affect clinical outcomes in the acute phase when being compared against each other.
Our study demonstrated no significant differences for bleeding outcomes when comparing prasugrel and ticagrelor. The PRAGUE-18 study21 and recent data from 12 European registries24 have shown similar findings.
Limitations
This study has several limitations. Data are observational and not randomised. Hence, we should exercise caution in interpretation of the results. The outcomes observed in the entire population cannot be translated to a substudy of patients undergoing platelet function testing without introduction of some bias. There was a significant mismatch between the baseline characteristics of the three groups. Figure 2 shows the trend of administration of P2Y12 inhibitors over the course of the trial. During the initial phase of the trial, prasugrel was the antiplatelet agent of choice. Administration of clopidogrel was at the operator’s discretion at some peripheral centres but was mainly given to patients older than 75 years, with a body weight <60 kg or a previous history of CVA. This diverted a higher risk population towards receiving clopidogrel rather than prasugrel. Some use of clopidogrel continued in the later phase when ticagrelor became the predominant P2Y12 inhibiting agent. The reasons include guideline recommendation at one peripheral hospital and occasionally operator discretion for patients who were already on long-term clopidogrel therapy or were presumed to have a higher bleeding risk.
There is also a significant mismatch between the median duration from time of P2Y12 inhibitor administration to EOP. The above-mentioned peripheral hospital is located farthest from the host institution which may explain the longer duration of LD administration to EOP for the clopidogrel group.
When comparing against prasugrel, the ticagrelor cohort had shorter duration between drug administration to the time a sample was taken for PFT. This could have influenced the level of platelet inhibition achieved and should be considered when evaluating the prasugrel advantage in this regard. Another limitation of the study is the lack of data regarding administration of morphine. Morphine administration has been shown to influence absorption of antiplatelet agents and, consequently, the level of platelet inhibition achieved10,12. The shorter duration of symptom onset to randomisation time for the ticagrelor cohort could have affected the platelet reactivity levels following morphine administration for symptom relief. An MLR model was used to adjust for the differences in baseline characteristics. Statistical significance for MACE was lost after adjustment for baseline risk profile. Another limitation is the lack of correction of the ADP-induced platelet aggregation results for the time differences from administration of LD to measurement of aggregation for each antiplatelet therapy.
Future considerations include an adequately powered randomised controlled trial comparing prasugrel and ticagrelor in a STEMI setting comparing clinical outcomes and assessing platelet function. The ISAR-REACT 5 trial is currently recruiting patients in this regard25.
Conclusions
In the HEAT-PPCI antiplatelet therapy observational substudy, patients who received prasugrel (rather than clopidogrel or ticagrelor) had significantly greater suppression of ADP-induced platelet aggregation at the end of the procedure. However, this did not translate into reduction in ischaemic events in comparison to ticagrelor or clopidogrel after adjusting the population for baseline characteristics. For bleeding outcomes, there were no significant differences among the antiplatelet therapies.
| Impact on daily practice The results of our study add to the available evidence supporting the use of oral antiplatelet agents in the PPCI setting. It gives an insight into the comparison between prasugrel and ticagrelor in terms of ADP-induced platelet aggregation and clinical outcomes. |
Funding
The main study was funded by Liverpool Heart and Chest Hospital, UK National Institute of Health Research, The Medicines Company, AstraZeneca, and The Bentley Drivers Club (United Kingdom).
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
Supplementary Table 1. Baseline characteristics.
Supplementary Table 2. Baseline characteristics for the platelet substudy cohort.
To read the full content of this article, please download the PDF.

