Abstract
Aims: Prior evaluations of endovascular cooling during primary percutaneous coronary intervention (PCI) for acute myocardial infarction (AMI) have suggested variability in treatment effect related to core temperature at the time of reperfusion, to infarct location and time from symptom onset to reperfusion. Recent results from a randomised feasibility study suggest rapid induction of hypothermia in primary PCI results in a significant reduction in infarct size (IS).
Methods and results: Outcomes from two randomised trials of hypothermia in primary PCI were pooled to examine IS as a percentage of left ventricular myocardium assessed by SPECT or magnetic resonance imaging. Compared with controls (n=103), hypothermia (n=94) was associated with a significant 24% relative reduction (RR) in IS (10.7±1.3% vs. 14.1±1.6%, mean±SEM, p=0.049). Among hypothermia-treated patients for whom core temperature <35C° was achieved before reperfusion, IS was reduced by 37% (8.8±1.7% vs. 14.1±1.6%, p=0.01), a benefit observed for both anterior (14.9±2.9% vs. 22.2±2.7%, RR 33%; p=0.03) and inferior infarcts (4.5±1.4% vs. 7.7±1.3%, RR 42%; p=0.04).
Conclusions: In a pooled analysis of randomised trials evaluating adjunctive hypothermia in primary PCI, achievement of core body temperature <35°C before reperfusion may reduce infarct size with a similar efficacy for both anterior and inferior MI.
Introduction
Current treatment of patients with acute ST-elevation myocardial infarction (STEMI) is to reperfuse the ischaemic myocardium with primary percutaneous coronary intervention (PCI) as soon as possible to reduce infarct size and associated complications. Infarct size is one of the main predictors of both short-term and long-term outcome in patients with acute myocardial infarction (AMI)1,2. Although reperfusion therapy is a prerequisite for myocardial salvage, the process itself may cause irreversible damage to the myocardium, referred to as reperfusion injury3-5. Experimental studies have shown that mild hypothermia (<35°C), induced before reperfusion of acute coronary occlusion, reduces infarct size and limits microvascular injury6-12. However, in animal experiments hypothermia has failed to reduce infarct size if initiated after the onset of reperfusion12,13.
Two clinical trials investigating mild hypothermia using endovascular cooling catheters alone as an adjunct therapy in AMI failed to show a reduction in infarct size14-16. Post hoc analysis of the data in those trials has shown that only a minority of patients were hypothermic (<35°C) at onset of reperfusion. However, a subgroup analysis of the two trials showed strong trends towards a positive effect in patients who did reach a temperature <35°C. In the safety and feasibility study RAPID MI-ICE, hypothermia was induced more rapidly by a combination of a rapid infusion together with endovascular cooling as an adjunctive therapy in acute myocardial infarction17. The trial demonstrated that rapidly induced hypothermia was feasible and safe, and that the secondary endpoint of reduction of infarct size was significantly reduced by 38%17. In designing the efficacy trial, Rapid Endovascular Catheter Core Cooling combined with cold saline as an Adjunct to Percutaneous Coronary Intervention For the Treatment of Acute Myocardial Infarction (CHILL-MI), with the primary endpoint of reduced infarct size, three main questions were raised: 1) Is a core body temperature of <35°C before reperfusion necessary? 2) Is there a difference in the effect of hypothermia between anterior and inferior infarcts? 3) Is there a difference in the effect of hypothermia between infarcts with long compared to short duration of symptoms prior to reperfusion time? To guide the design of the trial we performed a pooled post hoc analysis of the two clinical trials, ICE-IT and RAPID MI-ICE, which used the same endovascular cooling catheter that will be used in the CHILL-MI study (InnerCool RTx; Philips Healthcare, Best, The Netherlands). To answer these specific questions regarding the effect of hypothermia on infarct size, only patients treated per protocol and who had undergone infarct size determination with SPECT or magnetic resonance imaging (MRI) were included for analysis.
Methods
STUDY POPULATION
The CHILL-MI trial will use a specific endovascular cooling system (InnerCool RTx; Philips Healthcare). Analysis was performed, therefore, on patients included in two trials – the Intravascular Cooling Adjunctive to Percutaneous Coronary Intervention (ICE-IT)15 trial and the Rapid Intravascular Cooling in Myocardial Infarction as Adjunctive to Percutaneous Coronary Intervention study (RAPID-MI-ICE, ClinicalTrials.gov Identifier: NCT00417638) - for whom the same endovascular cooling system was used. Raw data from the ICE-IT trial was obtained from Philips Healthcare, Andover, MA, USA. Statistical analysis was performed at the Department of Cardiology, Lund University, Lund, Sweden.
For initial inclusion and exclusion criteria and study protocols we refer to the studies14,15,17. The ICE-IT trial included patients with STEMI of less than six-hour duration and induced hypothermia with endovascular cooling maintained for six hours with three hours gradual rewarming. Infarct size was measured with SPECT at 30 days. No significant difference was observed between the groups but a trend towards reduced infarction was seen in patients cooled to less than 35 degrees Celsius before reperfusion, especially in patients with anterior infarctions.
The RAPID MI-ICE trial also studied patients with STEMI of less than six-hour duration, but induced hypothermia with a combination of cold saline and endovascular cooling. Hypothermia was maintained for three hours with three hours gradual rewarming. Infarct size relative to myocardium at risk was significantly reduced.
Infarct size as a percentage of the left ventricular myocardium was chosen to be the combined endpoint for this pooled analysis. Infarct size was stratified by infarct location (inferior vs. anterior) and with respect to the time interval between onset of symptoms and hospital presentation (<4 hours vs. 4-6 hours).
In both studies the patients assigned to the hypothermia group were given 30 mg of oral buspirone. Meperidine was given as an intravenous loading dose of 1 mg/kg. The loading dose was reduced to 0.5 mg/kg if the patient had been given morphine before enrolment in the study. The loading dose was followed by a continuous infusion of meperidine at 30 mg/h. Additional 25 mg intravenous bolus doses of meperidine were given if the patient started to shiver. As regards other medication they were well treated and received standard treatment with aspirin, clopidogrel, GP IIb/IIIa blockers or bivalirudin, ACE inhibitors, beta-blockers and statins.
Infarct size was evaluated with SPECT at day 30 in ICE-IT and with MRI after 4±2 days in RAPID MI-ICE. Patients for whom infarct size was not determined were not included in the analysis. Additionally, patients with a prior MI and/or repeat MI prior to SPECT were excluded. Furthermore, patients were excluded if cooling treatment was intended but never initiated, if PCI was not performed, or if the onset of symptoms to hospital presentation exceeded six hours.
The ICE-IT study included 228 patients and RAPID MI-ICE included 20 patients. Infarct size was evaluated in 204 patients in the ICE-IT study and in 18 patients in the RAPID MI-ICE study. For the pooled analysis 25 patients in the ICE-IT study were excluded according to the criteria above. Thus, in the final analysis, 179 patients from ICE-IT and 18 patients from RAPID MI-ICE were examined, giving a total of 197 patients.
STATISTICS
All statistical analyses are post hoc secondary analyses on a pooled set of data. Any significant differences should be considered hypothesis-generating and not conclusive. Calculations and statistics were performed using the GraphPad Prism 5.0 software (GraphPad Software Inc., La Jolla, CA, USA). Continuous variables were tested using Student’s t-test. Statistical significance was accepted when p<0.05.
Results
OVERALL POOLED RESULTS
In all the pooled data from ICE-IT and RAPID MI-ICE (n=103 control vs. 94 hypothermia), hypothermia resulted in a significant reduction in infarct size as a percentage of the left ventricular myocardium by 24%, p<0.049 (Figure 1A). In patients with anterior infarctions (n=45 control vs. 41 hypothermia), hypothermia resulted in a significant reduction in infarct size by 30%, p=0.04 (Figure 1B). However, in patients with inferior infarctions (n=58 control vs. 53 hypothermia), no significant effect relative to infarct size was observed (Figure 1C).
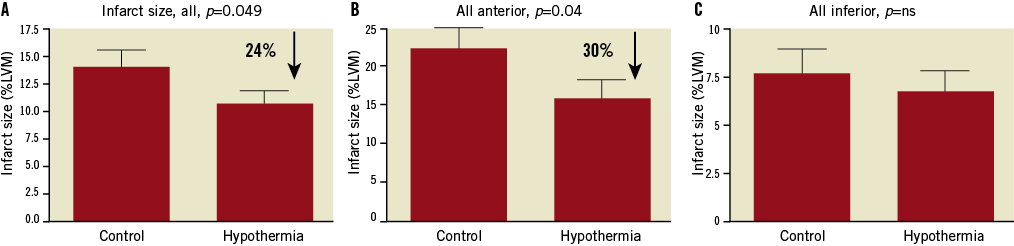
Figure 1. Infarct size as a percentage of the left ventricular myocardium. A) In all patients (both anterior and inferior infarctions) regardless of temperature at reperfusion (n=103 control vs. 94 hypothermia). B) In all patients with anterior infarctions regardless of temperature at reperfusion (n=45 control vs. 41 hypothermia). C) In all patients with inferior infarctions regardless of temperature at reperfusion (n=58 control vs. 53 hypothermia). LVM: left ventricular myocardium; ns: not significant
RESULTS IN PATIENTS <35°C VERSUS ≥35°C
In hypothermia-treated patients who reached <35°C before reperfusion, infarct size was reduced by 37%, p=0.01 (n=103 control vs. 36 hypothermia, Figure 2A). In patients who reached <35°C before reperfusion, the infarct size was significantly reduced for both anterior and inferior infarcts, by 33% (p=0.03, n=45 control vs. 15 hypothermia), and 42% (p=0.04, n=58 control vs. 21 hypothermia),
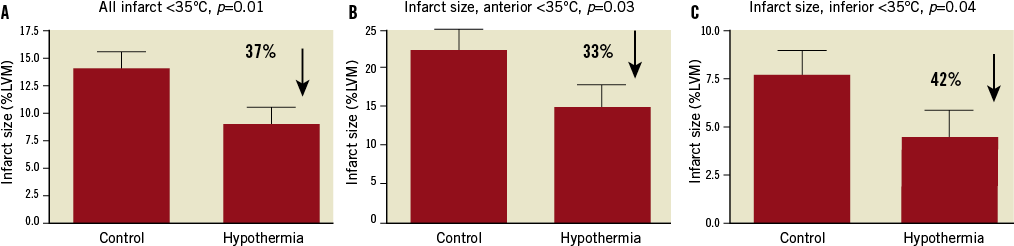
Figure 2. Infarct size as a percentage of the left ventricular myocardium. A) In all hypothermia-treated patients who reached <35°C before reperfusion compared with all control patients (n=103 control vs. 36 hypothermia). B) In patients with anterior infarctions (n=45 control vs. 15 hypothermia). C) In patients with inferior infarctions (n=58 control vs. 21 hypothermia). LVM: left ventricular myocardium
respectively (Figure 2A and Figure 2B).
In hypothermia-treated patients who did not reach <35°C before reperfusion, no significant reduction in infarct size was seen either in the whole group or in anterior or inferior groups separately (n=103 control vs. 58 hypothermia, Figure 3).
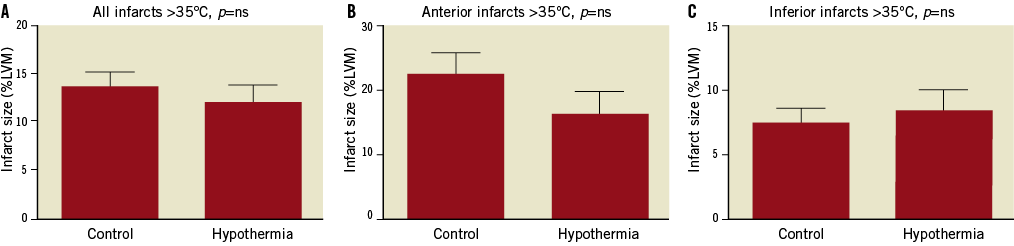
Figure 3. Infarct size as a percentage of the left ventricular myocardium. A) In all hypothermia-treated patients who did not reach <35°C before reperfusion compared with all control patients (n=103 control vs. 58 hypothermia). B) In patients with anterior infarctions (n=45 control vs. 26 hypothermia). C) In patients with inferior infarctions (n=58 control vs. 32 hypothermia). LVM: left ventricular myocardium; ns: not significant
In hypothermia-treated patients who reached <35°C before reperfusion with onset of symptoms to hospital presentation of less than four hours, infarct size was nonsignificantly reduced by 19%, p=0.15, (n=70 control vs. 28 hypothermia) (Figure 4A). In the 4-6 hours cohort, infarct size was non-significantly reduced by 33%, p=0.07 (n=33 control vs. 8 hypothermia) (Figure 4B).
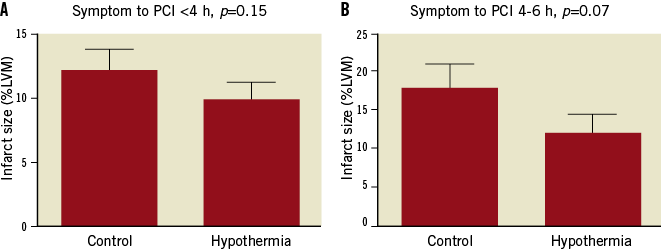
Figure 4. Infarct size as a percentage of the left ventricular myocardium in hypothermia-treated patients who reached <35°C before reperfusion compared with all control patients. Divided into those with a symptoms-to-hospital presentation of: A) less than 4 hours (n=70 control vs. 28 hypothermia); or B) 4-6 hours (n=33 control vs. 8 hypothermia). LVM: left ventricular myocardium; PCI: percutaneous coronary intervention
Discussion
This post hoc analysis of pooled data from ICE-IT and RAPID MI-ICE suggests that achieving a temperature of <35°C before reperfusion may reduce infarct size in patients with STEMI treated with primary PCI. The effect seems to be similar in anterior and inferior STEMI, and in long and short durations of ischaemia. It should be emphasised that this secondary analysis is hypothesis-generating; it is intended to help design an efficacy trial to study whether rapidly induced hypothermia prior to reperfusion in STEMI patients reduces infarct size.
The crucial determinant for success in the reduction of infarct size with hypothermia in STEMI patients seems to be the induction of hypothermia to <35°C before the actual reperfusion takes place. This was suggested in the post hoc analysis of both the COOL-MI and the ICE-IT trials14-16, and is consistent with animal experiments that showed failure to reduce infarct size if hypothermia is initiated after the onset of reperfusion, but success if <35°C is achieved before reperfusion12,13.
The present pooled analysis of patients in the ICE-IT and RAPID MI-ICE trials supports the goal of therapeutic hypothermia to below 35°C at the time of reperfusion in order to reduce infarct size significantly. Conversely, patients who did not achieve a core body temperature of <35°C at reperfusion did not have any significant effect of hypothermia, while patients who did reach that goal prior to reperfusion did have significant reduction in infarct size. Our conclusion, supported by animal data, is that it is crucial for future hypothermia trials to achieve a rapidly induced hypothermia to less than 35°C before reperfusion of the infarct-related artery, and this needs to be performed without significant delay in the “door-to-balloon-time” interval for patients treated with hypothermia.
A commonly raised question after failed cardioprotection trials is whether the effect could have been detected in the subgroup of anterior STEMI patients only18,19. The argument is that these infarctions are larger and more consistent in size. A study that includes a mixture of inferior and anterior infarcts without determination of myocardium at risk will involve larger variability and thus a greater standard deviation in infarct size. This will, in turn, necessitate a larger study to achieve the statistical power required to detect a difference between the groups. The current pooled analysis indicates that hypothermia may have an effect regardless of infarct location. Specifically, the study shows that both anterior and large inferior STEMI patients should be included in a future trial.
The ischaemia time or time of duration of symptoms to reperfusion may be an important determinant of the effect of cardioprotective therapies. A very short duration gives no infarction or a very small one. After a longer duration of ischaemia, the injury becomes established as a transmural infarction eventually consisting of 100% of the myocardium at risk. The time to reach 50% infarction of the myocardium at risk in man is approximately five hours20. Theoretically, an ischaemic time of <5 hours would thus be an optimal time period to evaluate the efficacy of a possible cardioprotective agent or therapy, such as hypothermia, compared to controls. Previous animal studies have demonstrated that hypothermia reduced infarct size in rats undergoing relatively short coronary artery occlusions (<45 min), but was lost when occlusion duration exceeded 45 min21. In our pooled data analysis, we therefore included only patients with less than six hours of ischaemia time to highlight the effect of hypothermia treatment. To be able to detect the impact of the ischaemia time we divided the STEMI subjects into those with less than 4-hour duration compared to those with 4 to 6-hour duration because this allowed fairly equal-sized groups for comparison. Although not significant, both groups showed a trend towards benefit.
Limitations
Although no differences existed in standard medication the hypothermia group received buspirone and meperidine. It is possible but not likely that these medications may have contributed to the cardioprotective effect.
All statistical analyses in this study are post hoc secondary analyses on a pooled set of data. Any significant difference should be considered hypothesis-generating and not conclusive. In this pooled analysis only the per protocol study population was analysed. Patients who did not receive catheter placement or treatment with PCI were excluded. In the ICE-IT design, patients who died were represented in infarct size with imputed values similar to the largest infarct of their infarct type (anterior or inferior). This was not performed in the pooled analysis.
These are exclusions that cannot be used in an intention-to-treat analysis but we felt justified in using them in this limited study in which we wanted to assess the possibly beneficial effect of hypothermia on patients who were treated with PCI, who were randomised to hypothermia, and for whom the infarct size was determined.
There are other randomised clinical trials of hypothermia for the treatment of STEMI: a pilot trial by Dixon and co-workers22, the COOL-MI trial15 and the pre-terminated COOL-MI 2 trial (results not yet reported). These trials were performed with another endovascular cooling system that will not be used in the CHILL-MI trial. These trials were therefore not included in the analysis.
Another limitation is that infarct size was determined with different methods (SPECT vs. cardiac MRI) and different time points (30 vs. 4 days) after infarction. However, the times were similar for the hypothermia and control groups in both studies.
Conclusions
Despite the limitations we feel that this pooled analysis could be helpful in the design of future trials of hypothermia as a treatment for acute myocardial infarction. The results indicate that reaching a temperature of less than 35°C before reperfusion is of paramount importance to reduce infarct size in the treatment of STEMI patients. The analysis suggests that hypothermia is of similar benefit in anterior and inferior infarcts. Furthermore, hypothermia seems to be beneficial up to six hours from onset of symptom to hospital presentation. Based on this, we have designed and recently started CHILL-MI (ClinicalTrials.gov identifier: NCT01379261) as a trial with endovascular cooling combined with rapid cold saline infusion including both anterior and large inferior STEMI of up to six hours of symptoms prior to inclusion. The trial will include 120 patients at 10 sites and measure myocardium at risk and infarct size at four days and six months with cardiac MRI. Hypothermia will be maintained for one hour after reperfusion.
Funding
This work was supported by the Swedish Research Council (VR 521-2009-2276), the Swedish Heart and Lung Foundation, and the Vascular Wall program (Lund University Faculty of Medicine).
Conflict of interest statement
D. Erlinge, M. Götberg and G.K. Olivecrona have received endovascular cooling catheters and funding for MRI exams in RAPID MI-ICE by way of a grant from InnerCool Therapies, Philips Healthcare. D. Kandzari has received consulting honoraria (minor) from Philips. The other authors have no conflicts of interest to declare.

