Introduction
Comatose survivors of out-of-hospital cardiac arrest (OHCA) undergoing percutaneous coronary intervention (PCI) and target temperature management (TTM) are at increased risk for stent thrombosis (ST)1. This may, in part, be explained by a delayed onset of P2Y12 inhibition after clopidogrel, as well as after novel P2Y12 inhibitors2. Such a delay may promote platelet aggregation on the exposed stent, ultimately leading to ST. We hypothesised that the P2Y12 inhibitor, cangrelor, would induce immediate platelet inhibition and thereby bridge the “P2Y12 inhibition gap”.
Methods
Our investigator-initiated, randomised, controlled trial (ClinicalTrials.gov: NCT04005729), approved by the National Ethics Committee (KME 0120-61/2019/8), enrolled consecutive comatose survivors of OHCA admitted to the Ljubljana University Medical Center (Slovenia) who underwent immediate PCI and TTM at 32-34º C. All patients were treated according to standardised PCI/TTM protocol with intubation/mechanical ventilation and sedation, including with parenteral opiates34.
Prior to PCI, patients were randomised 1:1 to cangrelor and control groups using a sealed envelope system. Exclusion criteria were age above 70 years, need for veno-arterial extracorporeal circulation (VA-ECMO), active bleeding, past ischaemic stroke and current P2Y12 inhibitor or anticoagulation treatment. All patients received intravenous boluses of acetylsalicylic acid (ASPÉGIC; Sanofi-Aventis) and unfractioned heparin (UFH) targeted to an activated clotting time of 250-300 seconds. The cangrelor group received an intravenous bolus of cangrelor (30 mcg/kg) followed by a 4-hour infusion (4 mcg/kg/min). A nasogastric tube was inserted immediately after intensive care admission and a 180 mg loading dose of ticagrelor (Brilique; AstraZeneca) was administered as a suspension of crushed tablets. The control group was treated equally except for cangrelor.
The primary efficacy endpoint was platelet inhibition at 1, 3, 5 and 8 hours after the passage of a PCI guidewire by VerifyNow P2Y12 (Accriva Diagnostics) and Multiplate (Roche)2. High on-treatment platelet reactivity (HPR) was defined as PRU >208 by VerifyNow and as >46 U by Multiplate. The primary safety endpoint was BARC 2, 3 or 5 bleeding.
Continuous variables are reported as mean±standard deviation and categorical variables as frequencies and proportions. Unpaired 2-tailed t-tests and the chi-square/Fisher’s exact tests were used for comparison. A p-value of <0.05 was considered significant.
Results
Between September 2019 and November 2021, 91 consecutive patients underwent immediate PCI. After excluding patients older than 70 years (n=30), with concomitant antiplatelet/anticoagulation (n=5), active bleeding (n=3), need for VA-ECMO (n=10), history of stroke (n=1), and for logistical reasons (n=12), 30 patients were randomised.
There was no significant difference between the cangrelor and control groups in patient characteristics, initial resuscitation, admission laboratory findings, acetylsalicylic acid administration (333±136 vs 357±149 mg; p=0.657), delay between PCI and ticagrelor (56±19 vs 64±19 minutes; p=0.252), angiographic/PCI features, final Thrombolysis in Myocardial Infaraction (TIMI) 3 flow, peak troponin I levels, ST at 30 days (14% vs 20%; p=1.000), hospital survival with neurological recovery (67% vs 60%; p=0.705) or BARC 2, 3 or 5 bleeding (6.7% vs 0%; p=1.000).
VerifyNow was significantly decreased in the cangrelor group at 1 hour (30±45 vs 221±45 PRU; p<0.001) and 3 hours (24±36 vs 180±67 PRU; p<0.001), without differences at 5 and 8 hours (Figure 1). These changes were mirrored in decreased HPR at 1 hour (0% vs 67%; p<0.001) and 3 hours (0% vs 47%; p=0.007), without significant differences at 5 and 8 hours. Multiplate was also significantly decreased at 1 hour (14±9 vs 48±24 U; p<0.001) and 3 hours (11±6 vs 42±29 U; p=0.001), without significant differences at 5 and 8 hours, and with corresponding changes in HPR at 1 hour (0% vs 39%; p=0.013) and 3 hours (0% vs 33%; p=0.044).
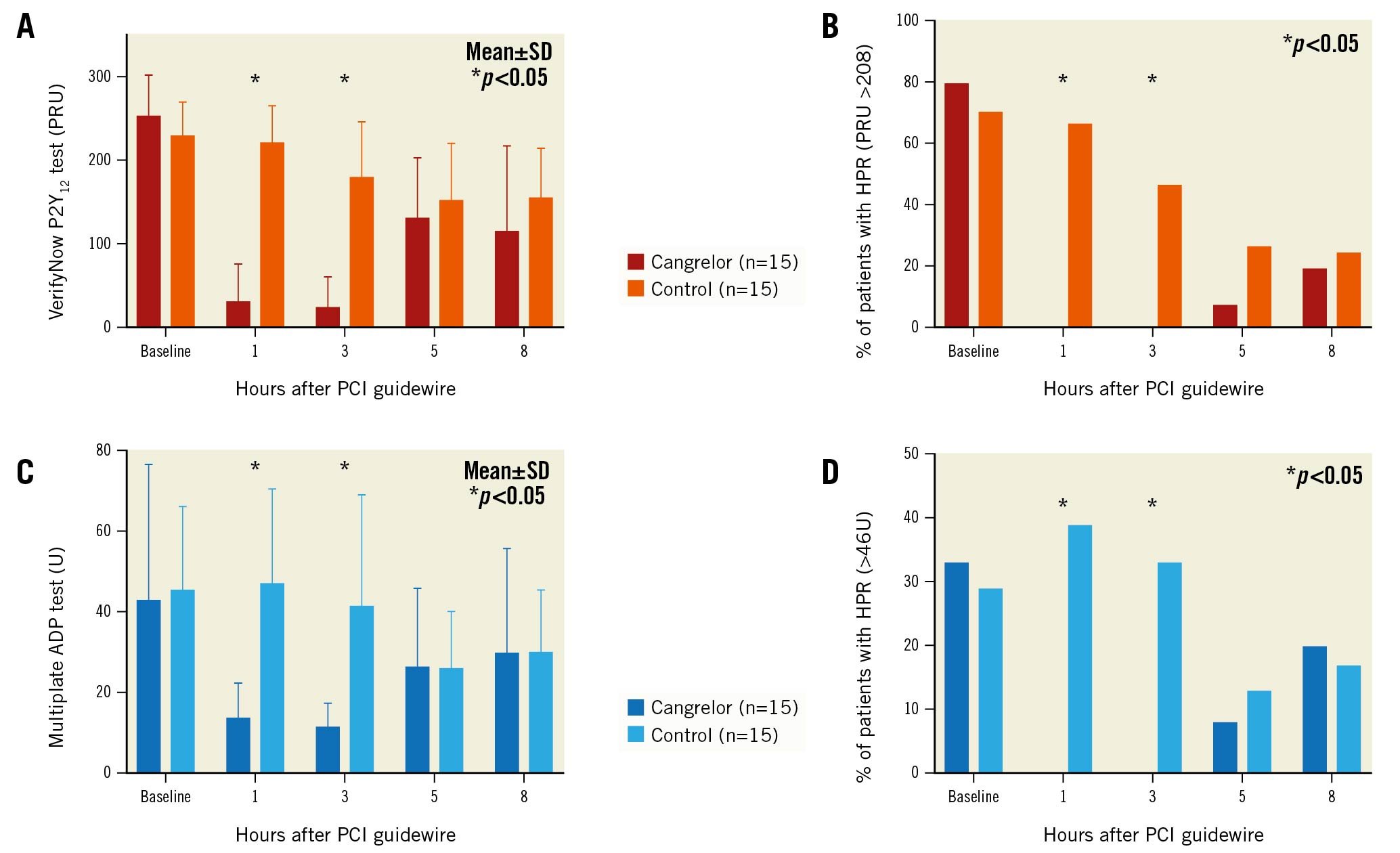
Figure 1. Cangrelor-induced platelet inhibition in comatose survivors of out-of-hospital cardiac arrest undergoing percutaneous coronary intervention and target temperature management. Platelet reactivity and high on-treatment platelet reactivity (HPR) measured at baseline and at 1, 3, 5 and 8 hours after passage of PCI guidewire according to VerifyNow P2Y12 (A, B) and Multiplate ADP (C, D) in cangrelor and control groups. ADP: adenosine-diphosphate; PCI: percutaneous coronary intervention; PRU: platelet reactivity units; SD: standard deviation
Discussion
Our study in comatose OHCA patients undergoing PCI and TTM at 32-34° C demonstrated that a periprocedural bolus of cangrelor followed by a 4-hour infusion induces immediate and profound platelet inhibition without significant drug-drug interaction with ticagrelor and with no excess of BARC 2, 3 or 5 bleeding. This replicates a favourable cangrelor profile in patients with ST-elevation myocardial infarction without cardiac arrest5. On the other hand, it is important to notice that up to 20% of patients with HPR after cangrelor discontinuation remained at increased risk for ST. A longer duration of infusion might therefore be needed to completely address delayed P2Y12 absorption. On the other hand, ST is multifactorial with inadequate P2Y12 inhibition representing only 1 component. The potential clinical benefit of cangrelor therefore needs to be investigated in an appropriately powered randomised trial.
Limitations
Our study is a single-centre and non-placebo-controlled trial, which is not powered for clinical endpoints. Because of a rather high exclusion rate, the findings cannot be generalised to the whole OHCA population. Our patients underwent TTM at 32-34° C; however, the “P2Y12 inhibition gap” might be shorter if normothermia (36-37°C) is used. We further hypothesise that the onset of P2Y12 inhibition might have been reduced by up to 1 hour if the insertion of a nasogastric tube and ticagrelor administration had already been performed in the catheterisation laboratory.
Conclusions
In comatose survivors of OHCA undergoing PCI and TTM, cangrelor safely induced immediate and profound platelet inhibition without significant drug-drug interaction with ticagrelor.
Funding
The study was an investigator-initiated trial sponsored by the University Medical Center, Ljubljana, and partially funded by an unrestricted grant from Chiesi Slovenia d.d., which covered the cost of cangrelor. Chiesi had no access to data or analysis and were not involved in the drafting or final version of the manuscript. VerifyNow platelet aggregation testing was funded in part by the Slovenian Research Agency research program (P3-0308).
Conflict of interest statement
The authors have no conflicts of interest to declare related to this study.

