
Abstract
Aims: In patients with acute coronary syndromes (ACS) undergoing a percutaneous coronary intervention (PCI), switching of oral P2Y12 receptor inhibitors may frequently occur. We aimed to assess the current incidence of switching of oral P2Y12 receptor inhibitors and its safety in consecutive ACS patients undergoing PCI over a three-month period.
Methods and results: The SCOPE registry was a multicentre, observational, prospective study. A total of 1,363 consecutive patients were enrolled in 39 PCI centres across Italy. Switching of oral antiplatelet therapies occurred in 2.3% in the cathlab, 3.3% at discharge and 5.1% at follow-up. The cumulative incidence of major adverse cerebrovascular events (MACE) and net adverse cerebrovascular events (NACE: a combination of MACE and bleeding events) was 1.6% and 5.6%, respectively. Among patients receiving an upgrade switching (change from old to novel P2Y12 receptor inhibitors), no ischaemic or bleeding events occurred during the whole study period. On the other hand, downgrade switching (from novel to old P2Y12 receptor inhibitors) was an independent predictor of NACE (OR 5.3; CI: 2.1-18.2; p=0.04).
Conclusions: Switching of oral antiplatelet therapies is not uncommon among ACS patients undergoing PCI. Notably, switching from clopidogrel to novel P2Y12 receptor inhibitors appears safe, while a downgrade switching in early phases of ACS is associated with adverse clinical events.
Abbreviations
ACS: acute coronary syndromes
BARC: Bleeding Academic Research Consortium
LD: loading dose
MACE: major adverse cerebrovascular events
MI: myocardial infarction
NACE: net adverse cerebrovascular events
NSTE-ACS: non-ST-elevation acute coronary syndromes
PCI: percutaneous coronary intervention
STEMI: ST-elevation myocardial infarction
TIA: transient ischaemic attack(s)
Introduction
Different oral antiplatelet therapies are indicated for the treatment of patients with acute coronary syndromes (ACS) undergoing percutaneous coronary intervention (PCI)1,2. In daily clinical practice, switching oral P2Y12 receptor inhibitors may occur in order to optimise platelet inhibition on a patient-by-patient basis, according to the subject’s clinical risk profile and the concomitant pharmacological and interventional strategies3,4. At the present time, outcome data on this common practice are limited and extrapolated from existing pivotal trials, meta-analyses or retrospective analyses3,4. Indeed, specifically designed studies evaluating the efficacy and safety with respect to clinical outcomes from switching of oral P2Y12 receptor inhibitors are not currently available.
Based on these assumptions, the Italian Society of Invasive Cardiology (SICI-GISE) designed the SCOPE (Switching from Clopidogrel to New Oral Antiplatelet Agents during PErcutaneous Coronary Intervention) registry in order to assess the current incidence of switching of oral P2Y12 receptor inhibitors and its safety in ACS patients undergoing PCI.
Methods
The SCOPE registry was a multicentre, observational, prospective study aimed at evaluating the incidence and short-term outcome of switching of oral P2Y12 receptor inhibitors in consecutive ACS patients undergoing PCI during a three-month period.
Consecutive patients ≥18 years old admitted with a diagnosis of ACS, either ST-elevation myocardial infarction (STEMI) or non-ST-elevation ACS (NSTE-ACS), and treated with a successful PCI were enrolled in this survey. The diagnosis of ACS was made if patients had cardiac ischaemia-related symptoms of ≥10 minutes duration and (a) concurrent biomarker evidence of ACS (elevated troponin [I or T] greater than the upper limit of normal at the study site, or [if troponin was not available] creatine kinase-MB > upper limit of normal) and/or (b) concurrent electrocardiographic changes (ST-segment elevation in ≥2 contiguous leads [≥0.2 mV in V1−V3, ≥0.1 mV in other leads], or ST-segment depression [≥0.05 mV] in ≥2 contiguous leads, or inverted T-waves, or new-onset left bundle branch block).
Patients with a diagnosis of ACS undergoing a surgical or conservative treatment during the index hospitalisation, STEMI patients treated with thrombolysis within 48 hours, those on chronic therapy with oral anticoagulation therapy or any P2Y12 receptor inhibitor or with known contraindications to oral antiplatelet agents, those with known pregnancy, breast-feeding, or intending to become pregnant during the study period (or other conditions that would contraindicate the use of some drugs) and those not giving informed consent were excluded from the study.
The SICI-GISE was the owner of the database and was responsible for the design and conduct of the registry. The SICI-GISE invited Italian hospitals with a medium to high PCI volume receiving ACS patients to participate. The SCOPE registry was funded by an unrestricted grant from Daiichi Sankyo Italy. The sponsor had no role in the design, conduct, data management, or statistical analysis of the registry, or in drafting the present paper. All patients were informed of the nature and aims of the study and asked to sign an informed consent for the management of their individual data. Local institutional review boards (IRB) approved the study.
DATA COLLECTION AND DATA QUALITY
Data on baseline characteristics, including demographics, risk factors and medical history, were collected. Information on coronary angiography and PCI, including different angiographic and procedural features, use of medications, and in-hospital major clinical events, were recorded on an electronic case report form (CRF). An emphasis was given to the collection of data regarding oral antiplatelet agents prescribed at the time of admission in the emergency department, during hospitalisation in cardiology wards, at discharge and at clinical follow-up.
Patterns of switching of antiplatelet agents included “upgrade” (change from old, namely clopidogrel or ticlopidine, to novel P2Y12 receptor inhibitors, namely prasugrel or ticagrelor), “downgrade” (change from novel to old P2Y12 receptor inhibitors), and simple “change” (change from prasugrel to ticagrelor and vice versa)4.
By using a validation plan, integrated in the data entry software, data were checked for missing or contradictory entries and values out of the normal range. At each site, the principal investigator was responsible for screening consecutive ACS patients undergoing PCI.
A blinded and independent central adjudication committee (CAC) of two expert physicians (not directly involved in the care of SCOPE study patients) adjudicated major bleeding, ischaemic events and all-cause deaths, when they occurred.
Cardiac death was defined as any death related to cardiac diagnosis or occurring because of a complication from a cardiac procedure. Any other death for which a clear non-cardiac cause was not identified was also considered as cardiac. Myocardial infarction (MI) was diagnosed in the presence of new ischaemic symptoms and an elevation or re-elevation (for subsequent ischaemic events ≥24 hours from the acute event) of biomarkers of myocardial necrosis (>20% above the prior documented level) with or without concurrent ECG changes. Stent thrombosis (ST) was defined according to the Academic Research Consortium definitions5. Stroke was identified as an acute neurologic deficit that lasted >24 hours and affected the ability to perform daily activities with or without confirmation by imaging techniques. Transient ischaemic attacks (TIA) were defined as episodes of temporary and focal dysfunction of vascular origin, commonly lasting from two to 15 min, but occasionally lasting as long as a day (24 hours). Bleeding events were classified according to the Bleeding Academic Research Consortium (BARC) criteria6.
Major adverse cerebrovascular events (MACE) were defined as the combination of death, MI, ST and stroke/TIA. Net adverse cerebrovascular events (NACE) were defined as the combination of MACE and any bleeding event.
FOLLOW-UP
A telephonic or clinical follow-up was planned one month after hospital discharge. Physicians were specifically requested to document whether any death, MI, ST, stroke/TIA, recurrent revascularisation or bleeding events had occurred. This was captured as a “yes/no” response for each event of interest. If any event occurred, the patient was asked to present the documentation of hospital admission for independent CAC adjudication. At follow-up, all switching of oral antiplatelet therapies was also assessed.
STATISTICAL ANALYSIS
Considering the explorative and observational nature of the current study, no formal sample size calculation was performed. However, the SCOPE registry was aiming for a sample of at least 1,000 patients from 40 high-volume PCI centres to allow a representative national cohort. Considering the number of patients undergoing oral antiplatelet switching in a recent snapshot performed in Italy7, it was estimated that approximately 1,000 patients would be included in three months of enrolment.
Categorical variables are reported as counts and percentages, whereas continuous variables are reported as means and standard deviations or interquartile range (IQR). Gaussian or non-Gaussian distribution was evaluated by the Kolmogorov-Smirnov test. The t-test was used to assess differences between parametric continuous variables, the Mann-Whitney U test for non-parametric variables, the chi-square test for categorical variables and Fisher’s exact test for 2×2 tables. Three different models of logistic regression were performed to appraise predictors of the cumulative switching (upgrade and downgrade) and in-hospital NACE. Variables included in the model were those that resulted in being significant at univariate analysis. Accuracy and discrimination of the model were evaluated with area under the curve (AUC) analysis and the Hosmer-Lemeshow test, respectively.
Missing data were handled in two ways. First, all individuals with missing data were excluded from the analyses. Second, missing binary data were coded as not present, while missing categorical data were coded as a “missing” category. The fit of these models was compared with those in which missing data were excluded. Results were not appreciably different in any case.
A two-sided p-value <0.05 was considered statistically significant. All analyses were performed with SPSS, Version 21.0 (IBM Corp, Armonk, NY, USA).
Results
Thirty-nine PCI centres across Italy participated in the study. Data were collected from February to April 2016. During the three-month study period, a total of 1,363 ACS patients naïve to P2Y12 receptor inhibitors and treated with a successful PCI were enrolled: 331 (24.3%) had an initial diagnosis of STEMI and 1,032 (75.7%) of NSTE-ACS.
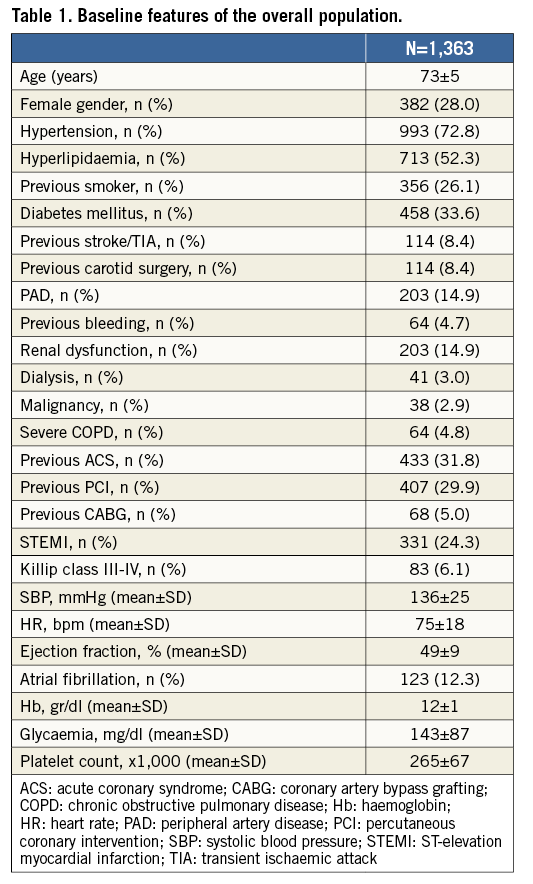
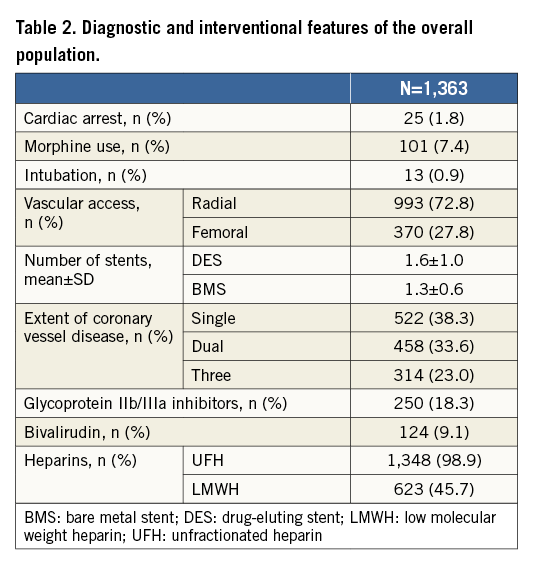
Baseline clinical characteristics and procedural variables are summarised in Table 1 and Table 2. Of note, the mean age of the patients enrolled was 73 years, 28% were female, 34% had diabetes mellitus, 34% had a history of previous ACS episodes and 3% were on dialysis. Coronary angiography was performed using a radial approach in 78% of cases and morphine was used in only 8% of patients before or during PCI. Single-vessel disease was present in 38% of the patients, while two-vessel disease and three-vessel disease were present in 34% and 23% of cases, respectively. A drug-eluting stent was implanted in 93.7% of patients, while only 6.3% received a bare metal stent.
ORAL ANTIPLATELET THERAPIES BEFORE AND DURING PCI
A P2Y12 receptor inhibitor was administered in association with aspirin before PCI in 67.4% of cases (55.5% and 80.4% in STEMI and NSTE-ACS, respectively). Among patients receiving a P2Y12 receptor inhibitor as a pre-treatment strategy, 39.6% were treated with clopidogrel, 0.3% with ticlopidine, 11.7% with prasugrel and 48.2% with ticagrelor. Time delay from hospital admission to PCI while on treatment was 1.1±0.3 hour for STEMI and 16.3±4.4 hours for NSTE-ACS.
In the cathlab, a switch from old to novel oral P2Y12 receptor inhibitors (upgrade) occurred in 31 (2.3%) cases. No downgrade or change occurred at the time of PCI.
SWITCHING AT DISCHARGE AND IN-HOSPITAL CLINICAL EVENTS
At discharge, 46 (3.3%) patients switched oral antiplatelet therapies: upgrade occurred in 1% of cases, downgrade in 1.8% and change in 0.5% of patients.
The in-hospital incidence of adverse events is shown in Table 3. The cumulative incidence of NACE and MACE was 4.5% and 1.7%, respectively. The incidence of NACE, MACE, and single adverse events in patients treated with old or novel P2Y12 receptor inhibitors during the whole hospitalisation and in patients who received a switch of oral antiplatelet therapies is shown in Figure 1.
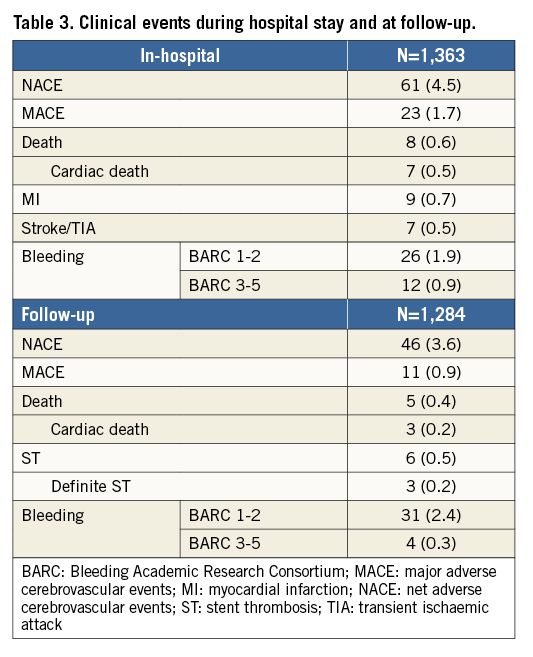
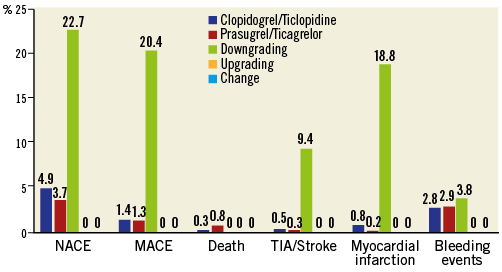
Figure 1. In-hospital relative incidence of NACE, MACE, and single adverse events in patients treated with old or novel P2Y12 receptor inhibitors (without switching) and in patients who received a switch (downgrade, upgrade or change) of oral antiplatelet therapies during the hospitalisation.
SWITCHING AND CLINICAL EVENTS AT FOLLOW-UP
Clinical follow-up was completed in 1,284 (94.2%) patients at a mean of 42±11 days from hospital discharge. The use of different oral antiplatelet agents in the cathlab, at discharge and at follow-up in NSTE-ACS and STEMI patients is displayed in Figure 2A and Figure 2B.
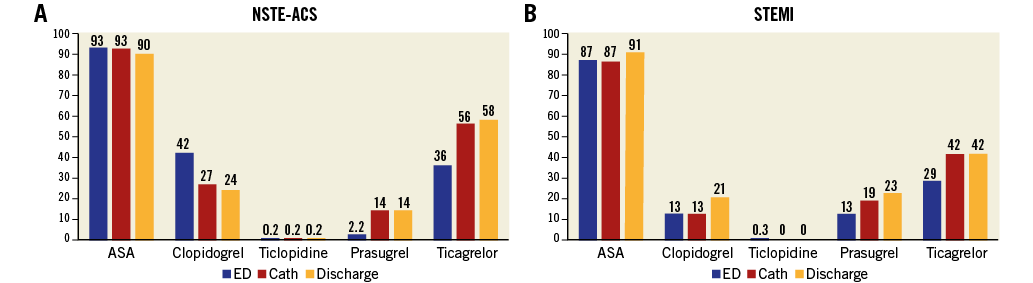
Figure 2. Use of different oral antiplatelet agents in the emergency department (ED), cathlab, and at discharge in patients with an initial diagnosis of NSTE-ACS (A) or STEMI (B).
Sixty-six (5.1%) patients switched oral antiplatelet therapy: upgrade occurred in 0.6% of cases, downgrade in 1.5% and change in 3.0% of patents. The overall rate of switching from hospital admission to one-month follow-up was 9.6%. Nobody declared using platelet inhibition testing before switching at any time. A standard (180 mg of ticagrelor, 60 mg of prasugrel and 300/600 mg of clopidogrel) loading dose (LD) at the time of switching was used in 92.4% of cases receiving upgrade, 47.7% for downgrade and 41.3% for change.
During follow-up, the main reasons for switching from ticagrelor were dyspnoea in 75% and minor bleeding in 25% of cases, while major bleeding was the only reason for switching down from prasugrel to clopidogrel. The rates of switching (upgrade, downgrade and change) that occurred in the cathlab, at discharge, at follow-up and during the whole study period are shown in Figure 3. At multivariate analysis, the predictors of cumulative upgrade switching were the presence of multivessel disease (OR 4.0; CI: 1.1-12.1; p=0.03) and the diagnosis of STEMI (OR 8.8; CI: 1.2-30.2; p=0.04), while the independent predictors of downgrade switching were a diagnosis of malignancy (OR 9.0; CI: 5.6-17.2; p=0.006), a history of stroke/TIA (OR 4.9; CI: 1.3-12.7; p=0.02) and age ≥75 years (OR 3.2; CI: 1.1-10.8; p=0.03).
The incidence of NACE and MACE from hospital discharge to follow-up was 3.6% and 0.9%, respectively, while the cumulative incidence of NACE and MACE from admission to follow-up was 5.6% and 1.6%, respectively. The relative incidence of MACE, NACE, and single adverse events in patients treated with old or novel P2Y12 receptor inhibitors and in patients who received a switch of oral antiplatelet therapies during the whole study period is shown in Figure 4.
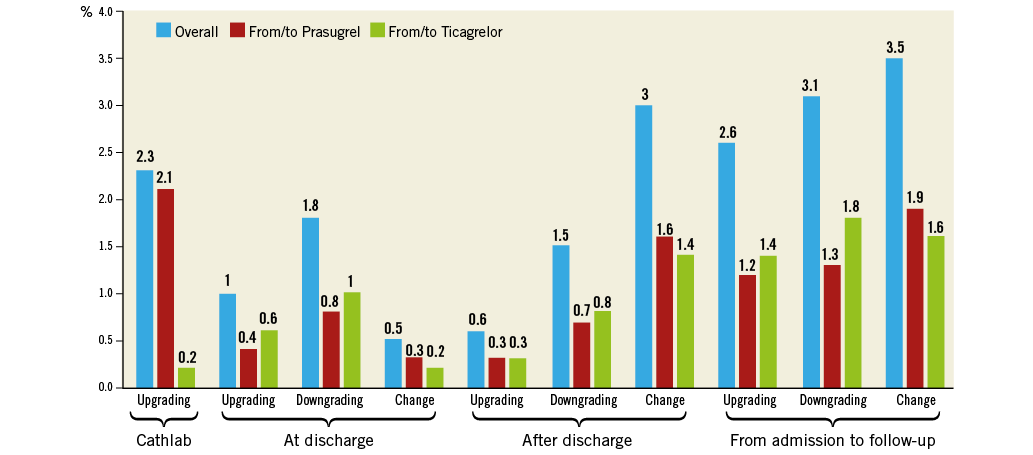
Figure 3. Absolute rate of switching (upgrade, downgrade and change) in the cathlab, at discharge, and at follow-up, and cumulative rate of switching during the whole study period.
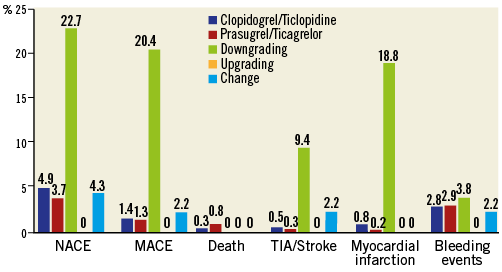
Figure 4. Cumulative relative incidence at one-month follow-up of MACE, NACE, and single adverse events in patients treated with old or novel P2Y12 receptor inhibitors (without switching) and in patients who received a switch (downgrade, upgrade or change) of oral antiplatelet therapies during the whole study period.
In particular, NACE occurred in 10 (22.7%) patients receiving downgrade and in two (4.3%) patients receiving a change, respectively (OR: 5.44; 95% CI: 1.1-18.2; p=0.03). No NACE occurred among patients receiving an upgrade switching (upgrade vs. downgrade: OR: 25.2; 95% CI: 1.4-242.9; p=0.02).
At multivariate analysis, the major predictors of cumulative NACE were the downgrade switching of oral antiplatelet therapies (OR 5.3; CI: 2.1-18-2; p=0.04) and the use of a femoral access during PCI (OR 2.2; CI: 1.1-4.6; p=0.04). Logistic regression analysis produced an area under the curve of 0.72, 0.73 and 0.76 for switching in the cathlab, at discharge and in-hospital NACE, and with non-significant Hosmer-Lemeshow tests confirming the reliability of the model.
Discussion
In SCOPE, a large, contemporary, prospective registry of consecutive ACS patients enrolled over three months in 39 Italian PCI centres, we observed that: 1) the rate of switching antiplatelet agents is low before and during PCI and increases slightly within the first month; 2) a strategy of upgrading from old to novel P2Y12 receptor inhibitors seems safe, while a downgrading is associated with a high incidence of adverse cardiovascular events.
In daily practice it is not uncommon for clinicians to consider switching patients previously loaded with clopidogrel and at high risk of recurrent ischaemic events3,4. In the last few years, several registries have been conducted in order to define the prevalence and reasons for switching oral antiplatelet therapies in real-world settings7-11. Overall, the reported prevalence of switching from clopidogrel to a new-generation P2Y12 receptor inhibitor ranges from 5% to 35% of cases during the index hospitalisation3,4. In our registry, the rate of switching was lower than previously observed in our country9 and in other surveys7-11, most likely because the use of novel P2Y12 receptor inhibitors has recently increased, due to ACS local protocol development and implementation of guidelines, reducing the clinical need for switching.
Several pharmacodynamic studies, including healthy volunteers, subjects with stable coronary artery disease and patients with ACS, have shown the feasibility of both upgrade switching and change12-15. All studies are consistent in showing enhanced platelet inhibition when upgrade switching is achieved, even compared to a higher clopidogrel maintenance dose or after switching from a high clopidogrel LD, while similar platelet inhibition is observed when change between ticagrelor and prasugrel is performed3,12,13. Specifically designed studies evaluating efficacy and safety with respect to clinical outcomes from the switching of oral P2Y12 receptor inhibitors are not currently available, but some observational analyses assessing clinical outcomes associated with switching from clopidogrel to novel P2Y12 receptor inhibitors have recently been reported3,4,14,15. A study-level meta-analysis of 15 studies including patients undergoing PCI did not show any increase in bleeding risk with a switching strategy from clopidogrel to prasugrel vs. prasugrel only (overall bleeding: OR 1.07, 95% CI: 0.69-1.66; p=0.77; major bleeding: OR 0.69, 95% CI: 0.32-1.49; p=0.34)16. The incidence of safety endpoints was also similar in the switching and clopidogrel only groups (overall bleeding: OR 1.27, 95% CI: 0.75-2.15; p=0.37; major bleeding: OR 0.70, 95% CI: 0.29-1.68; p=0.42)16. On the other hand, a recent large meta-analysis including more than 16,000 ACS patients suggested that switching to a novel P2Y12 agent significantly reduced the rate of MACE versus continuing clopidogrel (OR 0.77, 95% CI: 0.63-0.96, p=0.02), but increased bleeding events (OR 1.55, CI: 1.29-1.85, p<0.01)17. These conflicting results may be ascribed to the different patient populations included, or to different study designs and bleeding definitions employed. Our registry, the first prospectively designed to assess the outcome of switching in consecutive ACS patients undergoing PCI, clearly showed the in-hospital and short-term safety of upgrading from clopidogrel to novel P2Y12 receptor inhibitors and change between different novel P2Y12 receptor inhibitors, using the standardised BARC definition for bleeding and an independent committee for the evaluation of clinical events.
Information on downgrading from the novel oral antiplatelet agents to clopidogrel is scarce since this is a less frequent occurrence, usually considered in the case of relevant side effects (mainly bleeding), the need for concomitant oral anticoagulation and the increased costs of these new agents compared with generic clopidogrel. Some small studies evaluated the pharmacodynamic effects of switching from prasugrel to clopidogrel in ACS patients, suggesting that this downgrading is associated with a significant 10-fold increase in platelet reactivity18,19. Downgrading from ticagrelor to clopidogrel is associated with an increase in platelet reactivity, which occurs even if a 600 mg clopidogrel LD is given12. Moreover, a possible drug-to-drug interaction is highly probable with this strategy, given the different site of action and the different affinity of the active metabolites of ticagrelor and clopidogrel at the level of the P2Y12 platelet receptor. In particular, the occupancy of P2Y12 receptors by ticagrelor might prevent the active metabolites of clopidogrel from binding to the receptor during the early switching phase. Even the switching from ticagrelor to prasugrel20 and from cangrelor to oral thienopyridines, but not with ticagrelor, seems to be associated with an increase in platelet reactivity and needs further investigation. In our series, which did not include patients requiring anticoagulation, a downgrade switching strategy with both prasugrel and ticagrelor was associated with a high rate of MACE, particularly driven by an increased incidence of recurrent MI, and was an independent predictor of NACE.
Limitations
Our study must be evaluated in the light of some limitations. First, despite the considerable number of prospectively enrolled patients, causality cannot be inferred from this study that was probably underpowered for detecting differences in rarely occurring endpoints such as stent thrombosis, and the relative findings need to be confirmed in even larger cohorts. Second, potential unmeasured confounders cannot be excluded; thus, whether the downgrading strategy (switching from more potent P2Y12 platelet receptor inhibitors to clopidogrel) is truly an independent predictor of outcome or a marker of high risk needs to be confirmed in further studies. Third, our observation was limited to one month by study protocol. We should acknowledge that antiplatelet agent switching may also occur later on; however, the probability of bleeding events with dual antiplatelet therapy and ticagrelor-related dyspnoea, which represent the most frequent causes of switching besides the patient’s clinical characteristics, is generally more frequent in the first weeks after PCI. Finally, all patients in our study were treated at high-volume PCI Italian centres; therefore, the generalisation of our study results to other countries and centres is uncertain.
Conclusions
The SCOPE registry provided new data on the incidence and outcome of switching of oral P2Y12 receptor inhibitors in a real-world scenario. Switching of oral antiplatelet therapies occurred in approximately 10% of ACS patients within the first month from the acute event. The practice of switching from clopidogrel to novel P2Y12 receptor inhibitors appeared safe, while a switch from prasugrel or ticagrelor to clopidogrel in early phases of ACS was associated with adverse clinical events at short-term follow-up.
| Impact on daily practice This real-world registry suggests that a strategy of upgrading from old to novel P2Y12 receptor inhibitors is safe, while a downgrading is associated with a high incidence of adverse cardiovascular events. |
Conflict of interest statement
L. De Luca reports receiving personal fees from AstraZeneca, Bayer, Boehringer-Ingelheim, Eli Lilly, Daiichi Sankyo, PharmEvo, Menarini, and The Medicines Company, outside the submitted work. F. D’Ascenzo reports receiving personal fees from AstraZeneca, Abbott, Chiesi, Medtronic, and Servier, outside the submitted work. G. Musumeci reports receiving personal fees from Abbott Vascular, AstraZeneca, Daiichi Sankyo, Menarini, MSD, St. Jude Medical, and The Medicines Company, outside the submitted work. F. Saia reports receiving personal fees from AstraZeneca, Eli Lilly, Daiichi Sankyo, Menarini, Servier, The Medicines Company, St. Jude, Abbott Vascular, and Medtronic, outside the submitted work. G. Parodi reports receiving personal fees from AstraZeneca, Bayer, Daiichi Sankyo/Eli Lilly, and The Medicines Company, outside the submitted work. F. Varbella reports receiving personal fees from Abbott Vascular, AstraZeneca, Bayer, Boehringer-Ingelheim, Daiichi Sankyo, Maquet, Medtronic, Pfizer, and Stentys, outside the submitted work. S. De Servi reports receiving personal fees from AstraZeneca, Eli Lilly, Daiichi Sankyo, and The Medicines Company, outside the submitted work. L. Bolognese reports receiving personal fees from AstraZeneca and Daiichi Sankyo, outside the submitted work. The other authors have no conflicts of interest to declare.

