Abstract
Background: Incidence of stent thrombosis (ST) in comatose survivors of out-of-hospital cardiac arrest (OHCA) undergoing immediate percutaneous coronary intervention (PCI) and therapeutic hypothermia (TH) varies considerably, from 2.7% to 31.2%, in retrospective studies.
Aims: We aimed to investigate occurrence, timing and predictors of definite ST.
Methods: We prospectively investigated consecutive comatose survivors of OHCA with presumed cardiac aetiology undergoing immediate PCI with drug-eluting stents (DES) and TH targeted at 32-34°C admitted between August 2016 and July 2021. Repeat coronary angiography (CAG) was performed if ST was suspected and systematically between day 8-12 in the absence of clinical signs. All deceased patients underwent autopsy and histopathological analysis.
Results: Among 362 comatose survivors of OHCA, immediate PCI with stenting was performed in 169 patients (47%). Since 18 patients did not complete follow-up, 151 patients were ultimately enrolled in ST analysis. Definite ST was confirmed in 29 patients (19.2%; 95% confidence interval [CI]: 12.9%-25.6%) either by CAG (n=18) or autopsy (n=11). ST occurred within 3 days in 62% and presented with at least one clinical sign in 79%. Survival with good neurological recovery was observed in 17% of patients with ST and in 60% of patients without ST (p<0.001). Independent predictors of ST were longer prehospital resuscitation, lower arterial pH and increased creatinine on admission.
Conclusions: The incidence of definite ST in comatose survivors of OHCA undergoing immediate PCI and TH targeted at 32-34°C is substantial (19.2%) and significantly higher than in other PCI subsets despite systematic use of contemporary DES and anticoagulation/antiplatelet treatment.
Introduction
Immediate coronary angiography (CAG) followed by percutaneous coronary intervention (PCI) is increasingly performed in comatose survivors of out-of-hospital cardiac arrest (OHCA) of presumed cardiac origin, in particular if ST-elevation myocardial infarction (STEMI) is present on the post-resuscitation electrocardiogram (ECG)1. These patients routinely undergo therapeutic hypothermia (TH), aimed at reducing post-resuscitation brain injury2.
While the incidence of stent thrombosis (ST) in patients with stable coronary artery disease or even with acute coronary syndrome undergoing PCI with contemporary drug-eluting stents (DES) rarely exceeds 1%3, its occurrence in comatose survivors of OHCA is largely unknown and varies from 2.7% to 31.2% in retrospective studies45678 (Table 1). Such wide variation may be attributed to the retrospective design of available studies, the challenging diagnosis of ST in comatose patients, and differences in PCI timing, strategy, techniques, and periprocedural anticoagulation/antiplatelet treatment. Last but not least, the description of ST in these studies is rather inconsistent without distinguishing between possible, probable and definite ST9. Because of these uncertainties, we prospectively investigated the incidence, timing and predictors of definite ST in comatose survivors of OHCA undergoing immediate PCI and TH targeted at 32-34°C.
Methods
Our study (ClinicalTrials.gov: NCT03312296) enrolled consecutive comatose survivors of OHCA of presumed cardiac aetiology admitted between August 1, 2016 and July 31, 2021 to the University Medical Centre Ljubljana (Slovenia), who underwent CAG, PCI and TH on admission. The study protocol was approved by the National Ethics Committee (KME 0120-122/2015-2).
CAG on admission was performed in patients with OHCA of suspected acute coronary origin, as described in the European Association for Percutaneous Cardiovascular Interventions/Stent for Life (EAPCI/SFL) consensus document1. PCI immediately after CAG, defined as index PCI, was advised if an acute culprit lesion was identified. Prior to the index PCI, patients received an intravenous bolus of unfractionated heparin (UFH), to achieve a target activated clotting time between 250-300 seconds, and a 250-500 mg intravenous bolus of acetylsalicylic acid. Eptifibatide and cangrelor were used during the index PCI at the discretion of the interventional cardiologist. Only contemporary DES were implanted. Mechanical circulatory support was used at the discretion of the acute cardiac care intensivist. Immediately after admission to the intensive care unit (ICU), a nasogastric or orogastric tube was inserted and a loading dose of a preferably novel P2Y12 inhibitor was administered as crushed and dissolved tablets1011. Thereafter, daily maintenance doses of intravenous acetylsalicylic acid (100 mg) and crushed/dissolved tablets of the selected P2Y12 inhibitor via nasogastric/orogastric tube were given. If the patient regained consciousness, antiplatelet drugs were administered per os.
All patients were already intubated and mechanically ventilated prior to their arrival at the catheterisation laboratory. Other intensive care procedures were used according to contemporary practice and standardised post-resuscitation protocol1213 . TH, targeted at 32-34°C, was initiated either in the catheterisation laboratory or immediately upon ICU arrival and maintained for 24 hours. Thereafter, patients were slowly rewarmed to normothermia14.
Routine 12-lead ECG was recorded every 6 hours until patients were comatose and intubated. Cardiac troponin I (cTnI) was measured every 6-12 hours. ST was suspected in cases of new ischaemic changes in 12-lead ECG, unexplained haemodynamic deterioration, malignant cardiac arrhythmias including recurrent cardiac arrest, and if cTnI values, after the initial fall following index PCI, increased by more than 20%. In the presence of any of these clinical signs, clinically-driven CAG was immediately performed. In patients without clinical signs of suspected ST or negative clinically-driven CAG, control CAG, aimed at documenting possible clinically silent ST, was performed between days 8 and 12. Definite ST was defined according to the Academic Research Consortium (ARC)-2 document as the presence of occlusive or non-occlusive thrombus that originated in the coronary stent or in a 5 mm segment proximal or distal to the stent position9. If ST was confirmed by either clinically-driven or control CAG, rescue PCI, which is not included in the herein presented analysis, was performed at the discretion of the interventional cardiologist and acute cardiac care intensivist. All coronary angiograms were evaluated by an experienced interventional cardiologist who was part of the study team.
An autopsy was performed in all deceased patients. Coronary artery segments were removed and referred to the histopathological laboratory at Deutsches Herzzentrum München. Stented arteries were initially photographed, X-rayed in total and embedded into methyl methacrylate. Afterwards, each coronary artery was cut into five segments (proximal, proximal-mid, mid, mid-distal, and distal). For each segment, two slides were cut using a laser microtome (TissueSurgeon; LLS ROWIAK LaserLabSolutions GmbH). Histological sections were stained using haematoxylin-eosin and Verhoeff-Van Gieson stains and analysed by an expert in vascular pathology. Definite ST was defined as a platelet-rich obstructive (occupying >30% of the cross-sectional area of the stent lumen) or non-obstructive (occupying <30% of the cross-sectional area of the stent lumen) thrombus. Differentiation of ante mortem stent thrombus from post mortem thrombus was performed as previously reported15. If a detailed histopathological analysis could not be performed, the pathologist performing the autopsy conducted a standard analysis. In brief, stents were opened longitudinally and contents, if present, were removed and analysed microscopically for the presence and composition of thrombus16.
Continuous data are reported as mean±standard deviation unless otherwise stated. Categorical data are shown as frequencies and percentages. Differences between the groups were analysed using the unpaired t-test or Mann-Whitney U test, according to distribution. For comparison of categorical variables, Fisher’s exact test or the chi-square test were used. Independent predictors of ST were assessed by binominal logistic regression, which included variables with p<0.1 in univariate analysis. A p-value of <0.05 was considered statistically significant. Data were analysed using the Statistical Package for the Social Sciences (SPSS) version 22.0 (IBM).
Results
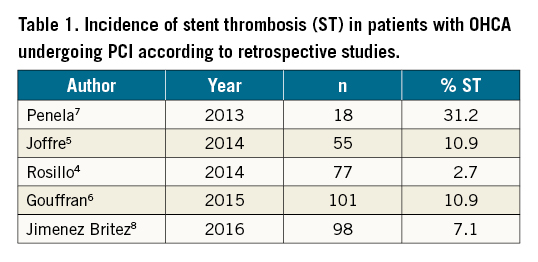
Among 362 consecutive comatose survivors of OHCA, CAG on admission was performed in 270 patients (75%) followed by the index PCI with stenting in 169 patients (47%) (Central illustration). Since 18 patients did not undergo clinically-driven/control CAG or an autopsy because of participant/relative refusal (n=17) and immobilisation due to spine fracture (n=1), 151 patients were ultimately included in the analysis for possible ST after the index PCI.
Central illustration. Study flowchart. CAG: coronary angiography; OHCA: out-of-hospital cardiac arrest; PCI: percutaneous coronary intervention; POBA: plain old balloon angioplasty; ST: stent thrombosis
Definite ST was confirmed in 13 of 29 patients (45%) who underwent clinically-driven CAG. The causes of clinical deterioration in the 16 patients in whom ST was not confirmed were onset of infection (n=7), ventricular arrhythmia associated with index myocardial infarction (n=6) and worsening of left ventricular function (n=3). Control CAG, which was performed in 108 patients, revealed ST in 5 patients (4.6%). Accordingly, ST was angiographically confirmed in 18 of 137 patients (13.1%). Of these patients, acute thrombotic occlusion with Thrombolysis in Myocardial Infarction (TIMI) 0-1 was confirmed in 94%.
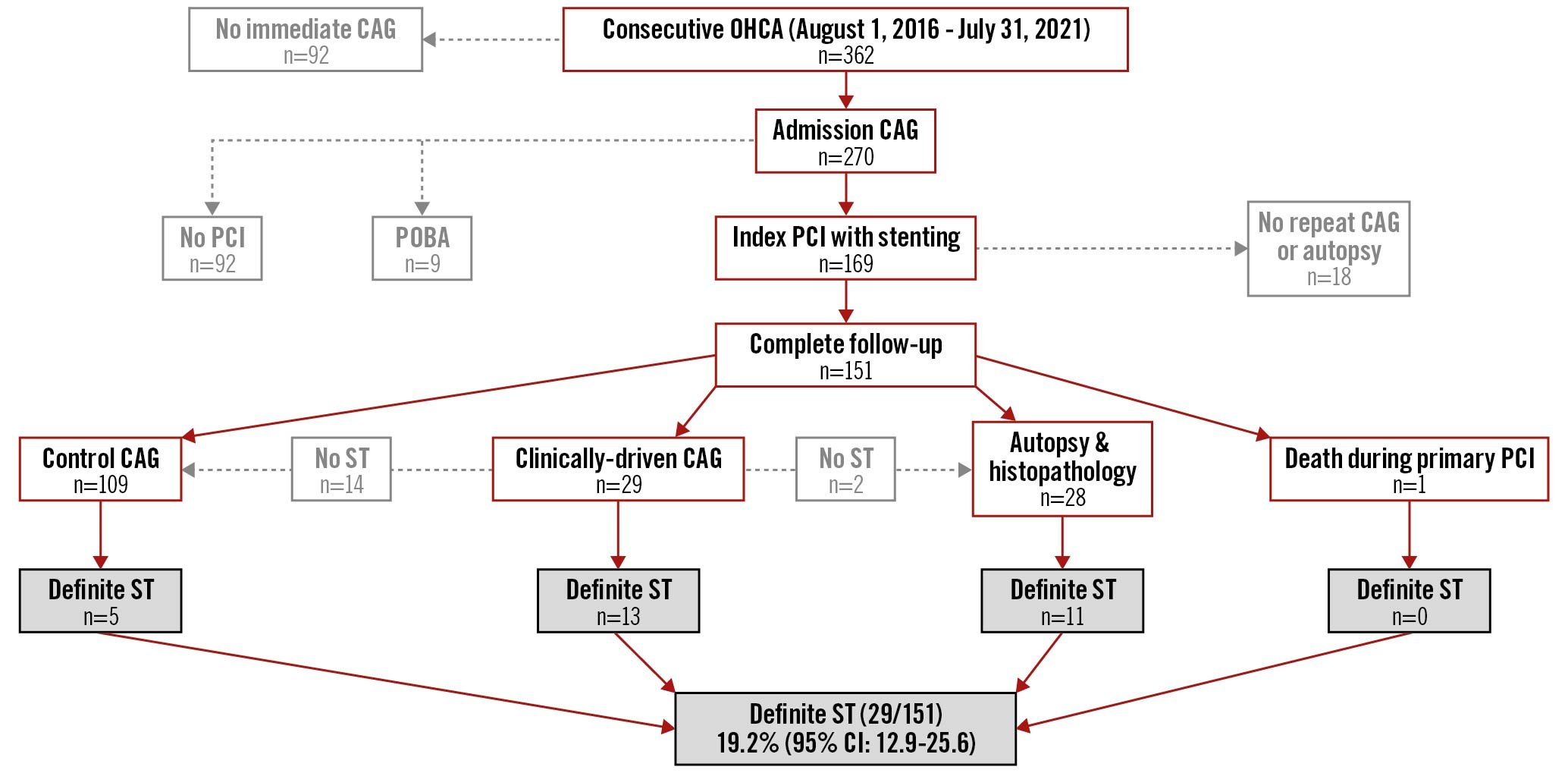
Of the 28 patients who died and underwent an autopsy, a standard analysis of stented segments was performed in 13 patients and revealed ST in 3 patients (23.1%). Detailed histopathological analysis was performed in 15 patients and revealed ST in 8 patients (53.3%). Accordingly, ST was confirmed by autopsy in 11/28 (39.3%). The ST that was assessed by detailed histopathological analysis in 8 patients was either obstructive ST (n=5) or non-obstructive (n=3) (Figure 1).
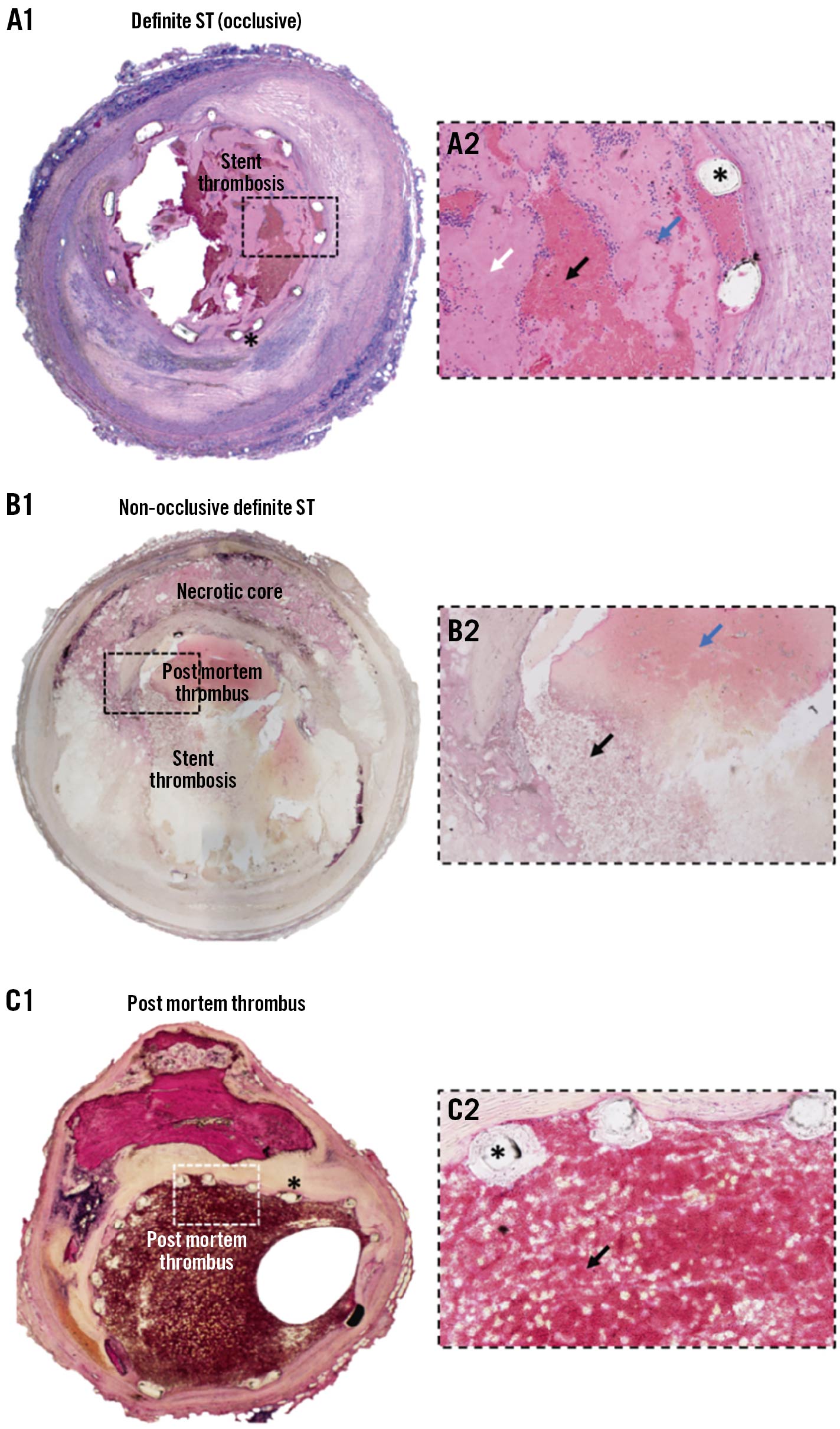
Figure 1. Detailed histological analysis of definite ST, post mortem thrombus and non-occlusive definite ST. H&E staining, A1/B1/C1: 2x magnification, A2/B2/C2: 20x magnification of boxed area. (*)=stent struts. A1/A2) Definite acute occlusive stent thrombosis. Higher magnification (A2) reveals platelet-rich thrombotic material (black arrow) and fibrin-rich appositional thrombus (white arrow) with interspersed deposits of inflammatory cells (blue arrow), occluding approximately 75% of the lumen. B1/B2) Definite non-occlusive stent thrombosis. Higher magnification (B2) reveals non-occlusive stent thrombosis (black arrow) together with post mortem thrombus consisting mainly of erythrocytes (blue arrow). C1/C2) Post mortem thrombus. Higher magnification (C2) shows thrombus consisting mainly of erythrocytes (black arrow). H&E: haematoxylin & eosin; ST: stent thrombosis
In summary, definite ST was confirmed by either CAG or autopsy in 29 of 151 patients (19.2%; 95% confidence interval [CI]: 12.9%-25.6%). The majority of ST occurred within the first 3 days (62%) (Figure 2). Clinical signs of ST included new ischaemic ECG changes (17%), malignant arrhythmias (14%), unexplained haemodynamic deterioration (34%), and significant cTnI rise (14%) (Table 2).
Figure 2. Timing of definite ST. CAG: coronary angiography; OHCA: out-of-hospital cardiac arrest; ST: stent thrombosis
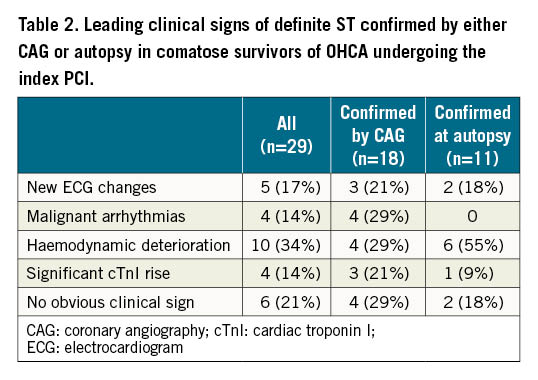
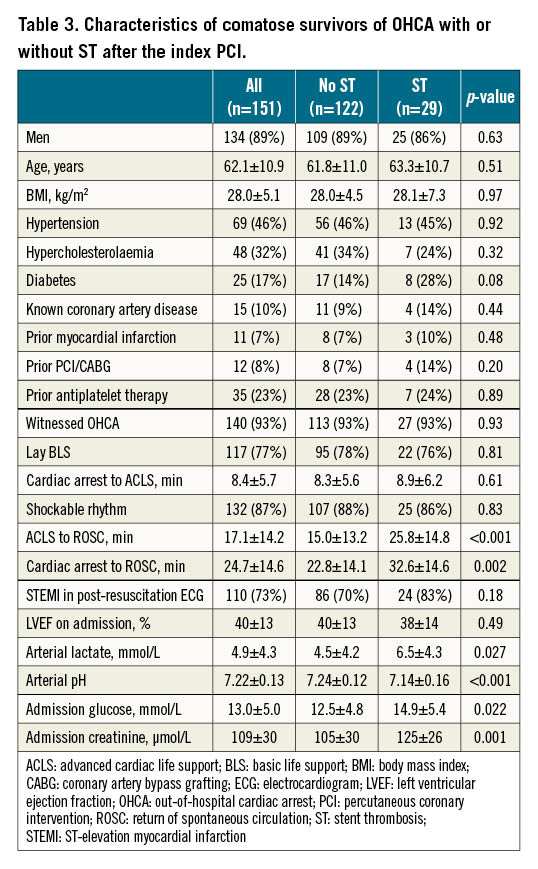
We then compared different features between patients with or without ST after the index PCI (Table 3). The duration of advanced cardiac life support (25.8 versus 15.0 minutes) and time interval between the onset of cardiac arrest and the re-establishment of spontaneous circulation (32.6 versus 22.8 minutes) were significantly longer in patients with ST. Longer prehospital resuscitation was associated with significantly increased admission concentrations of arterial lactate (6.5 versus 4.5 mmol/L), glucose (14.9 versus 12.5 mmol/L) and creatinine (125 versus 105 microg/L), and decreased arterial pH (7.14 versus 7.24).
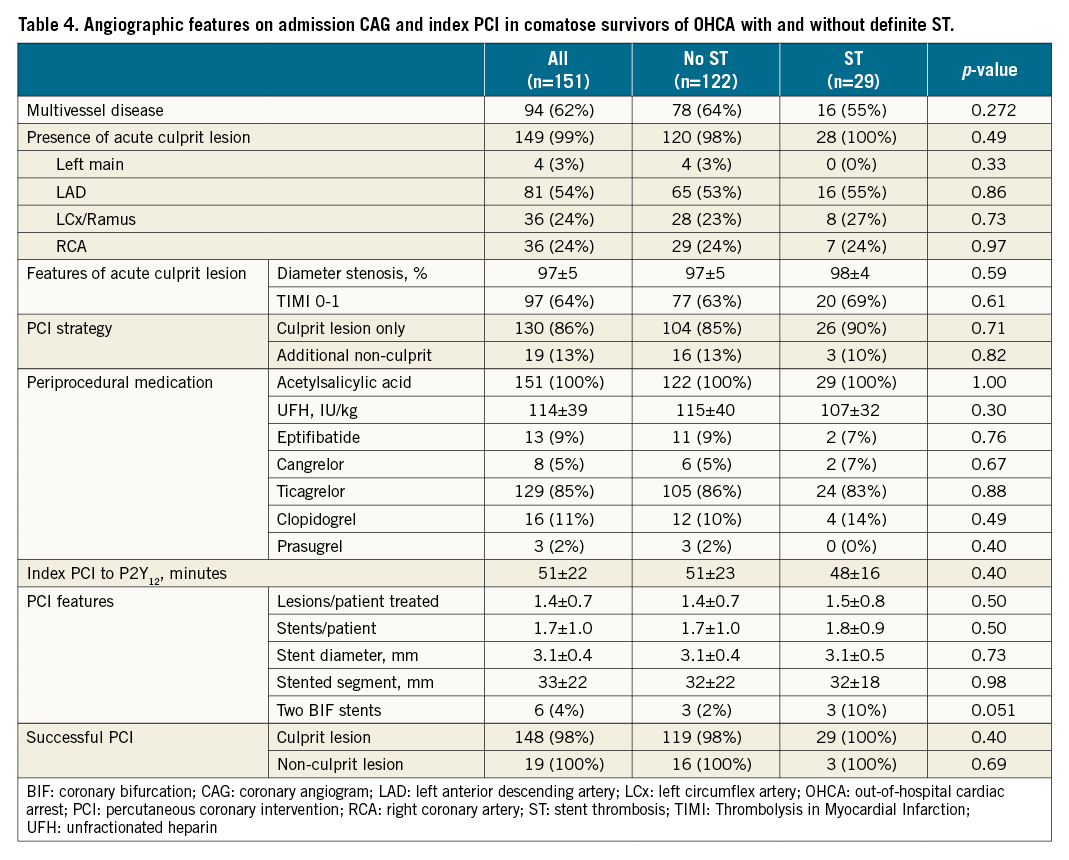
CAG on admission showed multivessel disease in 62% with the presence of an acute culprit lesion in 99% (Table 4). Acute occlusion of the culprit vessel (TIMI flow 0-1) was documented in 64%. There was no significant difference between the groups. Index PCI was mainly performed on acute culprit lesions. Periprocedural anticoagulation and antiplatelet treatment did not differ between the groups. Mean time delay between the index PCI and P2Y12 administration via nasogastric/orogastric tube in the ICU was also comparable (51 versus 48 minutes; not significant [NS]). Eptifibatide and cangrelor were used in 9% and 5%, respectively (NS). Except for more bifurcation stenting with two DES in the ST group (10% versus 2%; p=0.051), there was no difference in procedural characteristics. Good angiographic results were achieved in more than 98% of patients regardless of the lesion characteristics and the study group.
In terms of ICU treatment, there was no significant difference between patients with and without ST in the utilisation of vasoactive drugs (93% versus 97%; p=0.37), intra-aortic balloon pump (IABP; 31% versus 22%; p=0.31), veno-arterial extracorporeal membrane oxygenation (VA ECMO; 3% versus 7%; p=0.45) and antimicrobial agents (86% versus 93%; p=0.27). Haemodialysis was more often needed in patients with ST (14% versus 4%; p=0.048). Maximal median cTnI during the ICU stay was 143.765 ng/L (25th-75th interquartile [IQ] range 43.700-260.456 ng/L) in patients with ST and 40.822 ng/L (25th-75th IQ range 10.452-88.732 ng/L) in patients without ST (p<0.001). Survival with good neurological recovery was significantly decreased in patients with ST (17% versus 60%; p<0.001).
Binomial logistic regression identified a longer duration of prehospital resuscitation (OR 1.05; 95% CI: 1.01-1.09; p=0.018), lower arterial pH (OR 0.002; 95% CI: 0-0.51; p=0.027) and increased creatinine (odds ratio [OR] 1.02; 95% CI: 1.00-1.04; p=0.018) on admission as independent predictors of ST.
Discussion
Our study revealed a surprisingly high incidence of definite ST in comatose survivors of OHCA undergoing immediate PCI and TH. Importantly, an increased ST rate was observed despite systematic use of contemporary DES as well as periprocedural anticoagulation and antiplatelet treatment. We believe that ST in comatose survivors of OHCA has probably been underestimated in previous retrospective studies because clinical diagnosis is rather challenging without dedicated prospective protocols, including repeat CAG and autopsy with histopathological analysis, such as in our study. However, despite a well-defined prospective protocol, every fifth case of ST was clinically silent and in fact represented an incidental finding.
Increased incidence of definite ST in comatose survivors of OHCA is probably related to post-resuscitation syndrome, TH, or both. A longer duration of prehospital resuscitation resulting in more profound haemodynamic compromise and metabolic disarrangement on hospital admission seems to predispose to ST. Neither baseline angiographic characteristics nor index PCI features differed significantly between the patients with and without definite ST. Only the two-stent bifurcation strategy showed borderline significance, indicating that provisional stenting with only one stent might be the preferred PCI technique in this subset of patients.
Since our patients underwent TH targeted at 32-34°C, it is important to notice that hypothermia may facilitate platelet aggregation by increasing adenosine diphosphate (ADP) levels1718. This was demonstrated in vitro19 and in healthy volunteers20. Moreover, during hypothermia, absorption and onset of P2Y12 inhibition is significantly delayed after clopidogrel10, as well as after novel oral P2Y12 inhibitors, which were predominantly used in our study. An almost 3-hour “P2Y12 inhibition gap” was demonstrated after administration of crushed ticagrelor tablets via nasogastric tube11. Such a delay in P2Y12 inhibition may promote early platelet aggregation on stent struts, which may ultimately lead to acute ST. Indeed, ST occurred within the first day in 45% and within 3 days in 62% of our patients, with a substantial number of clinically silent ST cases which may also have occurred early after the index PCI. Unfortunately, we did not perform systematic platelet function testing to investigate for possible differences between the groups with and without definite ST. Our hypothesis that TH may create a prothrombotic environment is supported by recent clinical trials on myocardial salvage using ultrafast cooling as an adjunct to primary PCI in conscious STEMI patients. Even though the TH protocols in these trials were much faster and shorter, there was a numerical increase in ST in the VELOCITY trial using peritoneal cooling (11% versus 0%; p=0.09)21 and in combined analysis of the EU COOL AMI Pilot/Pivotal trials using intravascular cooling with a catheter in the inferior vena cava (6.0% versus 1.3%; p=0.11)2223.
To curtail the issue of ST in comatose OHCA survivors, novel approaches may be needed. First, based on recent randomised trials, including COACT24, PEARL25 and TOMAHAWK26, delayed/selective rather than admission CAG/PCI should be used in haemodynamically stable comatose OHCA patients without signs of STEMI on post-resuscitation ECG. In these patients, ST is probably less likely if PCI is delayed and performed in a more elective situation before hospital discharge, when patients regain consciousness and do not need intensive treatment. If PCI is needed already upon admission, such as in haemodynamically unstable patients or in patients with STEMI, it should probably follow the “culprit lesion PCI only” strategy, as shown in the CULPRIT-SHOCK trial which importantly enrolled about 50% of patients with resuscitated cardiac arrest27. Along with the systematic use of contemporary DES, complex bifurcation techniques and extended stenting should probably be avoided, at least in the acute post-resuscitation phase. Furthermore, achievement of optimal angiographic results with adequate stent sizing and expansion by using intravascular imaging, which was not systematically used in our study, is of paramount importance. Because of the presumed prothrombotic environment and the “P2Y12 inhibition gap” of up to 3 hours, the naso/orogastric tube should probably be inserted in the catheterisation laboratory and the novel P2Y12 agent administered when PCI is decided. This would eliminate approximately 50 minutes of the delay associated with P2Y12 administration after arrival to the ICU as documented in our study. On the other hand, because of significantly delayed inhibition, even if P2Y12 were administered in the catheterisation laboratory, systematic intraprocedural platelet inhibition may be needed. Even a single intravenous bolus of the glycoprotein llb/llla inhibitor eptifibatide has been shown to effectively achieve immediate and profound platelet inhibition10. Unfortunately, comatose OHCA patients also have a significantly increased risk of bleeding related to chest compression and intubation. In this respect, the new intravenous P2Y12 inhibitor cangrelor with immediate onset-offset of platelet inhibition might be preferred over the conventional glycoprotein IIb/IIIa inhibitors. Last but not least, because TH at 32-34°C is thought to produce a prothrombotic environment and promote ST without further benefits in neurological recovery, as shown in the recent randomised control trials2829, target temperature management should be aimed for normothermia.
Limitations
Our study has several potential limitations. Although performed in a high volume “24/7” interventional facility with more than 800 primary PCI for STEMI per year30, it remains a single-centre study and the observed rate of definite ST may not universally apply to other interventional centres. Systematic intravascular imaging, which would optimise stent deployment, was not used in our study. Furthermore, due to lack of intravascular imaging, we also could not adequately address possible mechanisms of ST including stent underexpansion, undersizing and edge dissection. We did not perform systematic testing of platelet reactivity to identify possible P2Y12 non-responders. Importantly, since our patients underwent TH targeted at 32-34°C, the findings may not be generalisable to target temperature protocols aimed for post-resuscitation normothermia. We further acknowledge a limited number of patients, with an 11% dropout rate because of incompletion of the study protocol. Moreover, due to logistic problems related to the COVID-19 pandemic, a detailed histopathological analysis could not be performed in all stented coronary segments as planned. Considering an ST rate of only 23% identified by standard histopathological analysis and 53% by detailed analysis, the absolute ST rate in deceased patients may have even been underestimated.
Conclusions
The incidence of definite ST in comatose survivors of OHCA undergoing immediate PCI and TH between 32-34°C is substantial (19.2%) and much higher than in other PCI subsets despite systematic use of contemporary DES and anticoagulation/antiplatelet treatment. ST, which typically occurred early (62% within 3 days), was associated with a longer duration of prehospital resuscitation, resulting in more profound haemodynamic compromise and metabolic disarrangement on admission. Clinical signs including new ischaemic ECG changes, malignant arrhythmias, haemodynamic deterioration and unexplained cTnI rise were present in almost 80% of patients.
Impact on daily practice
PCI upon admission is increasingly performed in comatose survivors of OHCA who also undergo TH aimed at reducing post-resuscitation brain injury. Our study demonstrated that the incidence of definite ST in these patients is substantial and significantly higher than in other PCI subsets despite systematic use of contemporary DES and anticoagulation/antiplatelet treatment. Novel approaches including better patient selection, PCI timing/strategy/intravascular imaging as well as improved early antiplatelet treatment are therefore needed. Because TH at 32-34°C may create a prothrombotic environment and facilitate ST without further benefits in neurological recovery, target temperature management should be aimed for normothermia.
Acknowledgements
The authors would like to acknowledge the significant contribution of the physicians and nurses of the Centre for Intensive Internal Medicine (Head: Asst. Prof. Peter Radsel, MD, PhD) and the interventional team of the Department of Cardiology at the University Medical Centre Ljubljana (Head: Prof. Matjaz Bunc, MD, PhD). We are grateful to the Department of Pathology (Head: Prof. Joze Pizem, MD, PhD) and the Department of Forensic Medicine (Head: Prof. Joze Balazic, MD, PhD) at the Medical School, University of Ljubljana for providing the stented coronary segments.
Funding
The study was funded by the University Medical Centre Ljubljana (Slovenia) and Deutsches Herzzentrum München, München (Germany).
Conflict of interest statement
The authors have no conflicts of interest to declare in relation to this study.
Supplementary data
To read the full content of this article, please download the PDF.

