
Abstract
Aims: We aimed to investigate the rapid induction of therapeutic hypothermia using the ZOLL Proteus Intravascular Temperature Management System in patients with anterior ST-elevation myocardial infarction (STEMI) without cardiac arrest.
Methods and results: A total of 50 patients were randomised; 22 patients (88%; 95% confidence interval [CI]: 69-97%) in the hypothermia group and 23 patients (92%; 95% CI: 74-99) in the control group completed cardiac magnetic resonance imaging at four to six days and 30-day follow-up. Intravascular temperature at coronary guidewire crossing after 20.5 minutes of endovascular cooling decreased to 33.6°C (range 31.9-35.5°C). There was a 17-minute (95% CI: 4.6-29.8 min) cooling-related delay to reperfusion. In “per protocol” analysis, median infarct size/left ventricular mass was 16.7% in the hypothermia group versus 23.8% in the control group (absolute reduction 7.1%, relative reduction 30%; p=0.31) and median left ventricular ejection fraction (LVEF) was 42% in the hypothermia group and 40% in the control group (absolute reduction 2.4%, relative reduction 6%; p=0.36). Except for self-terminating paroxysmal atrial fibrillation (32% versus 8%; p=0.074), there was no excess of adverse events in the hypothermia group.
Conclusions: We rapidly and safely cooled patients with anterior STEMI to 33.6°C at the time of coronary guidewire crossing. This is ≥1.1°C lower than in previous cooling studies. Except for self-terminating atrial fibrillation, there was no excess of adverse events and no clinically important cooling-related delay to reperfusion. A statistically non-significant numerical 7.1% absolute and 30% relative reduction in infarct size warrants a pivotal trial powered for efficacy. ClinicalTrials.gov Identifier: NCT02509832
Abbreviations
BSAS: bedside shivering assessment scale
CI: confidence interval
cMR: cardiac magnetic resonance imaging
EF: ejection fraction
IS: infarct size
LV: left ventricle
PCI: percutaneous coronary intervention
STEMI: ST-elevation myocardial infarction
Introduction
Experimental studies in different animal species have shown that mild hypothermia, induced before reperfusion of acute coronary occlusion, reduces infarct size (IS)1-6. Cooling prior to reperfusion therefore appeared a promising adjunct to primary percutaneous coronary intervention (PCI) in ST-elevation myocardial infarction (STEMI) to reduce IS further and thereby improve clinical outcome7,8. Despite promising experimental evidence, except for RAPID MI-ICE9, randomised clinical trials including COOL MI10, ICE-IT11, CHILL-MI12 and VELOCITY13 have failed to show a significant reduction in IS, although endovascular cooling appeared to be safe and well tolerated. Despite neutral overall results, subsequent unpublished post hoc subgroup analysis of COOL MI and ICE-IT, and combined analysis of RAPID MI-ICE and CHILL-MI14 showed significant reduction in IS in a subgroup of early presenters with anterior STEMI who were cooled below 35°C prior to reperfusion. Accordingly, benefits of therapeutic hypothermia may be achieved in properly selected patients by using a rapid cooling to decrease core temperature sufficiently prior to the opening of the infarct-related artery.
In the present pilot study, we therefore selected patients with anterior STEMI within six hours of symptom onset and tested the new ZOLL® Proteus™ Intravascular Temperature Management System™ (ZOLL Medical Corporation, Chelmsford, MA, USA) (Figure 1), which is, according to technical specifications, significantly more powerful (cooling rate 9.6°C/hour, 430 watts) than the Reprieve™ System (cooling rate 3.3°C/hour, 180 watts) (Radiant Medical, Redwood City, CA, USA) used in COOL MI10 and the InnerCool™ Accutrol™ System (cooling rate 3.6°C/hour, 175 watts) (ZOLL Medical Corporation) used in the ICE-IT11, RAPID MI-ICE9 and CHILL-MI12 trials. The primary goals of our pilot study were to investigate the feasibility and safety of more rapid and profound cooling than in previous trials and to gather information for sample size calculations for a large pivotal trial. Accordingly, this trial was not powered to demonstrate possible reductions in IS by hypothermia.
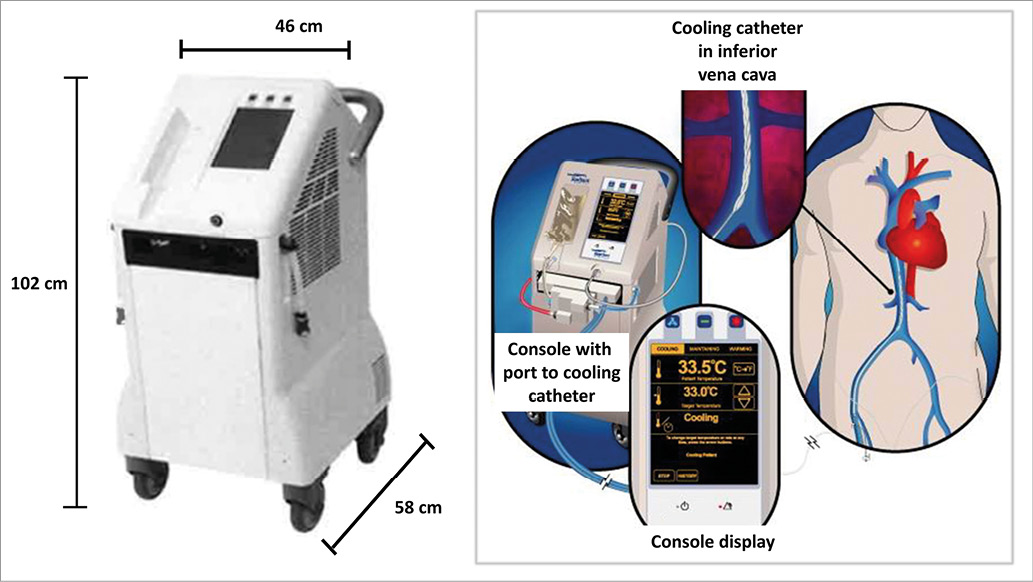
Figure 1. ZOLL Proteus Intravascular Temperature Management System.
Methods
This was a multicentre, prospective, interventional, randomised controlled, two-arm pilot trial performed at 16 sites in eight countries including Zemun, Belgrade, Novi Sad (Serbia), Vienna (Austria), Ljubljana (Slovenia), Budapest, Balatonfured, Pecs, Debrecen and Miskolc (Hungary), Zabrze, Lodz and Warsaw (Poland), Tallinn (Estonia), Basildon (UK) and Lund (Sweden). Each centre had to perform up to four successful roll-in patients before starting to randomise. All procedures were carried out in accordance with the Declaration of Helsinki and the local/national ethics committees approved the study protocol. All patients gave written informed consent prior to inclusion in the study. An independent Data and Safety Monitoring Board, consisting of physicians independent of the trial sponsor and operational leadership, monitored the safety of the study based on access to unblinded data.
PATIENTS
The study enrolled patients ≥18 years of age with a duration of symptoms of ≤6 hrs presenting with an anterior STEMI with persistent ST-segment elevation of >0.2 mV in two contiguous leads at arrival to the catheterisation laboratory and before randomisation. Patients with resuscitated cardiac arrest, previous acute myocardial infarction, PCI or coronary artery bypass grafting, Killip class II-IV at presentation, atrial fibrillation, end-stage kidney disease or hepatic failure, recent stroke, coagulopathy and pregnancy were excluded. Eligible patients were randomised 1:1 using a computer generating system to the hypothermia arm (primary PCI+cooling+standard care) or to the control arm (primary PCI+standard care alone).
All patients received acetylsalicylic acid, heparin and P2Y12 receptor blockade. Glycoprotein IIb/IIIa inhibitors were administered at the discretion of the treating physician.
HYPOTHERMIA PROTOCOL
Patients assigned to the hypothermia group were initially administered 60 mg of oral buspirone and pethidine (meperidine) as an intravenous loading dose of 1 mg/kg (maximum 100 mg) or 0.5 mg/kg if the patient had already received morphine. After 15 minutes, an additional dose of 0.5 mg/kg was given and continued as an infusion at 25 mg/hour (up to 80 kg patient) or 35 mg/hour (>80 kg patient) for the duration of the device deployment. Patients were placed on a Bair Hugger™ (3M, Maplewood, MN, USA) which covered the catheterisation table for skin counter warming. Continuous verbal contact with the patient was maintained. Respiratory rate and pulse oximetry were monitored with targets of >10 breaths/minute and arterial oxygen saturation of ≥90%. Cooling was initiated with a forced infusion of up to 1 L of cold saline (4°C) using pressure bags and continued by the ZOLL Proteus Intravascular Temperature Management System. The cooling catheter was inserted via the femoral vein into the inferior vena cava with the tip positioned at the level of the diaphragm. The Proteus temperature probe (X-Probe; ZOLL) was put through the catheter lumen to the right atrium for continuous measurement of core temperature. The console temperature was set to 32.0°C and cooling at maximum power started. Following placement and activation of the cooling catheter, arterial puncture was performed and coronary angiography/PCI conducted in a standard fashion. An interval of 18 minutes of endovascular cooling from catheter activation to coronary guidewire passing across the acute occlusion was advised. Cooling was maintained for three hours, followed by active rewarming at the rate of 1.0°C/hour to attain 36.0°C. The catheter was then removed. Shivering was continuously assessed by the bedside shivering assessment scale (BSAS) using the following categories:
0 - No shivering on palpation of the masseter, neck or chest wall.
1 - Shivering localised to the neck and/or thorax only.
2 - Shivering with gross movement of the neck, thorax and upper extremities.
3 - Shivering involving gross movements of the trunk, upper and lower extremities.
If BSAS was ≥2, additional boluses of pethidine (25 mg) were used and infusion increased to a maximum of 35 mg/hour. If shivering persisted, the Proteus target temperature was raised stepwise by 0.5°C until shivering disappeared.
CARDIAC MAGNETIC RESONANCE IMAGING
After four to six days, patients underwent cardiac magnetic resonance imaging (cMR) in the supine position. After initial scout images to locate the heart and the standard imaging planes, 0.2 mmol/kg of body weight of an extracellular gadolinium-based contrast agent was administered. For evaluation of left ventricular (LV) function, early contrast-enhanced steady-state free precession (CE-SSFP) cine images were obtained approximately five minutes after contrast injection (slice thickness 8 mm with no slice gap, temporal resolution 20 to 30 frames per cardiac cycle, in-plane resolution 1.5 mm×1.5 mm). For infarct visualisation, late gadolinium enhancement (LGE) images were acquired 15-20 minutes after administration of the contrast agent using an inversion-recovery gradient-echo sequence (slice thickness 8 mm with no slice gap, in-plane resolution 1.5 mm×1.5 mm). Inversion time was manually adjusted to null the signal from viable myocardium, typically 200-300 milliseconds. Cine and LGE images were acquired in the short-axis view, from base to apex, and in the three standard long-axis views (two-chamber, four-chamber and left ventricular outflow tract views). Analyses of cMR images were performed by an independent core lab (Imacor AB, Lund, Sweden) using post-processing software (Segment EWA)15. IS divided by LV mass (IS/LV mass) and LV ejection fraction (EF) were measured.
STUDY ENDPOINTS
The primary endpoint was the proportion of all subjects who completed the follow-up and cMR imaging requirements at 30 days following trial enrolment and randomisation.
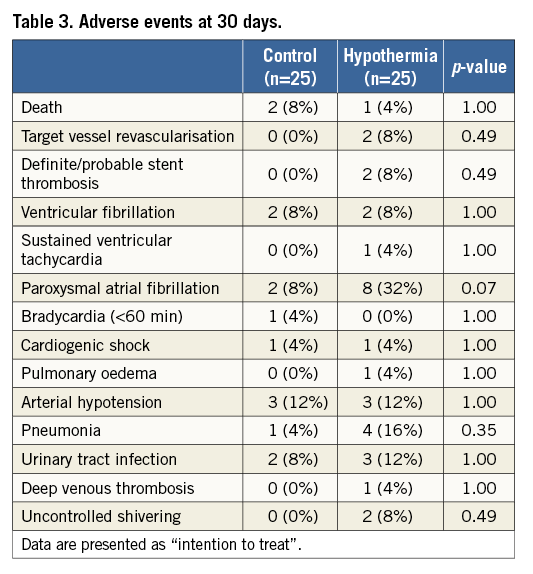
Secondary efficacy endpoints were IS/LV mass and LVEF at day 4-6. Secondary safety endpoints were followed within 30 days (±7 days) after the index procedure and included death, target vessel revascularisation, stent thrombosis, arrhythmias, cardiogenic shock, pulmonary oedema, deep venous thrombosis/pulmonary embolism, vascular complications requiring intervention, cooling catheter access site and systemic infection. Clinical events were collected by adverse event and serious adverse event reporting based on hospital charts reviewed by independent monitors.
STATISTICAL ANALYSIS
In this pilot study, evaluation of retention was calculated with number and proportion with exact 95% confidence interval. For all clinical, angiographic, periprocedural characteristics and safety outcomes, mean, standard deviation, median, range or frequency and proportion were reported. For categorical variables, Fisher’s exact test or the chi-square test was used to compare between the two treatment groups. For continuous variables including IS/LV mass and LVEF, the Wilcoxon rank-sum test and t-test were used to compare between the two treatment groups as appropriate. No imputation was carried out for missing data. All tests were two-sided. A p-value <0.05 was considered statistically significant. All statistical analyses were performed using SAS 9.4 (SAS Institute, Cary, NC, USA).
Results
From May 2016 to February 2017, among 273 screened patients with anterior STEMI, 50 patients were enrolled and randomised to either the cooling group (n=25) or the control group (n=25) (Figure 2). Because of acute respiratory failure and cooling system malfunction, respectively, two patients randomised to hypothermia did not undergo the cooling procedure. Cooling was completed according to protocol in 23 patients and 22 survived to day 30. cMR was performed in 22 patients at day 4-6. In the control group, 23 patients survived to day 30 with cMR performed in 22 patients at day 4-6. Accordingly, 22 patients (88%; 95% confidence interval [CI]: 69-97) in the cooling group and 23 patients (92%; 95% CI: 74-99) in the control group completed cMR at 4-6 days and follow-up at 30 days following enrolment and randomisation. Reasons for not performing cMR were patient discharge (n=1) in the cooling group and death (n=2) in the control group.
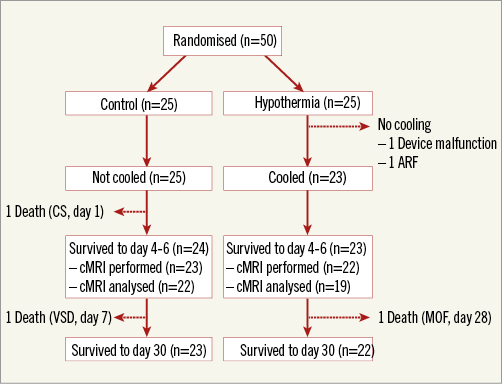
Figure 2. Study flow chart. ARF: acute respiratory failure; cMRI: cardiac magnetic resonance imaging; CS: cardiogenic shock; MOF: multiple organ failure; VSD: ventricular septal defect
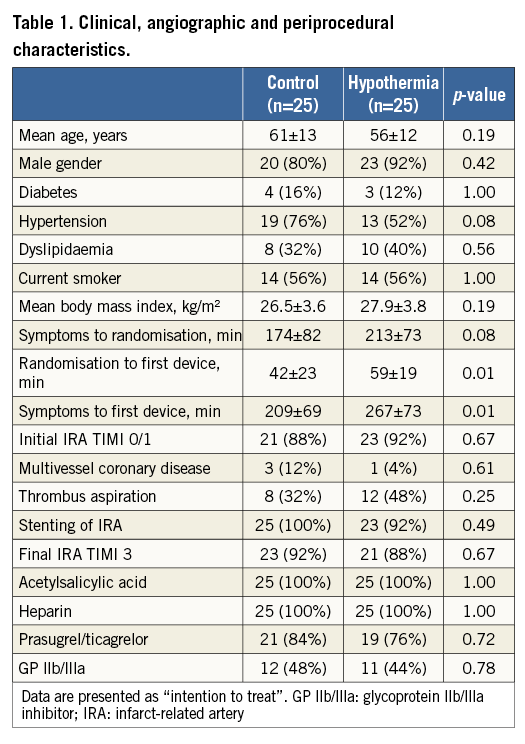
The hypothermia group and the control group were comparable in terms of age, gender, body mass index and risk factors for coronary disease (Table 1). Mean time delay from symptom onset to the first PCI device (balloon or thrombus aspiration catheter) was 267 minutes in the hypothermia group and 209 minutes in the control group (p=0.01). Increased total ischaemic time in the hypothermia group was predominantly caused by longer time from symptom onset to randomisation (213 minutes versus 174 minutes; p=0.08) and not by the delay from randomisation to the first PCI device (59 minutes versus 42 minutes; p=0.01). The cooling-related time delay to reperfusion was 17 minutes (95% CI: 4.6-29.8 minutes). There were no significant differences in angiographic features, primary PCI result or periprocedural medication between the groups.
Anti-shivering medication administered before and during the cooling included morphine, buspirone and pethidine (Table 2). The mean time interval between Proteus system activation and coronary guidewire crossing was 20.5 minutes. At this point, mean intravascular temperature reached 33.6°C (range 31.9-35.5°C). Uncontrolled shivering was documented in two patients (9%). The three-hour cooling phase was followed by gradual rewarming which increased the temperature back to normothermia documented in the control group during the whole study interval (Figure 3).
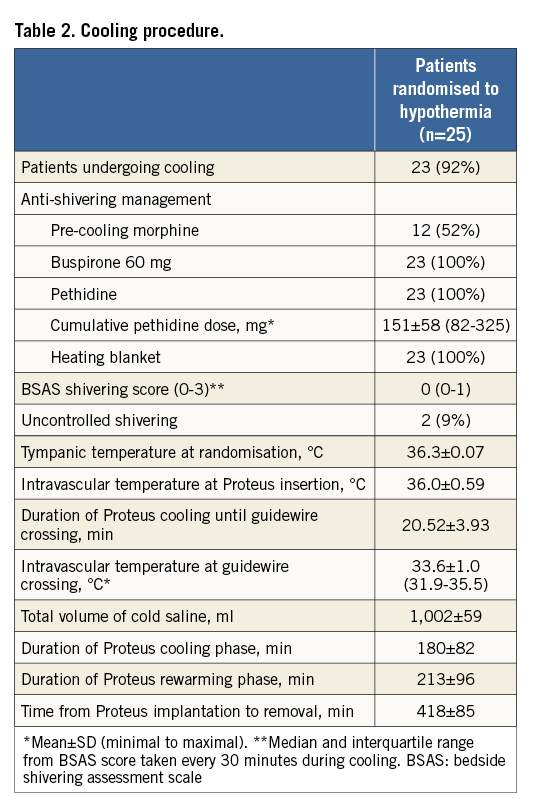
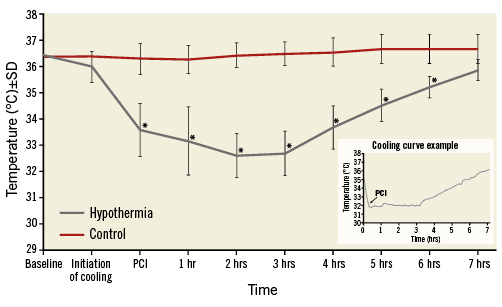
Figure 3. Temperature in hypothermia and control groups at baseline randomisation, initiation of endovascular cooling, and at guidewire crossing, after PCI and during rewarming. Intravascular temperature measured by the Proteus catheter in the right atrium for the hypothermia group, and tympanic temperature for the control group are shown. Representative cooling curve is shown in the right lower corner. *p<0.05 versus control.
cMR at day 4-6 was analysed in 19 of 22 patients in the hypothermia group and 22 of 23 patients in the control group. Reasons for no cMR analysis in the hypothermia group were technically inadequate image (n=2) and stent thrombosis with reinfarction before cMR (n=1) which would have made interpretation of the index IS impossible. In the control group, one image was not readable. The numerical difference in IS/LV mass (Figure 4) and LVEF (Figure 5) in favour of the hypothermia group at day 4-6 was not statistically significant. In “per protocol” analysis, median IS/LV mass was 16.7% in the hypothermia group versus 23.8% in the control group (absolute reduction 7.1%, relative reduction 30%; p=0.31) and median LVEF was 42% in the hypothermia group and 40% in the control group (absolute reduction 2.4%, relative reduction 6%; p=0.36).
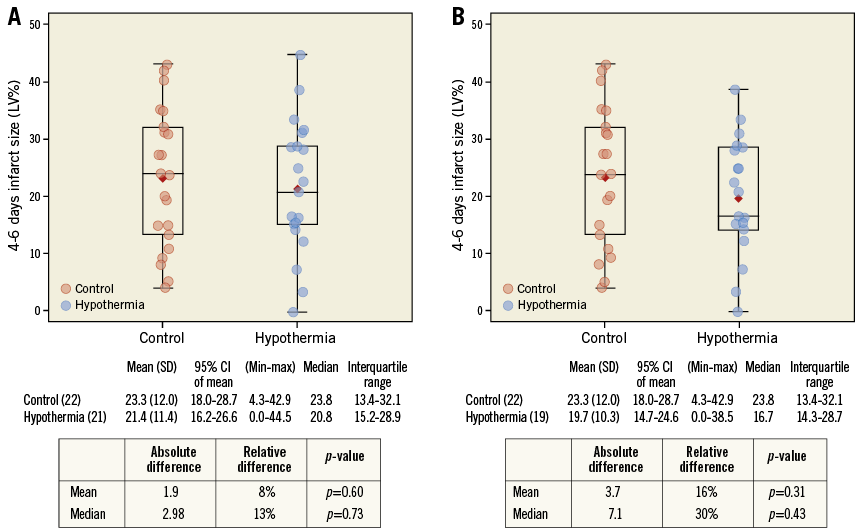
Figure 4. IS/LV mass (%) measured by cardiac magnetic resonance imaging in control and hypothermia patients at day 4-6. A) Intent-to-treat population. B) Per-protocol population.
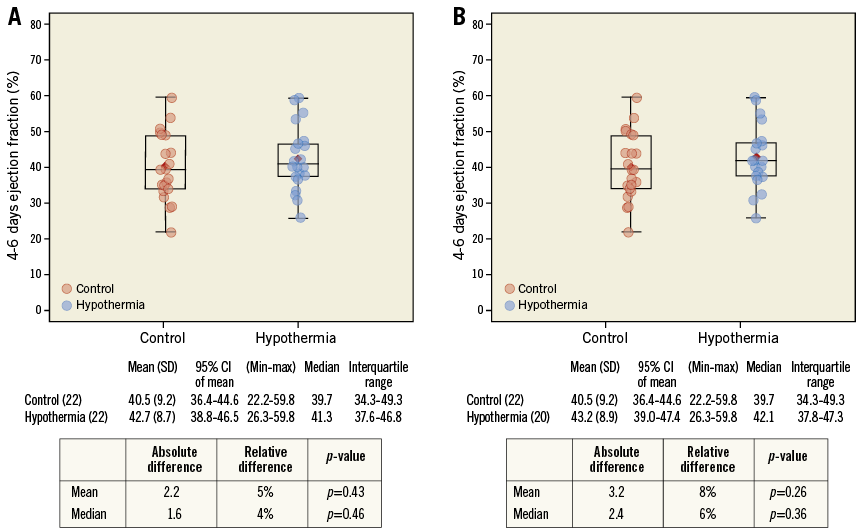
Figure 5. Left ventricular ejection fraction measured by cardiac magnetic resonance imaging in control and hypothermia patients at day 4-6. A) Intent-to-treat population. B) Per-protocol population.
There were no significant differences in adverse events except for a strong trend towards more self-terminating paroxysmal atrial fibrillation, documented in 32% of cooled patients and 8% in the control group (p=0.074) (Table 3). The atrial fibrillation resolved spontaneously to sinus rhythm in all patients during the rewarming phase. In the hypothermia group, two patients (8%; 95% CI: 1.1-28.0) had stent thrombosis. Acute stent thrombosis in one patient was probably related to stent malpositioning. Subacute stent thrombosis on day 5 in another patient might have been associated with clopidogrel resistance documented by platelet testing. There were no vascular complications, significant bleeding or infection related to the access site.
Discussion
In the present pilot study in patients with anterior STEMI, we were able to cool conscious patients to 33.6°C prior to coronary guidewire crossing using the ZOLL Proteus Intravascular Temperature Management System and up to 1 L of concomitant infusion of cold saline. Such a reduction in core temperature, achieved within approximately 20 minutes from endovascular catheter activation and with 17 minutes of cooling-related delay to reperfusion, is at least 1.1°C lower than documented in recent cooling trials including RAPID-MI ICE9, CHILL-MI12 and VELOCITY13.
Our cooling protocol with anti-shivering medication and skin counter warming appeared to be safe although the number of patients in the study is too small to draw a definite conclusion. The incidence of ventricular fibrillation and sustained ventricular tachycardia was comparable in both groups, while paroxysmal atrial fibrillation tended to be more frequent in the cooling group. Although an excess of atrial fibrillation has not been reported in any of the previous cooling studies, it might have been related to more rapid and profound cooling. However, atrial fibrillation appeared to be a short-lasting and self-terminating phenomenon requiring no additional treatment. We further documented two patients with stent thrombosis in the cooling group which might have been related to a suboptimal angiographic result or clopidogrel resistance, or might have been a random effect due to the small number of patients in our study. Of note, an excess of stent thrombosis has never been reported in any of the previous endovascular cooling studies9-12. However, core temperature in our study was ≥1.1°C lower and this might have increased platelet aggregation16,17. Furthermore, resorption and metabolism of clopidogrel, used in 24% of our hypothermia group, is slower in cooled and sedated patients18. Our observation may therefore point to possible prothrombotic conditions if intravascular temperature is so quickly and strikingly reduced. It also underscores the importance of selection of the least thrombogenic contemporary drug-eluting stents, optimal deployment and utilisation of novel P2Y12 inhibitors rather than clopidogrel in the planned pivotal study. Although shivering did not appear to be a significant issue, we observed two patients (9%) with uncontrolled shivering, indicating room for further improvement in anti-shivering medication to increase the comfort of patients.
Although not statistically significant, a numerical reduction in median IS/LV mass (7.1% absolute and 30% relative) was documented in the hypothermia group compared to controls in the per protocol population, warranting further evaluation for efficacy in a fully powered pivotal trial. Of note, a 5.1% absolute reduction in IS in the “Stent Versus Thrombolysis for Occluded Coronary Arteries in Patients With Acute Myocardial Infarction” trial is generally accepted as a clinically meaningful result for cardioprotection trials19. Moreover, IS of >29.8% was shown to be significantly correlated with increased mortality or heart failure hospitalisation within one year20. In our study, 16% of the patients in the hypothermia group had an IS >29.8% compared to 41% of patients in the control group (p=0.16).
Limitations
Our results on IS reduction, however, should be interpreted in the light of several limitations. Firstly, the study was obviously not powered to detect significant difference in IS/LV mass and LVEF. Based on the results of this pilot study, assuming a standard deviation of 12%, a two-tailed t-test of difference between the means, and a normal distribution, the sample size required is 372 patients (186 in each arm) for 80% power and 5% level of significance to detect a 20% relative (3.5% absolute) reduction in IS/LV mass. With a further assumption of up to 20% loss in cMR and follow-up, the pivotal trial would need to enrol 468 patients (234 in each arm). Secondly, the total ischaemic time was 60 minutes longer in cooled patients, mainly driven by longer time to randomisation because the cooling-related delay was only 17 minutes. This unfortunate imbalance, representing a significant bias against hypothermia, may happen in small trials such as ours despite randomisation. Thirdly, total ischaemic time in our cooled patients (260 minutes) was also significantly longer than in CHILL-MI (132 minutes)12, VELOCITY (172 minutes)13, RAPID MI ICE (174 minutes)9 and COOL MI (205 minutes)10, which again may represent a bias against hypothermia. Pooled analysis of CHILL-MI and RAPID MI ICE demonstrated that hypothermia before reperfusion has significantly better effectiveness in early presenters14. Last but not least, our study is lacking core laboratory measurements of cardiac troponin, which would represent a cMR-independent estimate of IS. These are all important lessons learned for the planned pivotal study.
Conclusions
Using the ZOLL Proteus Intravascular Temperature Management System and up to 1 L of cold saline, we were able to cool conscious patients with anterior STEMI to 33.6°C at the time of coronary guidewire crossing, which was ≥1.1°C lower than in previous cooling studies. Such rapid cooling resulted in 17 minutes of cooling-related delay to reperfusion. Our cooling protocol, including anti-shivering medication and skin counter warming, appeared to be safe and well tolerated. Although this pilot trial was underpowered, the improved delivery of hypothermia resulted in a numerical reduction of IS, which supports a further pivotal study to evaluate efficacy in a larger population.
| Impact on daily practice This pilot trial, which demonstrated that we can cool conscious patients with anterior STEMI to 33.6°C at the time of coronary guidewire crossing which is ≥1.1°C lower than in previous cooling trials, opens the door for an adequately powered pivotal trial with the ZOLL Proteus Intravascular Temperature Management System aimed at demonstrating a clinically relevant reduction in IS by inducing therapeutic hypothermia prior to reperfusion by primary PCI. |
Acknowledgements
Site investigators of the COOL AMI EU pilot trial were Dragan Petrovic, Ivan Ilic, Milivoje Cerovic, Dusan Milicevic, Predrag Milicevic, Milos Panic (Clinical Hospital Center Zemun, Belgrade, Serbia), István Édes, Krisztina Heltai, Árpád Lux, Timea Szigethi, Levente Molnár (Heart and Vascular Center, Semmelweis University, Budapest, Hungary), Ursa Mikuz, Pavel Berden, Peter Radsel, Miha Cercek, Matjaz Bunc (University Medical Center Ljubljana, Ljubljana, Slovenia), Milana Jarakovic, Milos Trajkovic, Mila Kovacevic, Ilija Srdanovic, Snezana Bjelic (Institute of Cardiovascular Diseases Vojvodina, Sremska Kamenica, Serbia), József Faluközy, Döme Dézsi, György Fogarassy, Csaba Bujáky (Heart Center Balatonfüred, Balatonfüred, Hungary), Toomas Marandi, Urmet Arus, Tuuli Teeäär, Boris Lapidus, Andrei Šamarin, Ruth Brandt (North-Estonia Medical Centre, Tallinn, Estonia), Milorad Zivkovic, Ana Uscumlic, Miodrag Dikic, Srdjan Aleksandric, Dejan Milasinovic, Milorad Tesic (Clinical Center of Serbia, Belgrade, Serbia), Paweł Francuz, Jan Kłyś, Jacek Kowalczyk, Karol Przyłudzki (Silesian Center for Heart Diseases, Zabrze, Poland), Alexander Nürnberger, Irene Lang, Stefan Stojković (Medical University of Vienna, Vienna, Austria), Grigorius Karamasis, Firas Al-Janabi, Shahed Islam (Essex Cardiothoracic Centre, Basildon and Anglia Ruskin University, Chelmsford, UK), Matthias Götberg, Lotta Cinthio (Department of Cardiology, Lund University, Lund, Sweden), Marcin Ojrzanowski (Medical University in Łódź, Łódź, Poland), Milosz Marona, Adam Witkowski, Janina Stepinska, Marek Banaszewski, Jerzy Pregowski, Malgorzata Celinska-Spodar (Institute of Cardiology, Warsaw, Poland), Balint Kittka, András Komócsi, Attila Kónyi, Balázs Magyari, Tünde Pintér, Mihály Simon, Brigitta Németh (Heart Institute - University of Pecs, Pecs, Hungary), Laszlo Fulop, Bertalan Kracsko, Edina Nagy-Balo, Laszlo Balogh, Ferenc Gyory, Annamaria Bodi (University of Debrecen, Debrecen, Hungary), Erika Csengo, Zsolt Ondrejko, Timea Uveges (1st Department of Internal Medicine and Cardiology, Miskolc, Hungary).
The authors would like to acknowledge the professional contribution of the Data Monitoring Committee including Graham Nichol (University of Washington-Harborview Center for Prehospital Emergency Care, Seattle, WA, USA), Marcus Ferrari (Helios Medical Center Wiesbaden, Wiesbaden, Germany), Stefano De Servi (Multimedica Hospital Sesto San Giovanni, Sesto San Giovanni, Italy) and Tim Collier (London School of Hygiene & Tropical Medicine, London, UK).
The authors would also like to thank the ZOLL team (Renee Kochevar, Michael Dae and Olivia Wilburn) for their support, and Anne Dee for statistical analysis.
Funding
The study was funded by ZOLL Circulation.
Conflict of interest statement
M. Noc, B. Średniawa, D. Aradi and M. Holzer have received consultation fees from ZOLL. The other authors have no conflicts of interest to declare.

