Abstract
Background: Despite primary PCI (PPCI), ST-elevation myocardial infarction (STEMI) can still result in large infarct size (IS). New technology with rapid intravascular cooling showed positive signals for reduction in IS in anterior STEMI.
Aims: We investigated the effectiveness and safety of rapid systemic intravascular hypothermia as an adjunct to PPCI in conscious patients, with anterior STEMI, without cardiac arrest.
Methods: Hypothermia was induced using the ZOLL® Proteus™ intravascular cooling system. After randomisation of 111 patients, 58 to hypothermia and 53 to control groups, the study was prematurely discontinued by the sponsor due to inconsistent patient logistics between the groups resulting in significantly longer total ischaemic delay in the hypothermia group (232 vs 188 minutes; p<0.001).
Results: There were no differences in angiographic features and PPCI result between the groups. Intravascular temperature at wire crossing was 33.3+0.9°C. Infarct size/left ventricular (IS/LV) mass by cardiac magnetic resonance (CMR) at day 4-6 was 21.3% in the hypothermia group and 20.0% in the control group (p=0.540). Major adverse cardiac events at 30 days increased non-significantly in the hypothermia group (8.6% vs 1.9%; p=0.117) while cardiogenic shock (10.3% vs 0%; p=0.028) and paroxysmal atrial fibrillation (43.1% vs 3.8%; p<0.001) were significantly more frequent in the hypothermia group.
Conclusions: The ZOLL Proteus intravascular cooling system reduced temperature to 33.3°C before PPCI in patients with anterior STEMI. Due to inconsistent patient logistics between the groups, this hypothermia protocol resulted in a longer ischaemic delay, did not reduce IS/LV mass and was associated with increased adverse events.
Introduction
Experimental studies in different animal species have consistently shown that mild hypothermia, induced prior to reperfusion of acute coronary occlusion, reduces infarct size (IS)1,2,3,4,5,6. Cooling before reperfusion therefore appeared to be a promising adjunct to primary percutaneous coronary intervention (PPCI) in ST-elevation myocardial infarction (STEMI)7,8. Several randomised clinical trials7,8,9,10,11 showed mixed results, although intravascular cooling appeared to be safe and well tolerated. Subsequent combined analysis of RAPID MI-ICE and CHILL MI12 revealed significant reduction in IS in a subgroup of early presenters with anterior STEMI who were cooled below 35°C prior to reperfusion. A similar favourable signal was also observed in the COOL AMI pilot randomised trial, which enrolled 50 patients with anterior STEMI13. With up to 1,000 ml intravenous infusion of cold saline using the ZOLL® Proteus™ intravascular cooling system (ZOLL Medical, Chelmsford, MA, USA), patients were safely cooled to 33.6°C at the time of coronary guidewire crossing, which resulted in numerical reduction in IS from 23.8% to 16.7% (p=0.31). We therefore performed the COOL AMI EU Pivotal Trial powered to demonstrate a clinically meaningful reduction in IS using rapid intravascular systemic hypothermia prior to PPCI compared to standard treatment with PPCI only.
Methods
The COOL AMI EU Pivotal Trial (NCT03173313) was a multicentre, prospective, interventional, randomised controlled two-arm pivotal trial performed at 24 sites in 11 countries. Each centre had to perform up to four successful roll-in patients before starting to randomise. All procedures were carried out in accordance with the Declaration of Helsinki and the local/national ethics committees approved the study protocol. All patients gave written informed consent prior to inclusion in the study. An independent clinical events committee (Icahn School of Medicine at Mount Sinai, New York, NY, USA) adjudicated major adverse cardiac events (MACE). An independent data monitoring committee (DMC), consisting of three physicians and one statistician independent of the trial sponsor and operational leadership, monitored the safety of the study based on access to unblinded data. An advisory board comprised the study principal investigator and the most experienced study investigators.
The study enrolled conscious patients >18 years of age with <4.5 hours duration of symptoms presenting with an anterior STEMI with persistent ST-segment elevation of >0.2 mV in two contiguous leads. Patients with resuscitated cardiac arrest, previous acute myocardial infarction, PCI or coronary artery bypass grafting, Killip class II-IV, atrial fibrillation, end-stage kidney disease, hepatic failure, recent stroke, coagulopathy and pregnancy were excluded. Eligible patients were randomised 1:1 using a computer-generated system to hypothermia (hypothermia + PPCI + standard care) or to control (PPCI + standard care) groups. All patients received acetylsalicylic acid, heparin and a P2Y12 inhibitor. Glycoprotein IIb/IIIa inhibitors were administered at the discretion of the treating physician.
Patients assigned to hypothermia initially received 60 mg of oral buspirone and pethidine (meperidine) as an intravenous loading dose of 1 mg/kg (maximum 100 mg) or 0.5 mg/kg if the patient had already received morphine. After 15 minutes, an additional dose of 0.5 mg/kg pethidine was given and continued as an infusion at 25 mg/hour (<80 kg body weight) or 35 mg/hour (>80 kg body weight) for the duration of the device deployment. Patients were placed on a Bair Hugger™ (3M Medical, St. Paul, MN, USA) which covered the catheterisation table for skin counter-warming. Continuous verbal contact with the patient was maintained. Respiratory rate and pulse oximetry were monitored with targets of >10 breaths/minute and arterial oxygen saturation of >90%. Hypothermia was initiated with a forced intravenous infusion of up to 1 L of cold saline (4°C) using pressure bags and continued by intravascular cooling with the ZOLL Proteus cooling system (Figure 1). A cooling catheter was inserted via the femoral vein into the inferior vena cava with the tip positioned at the level of the diaphragm. A Proteus temperature probe was put through the catheter lumen into the right atrium for continuous measurement of blood temperature. The console temperature was set to 32.0°C and cooling at maximum power started. An interval of 18 minutes of endovascular cooling from catheter activation to coronary guidewire passing across the acute occlusion was advised. Following placement and activation of the cooling catheter, arterial puncture, coronary angiography, administration of anticoagulation/antiplatelet therapy and PPCI were performed. Cooling was maintained up to three hours followed by active rewarming at the rate of 1.0°C/hour to attain 36.0°C. The catheter was then removed.
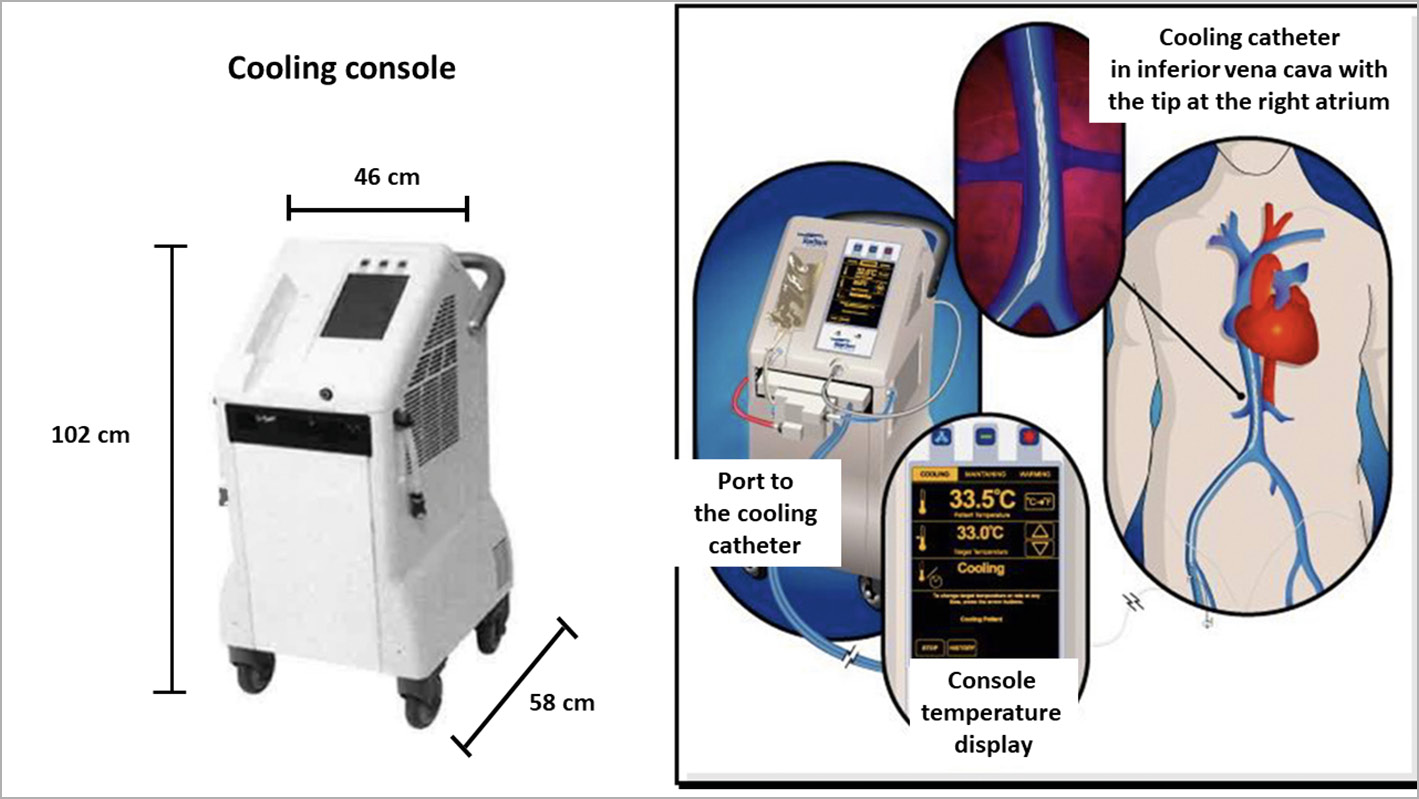
Figure 1. ZOLL Proteus intravascular cooling system.
Shivering was continuously assessed using the bedside shivering assessment scale (BSAS) with the following categories:
0 – No shivering on palpation of the masseter, neck or chest wall
1 – Shivering localised to the neck and/or thorax only
2 – Shivering with gross movement of the neck, thorax and upper extremities
3 – Shivering involving gross movements of the trunk, upper and lower extremities.
If BSAS was >2, additional boluses of pethidine (25 mg) were used and infusion increased to a maximum of 35 mg/hour. If shivering persisted, the device target temperature was raised stepwise by 0.5°C until shivering disappeared.
After 4-6 days, patients underwent cardiac magnetic resonance imaging (CMR) in the supine position. After initial scout images to locate the heart and the standard imaging planes, 0.2 mmol/kg of body weight of an extracellular gadolinium-based contrast agent was administered. For evaluation of left ventricular (LV) function, early contrast-enhanced steady-state free precession (CE-SSFP) cine images were obtained approximately five minutes after contrast injection (slice thickness 8 mm with no slice gap, temporal resolution 20 to 30 frames per cardiac cycle, in-plane resolution 1.5 mm x 1.5 mm). For infarct visualisation, late gadolinium enhancement (LGE) images were acquired 15-20 minutes after administration of the contrast agent using an inversion-recovery gradient-echo sequence (slice thickness 8 mm with no slice gap, in-plane resolution 1.5 mm x 1.5 mm). Inversion time was manually adjusted to null the signal from viable myocardium, typically 200-300 milliseconds. Cine and LGE images were acquired in the short-axis view, from base to apex, and in the three standard long-axis views (two-chamber, four-chamber and left ventricular outflow tract views). Analyses of CMR images were performed by an independent core lab (Imacor AB, Lund, Sweden) using post-processing software (Segment EWA)14. IS divided by LV mass (IS/LV mass) and LV ejection fraction (EF) were measured. Microvascular obstruction was quantified from LGE images using an EWA algorithm with manual adjustments when needed and expressed as percentage of LV mass14. For visualisation of the area at risk, early CE-SSFP cine images were obtained approximately five minutes after contrast injection, as previously described15.
The trial was considered to have met the primary effectiveness endpoint if the hypothermia arm demonstrated a statistically significant reduction in IS/LV mass (IS/LV%) measured by CMR on day 4-6 compared to the control arm. The primary safety endpoint was a non-inferiority comparison with a 6% non-inferiority margin of MACE at day 30, defined as cardiac death, myocardial reinfarction and clinically driven target lesion revascularisation. Additional secondary safety endpoints included stent thrombosis, cardiogenic shock, pulmonary oedema, arrhythmias, deep venous thrombosis, pulmonary embolism, vascular complications requiring intervention and infections. Adverse events were collected and described as serious adverse events (SAE) and non-serious adverse events (NSAE) based on hospital charts reviewed by independent monitors.
Based on an assumed standard deviation of 12% and two-sided alpha of 0.044, the required total sample size to detect a 20% relative reduction in IS/LV% with 80% power was 384 subjects. Because of an expected dropout of 24% due to missing CMR data, the planned total sample size was increased to 500 patients (250 patients per group).
STATISTICAL ANALYSIS
Continuous data were reported as mean±standard deviation or median with interquartile range. Categorical data were shown as frequencies and proportions. For continuous variables, the Wilcoxon rank-sum test and t-test were used for comparison between the two treatment groups, as appropriate. For comparison of categorical variables, Fisher’s exact test or the chi-square test was used. No imputation was carried out for missing data. The data are presented as intention-to-treat except for the primary effectiveness endpoint for which per-protocol analysis was also performed. A p-value of <0.05 was considered statistically significant. All statistical analyses were performed using SAS 9.4 (SAS Institute, Cary, NC, USA).
Results
From April 2016 to August 2018, among 1,815 screened patients, 166 fulfilled the inclusion criteria. Fifty-five patients were included in the roll-in group and 111 patients were randomised to either the hypothermia (n=58) or the control (n=53) group. Follow-up was 98% and 100% at 4-6 days and 90% and 96% at 30 days in the hypothermia and control groups, respectively. In August 2018, the trial was prematurely discontinued by the sponsor with the support of the DMC due to operational issues related to different patient pathways through the hospital between the hypothermia and control arms resulting in significant difference in ischaemic delay to reperfusion.
The hypothermia and control groups were comparable in terms of age, gender, body mass index and risk factors for coronary disease (Table 1). The mean delay from symptom onset to balloon was 232 minutes in the hypothermia group and 188 minutes in the control group (p<0.001). The observed increase in total ischaemic time in the hypothermia group was caused by both a numerically increased delay from symptom onset to randomisation (175 minutes versus 157 minutes; p=0.125) and a significantly longer delay from randomisation to balloon (61 minutes versus 32 minutes; p<0.001). There were no differences in angiographic features, primary PCI result or periprocedural medication between the groups.
Among 58 patients randomised to hypothermia, 55 were cooled (95%). Anti-shivering medication administered before and during the cooling included buspirone and pethidine (Table 2). The mean total volume of infused cold saline was 1,028 ml and the interval between cooling catheter activation and coronary guidewire crossing was 20 minutes. At this point, the mean intravascular temperature reached 33.3°C. Uncontrolled shivering was documented in 12 patients (20.7%).
As shown in Table 3, mean IS/LV% by CMR at day 4-6 was comparable between the hypothermia and control groups using intention-to-treat (21.3% vs 20.0%; p=0.540) and per-protocol analyses (21.6% vs 20.9%; p=0.680) (Central illustration). Additional CMR measurements including IS/area at risk (46.9% vs 42.8%; p=0.356), myocardial salvage index (0.5 vs 0.6; p=0.356), microvascular obstruction (3.3% vs 3.0%; p=0.598) and left ventricular ejection fraction (41% vs 43%; p=0.592) were also comparable between the hypothermia and control groups.
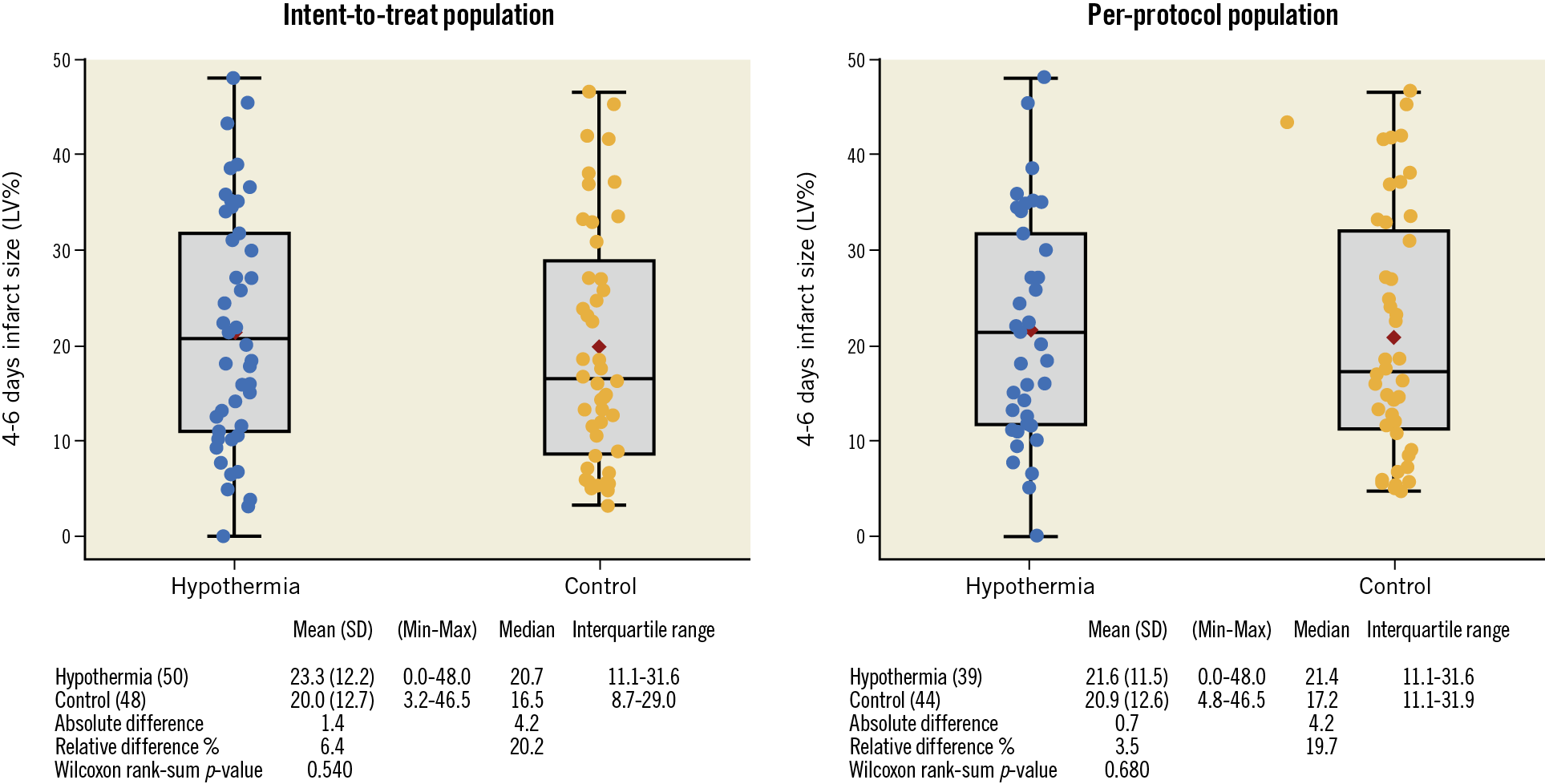
Central illustration. Infarct size by left ventricular mass (IS/LV%) measured by cardiac magnetic resonance (CMR) in the hypothermia and control groups at day 4-6 shown as intention-to-treat and per-protocol analyses.
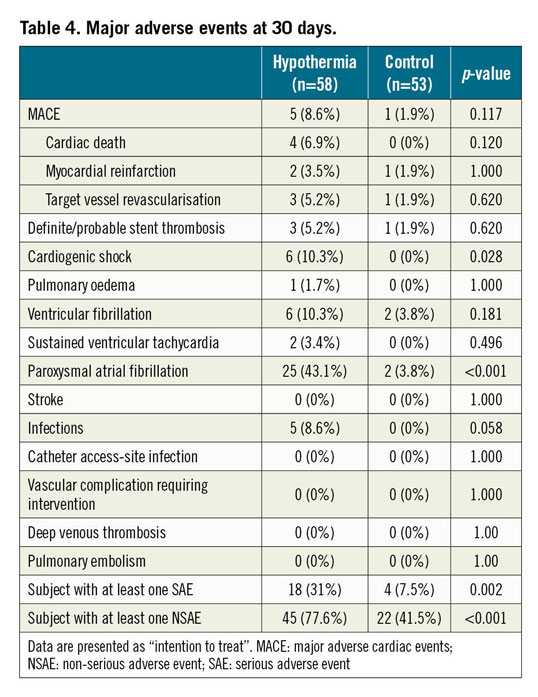
The MACE rate at 30 days was non-significantly increased in the hypothermia group (8.6% vs 1.9%; p=0.117), while cardiogenic shock (10.3% vs 0%; p=0.028) and paroxysmal atrial fibrillation (43.1% vs 3.8%; p<0.001) were significantly more frequent in the hypothermia group (Table 4). Paroxysmal atrial fibrillation resolved spontaneously in all patients during the rewarming phase and did not result in an increased stroke rate at 30 days. No significant increase in stent thrombosis, malignant ventricular arrhythmia or infections was observed in the hypothermia group. There were no cooling catheter-related complications.
Discussion
The present study using 1 L intravenous infusion of cold saline and the intravascular ZOLL Proteus cooling system showed that rapid cooling to 33.3°C did not reduce IS in patients with anterior STEMI undergoing primary PCI. Cooled subjects had longer ischaemic times and an excess of SAE and NSAE. Longer ischaemic times caused in part by different patient pathways led to the early discontinuation of the study and confounded the results. These results contrast with a successful pilot trial13. Several lessons can be learned for future hypothermia STEMI trials.
The key reason that reassuring animal data on IS reduction were not reproduced in this study may be related to the 44-minute increase in total ischaemic delay in the hypothermia group which was due predominantly to workflow logistics that resulted in a significantly longer delay from randomisation to passing of the guidewire across the occlusion. Comparable IS between the groups despite an unacceptable cooling-related delay may indirectly indicate that the detrimental effect of increased ischaemic time on IS may be nullified when subjects are treated with hypothermia. This is in accordance with a recent experimental study in pigs in which a 48-minute increase in ischaemic delay did not translate into a larger IS/area at risk if intravascular cooling was implemented before reperfusion16. However, for a clinically meaningful reduction in IS, the cooling-related delay must be minimised by improved logistics and a rapid and organised team approach in the catheterisation laboratory. This includes simultaneous and co-ordinated implementation of the anti-shivering protocol and priming of the cooling console. At the same time, the interventional team should be draping a patient and inserting a cooling catheter. After cooling catheter activation, the interventional cardiologist has 18 minutes to obtain arterial access, perform coronary angiography, administer anticoagulation/antiplatelet agents, place the guiding catheter and advance the guidewire up to the occlusion without crossing. Since these procedures are usually performed faster than in 18 minutes, further technological development should focus on more powerful intravascular cooling systems enabling shorter intravascular cooling and elimination of the need for concomitant cold saline infusion. It is also important to keep in mind that cooling prior to reperfusion is probably more likely to reduce IS in early STEMI presenters. Total ischaemic time in our cooled patients (232 minutes) was longer than in other trials including CHILL MI (132 minutes)10, VELOCITY (172 minutes)11, RAPID MI ICE (174 minutes)7 and COOL MI (205 minutes)8. All these tasks might be best achieved in mature high-volume STEMI networks with direct transfer of patients to the catheterisation laboratory.
As described, there was an excess of SAE and NSAE in our study. A significantly increased rate of periprocedural self-terminating paroxysmal atrial fibrillation, which did not increase stroke rate, was observed. Such a signal was already documented in the pilot trial but not in other studies with less rapid cooling. In addition, we documented a non-significant increase in ventricular fibrillation/tachycardia, also not evident in the pilot trial. Observed arrhythmias might have been related to more rapid and profound cooling. This possible association should be rigorously addressed in future trials. Both atrial and ventricular arrhythmias together with a numerically increased rate of acute and subacute stent thrombosis may also explain the significantly greater incidence of cardiogenic shock in the hypothermia group.
An excess of stent thrombosis was not reported in the pilot study or in any of the previous intravascular cooling trials7,8,9,10. A striking and rapid decrease in core temperature of 1.4°C more than in previous studies together with a three-hour extension of hypothermia after reperfusion may have facilitated platelet aggregation by increasing adenosine diphosphate (ADP) levels17,18 which have been demonstrated in vitro19 and in healthy volunteers20. In this setting, absorption and onset of P2Y12 inhibition may also be delayed, even with novel agents such as ticagrelor, as demonstrated in comatose survivors of cardiac arrest undergoing hypothermia between 32 and 34°C for 24 hours21. In these patients, an almost three-hour “P2Y12 inhibition gap” was documented following administration of crushed ticagrelor tablets via a nasogastric tube. Furthermore, concomitant pethidine administration as a part of our anti-shivering protocol is likely to impair gastrointestinal mobility and P2Y12 absorption further. A new intravenous P2Y12 inhibitor, cangrelor, with immediate onset of platelet inhibition and up to four-hour infusion, might therefore represent an effective bridging for future cooling studies. Since rapid intravascular cooling may create a more prothrombotic environment favouring stent thrombosis, achievement of an optimal angiographic result may be of even greater importance. We advise culprit-only PCI in the acute setting, selection of the least thrombogenic and most contemporary drug-eluting stent with adequate sizing/expansion using intravascular imaging, if necessary, and avoiding unnecessarily complex and long non-culprit lesion stenting.
Limitations
Importantly, our study was stopped prematurely due to site-specific issues, which limits the power of our results to detect significant difference in IS/LV at 4-6 days following the procedure. Overwhelmingly, the greatest study limitations were the large disparities observed in door-to-balloon and total ischaemic times between the study groups, which ultimately precluded meaningful group comparisons. We also acknowledge that measurements of blood temperature in the control group at the time of reperfusion would add additional scientific merit to our study protocol.
Conclusions
The present study using a 1 L intravenous infusion of cold saline and the intravascular ZOLL Proteus cooling system reduced temperature in patients with anterior STEMI to 33.3°C before reperfusion. Due to inconsistent patient logistics between the groups, this hypothermia protocol resulted in longer ischaemic delay in cooled subjects, did not reduce IS/LV mass and was associated with increased adverse events. Future hypothermia studies should therefore focus on reduction of cooling-related delay. Because such delay is likely to counterbalance potential myocardial salvage and increase adverse events, we advise implementing a predefined stopping rule not only for hypothermia but also for other trials investigating myocardial salvage beyond PPCI.
|
Impact on daily practice Cooling as an adjunct to primary PCI in STEMI remains experimental. Lessons learned from this prematurely discontinued trial should be implemented in the protocols of possible future trials. |
Acknowledgements
Additional site investigators of the COOL AMI EU Pivotal Trial were: Teele Pern, Julia Reiments, Toomas Marandi (North-Estonia Medical Centre Foundation, Tallinn, Estonia); Srdjan Kafedzic, Dragan Petrovic (Clinical Hospital Center Zemun, Belgrade, Serbia); Milenko Cankovic, Milana Jarakovic, Mila Kovacevic (Institute of Cardiovascular Diseases of Vojvodina, Sremska Kamenica, Novi Sad, Serbia); Ljiljana Kos, Sasa Loncar (University Clinical Center Banja Luka, Republic of Srpska); Zalan Gulyas, Andras Korda (Military Hospital, Budapest, Hungary); Endre Zima, Istvan Ferenc Edes (Heart and Vascular Center, Semmelweis University, Budapest, Hungary); Petra Poliacikova, Milan Trencan (Stredoslovenski Ustav Srdcovych a Cievnych Chorob, Banska Bystrica, Slovakia); Grigoris Karamasis, Ellie Gudde (Essex Cardiothoracic Centre, Basildon and Thurrock University Hospital NHS Foundation Trust, Basildon, UK); Ana Uscumilic, Vladan Dedovic, Milorad Zivkovic (Clinical Center of Serbia, Belgrade, Serbia); Alexandra-Maria Warenitis, Irene Lang, Matthias Mueller (Department of Emergency Medicine, Medical University of Vienna, Vienna, Austria); Gert Klug, Sebastjan Reinstadler (University Hospital of Internal Medicine lll/Cardiology and Angiology, Medical University Innsbruck, Innsbruck, Austria); Janina Stepinska, Michal Ciszewski (The Cardinal Stefan Wyszynski Institute of Cardiology, Warsaw, Poland); Ursa Mikuz, Peter Radsel (University Medical Center Ljubljana, Slovenia); Svetlana Ratobilska, Viktorija Skuja (Pauls Stradins Clinical University Hospital, University of Latvia, Riga, Latvia); Erika Csengo, Zsolt Koromi (Borsod-Abauj-Zemplen County Central Hospital and University Teaching Hospital, 1st Department of Internal Medicine and Cardiology, Miskolc, Miskolc, Hungary); Zvonimir Ostojic (University Hospital Center Zagreb, Zagreb, Croatia); Jaroslav D. Kasprzak, Marcin Ojrzanowski, Lukasz Jankowski (Medical University in Łódź, Bieganski Hospital, Łódź, Poland); Andrzej Swiatkowski, Jacek Kowalczyk (Silesian Center for Heart Diseases, Department of Cardiology, Medical University of Silesia, DMS in Zabrze, Zabrze, Poland). The authors would like to acknowledge the professional contribution of the DMC with Marcus Ferrari, MD, PhD (Germany), Stefano Servi, MD, PhD (Italy), Andreas Schaefer, MD, PhD (Germany) and Timothy Collier, MSc, MS (UK). The authors would also like to thank the ZOLL team for their continuous support.
Funding
The study was funded by ZOLL.
Conflict of interest statement
M. Noc has received consultation and speaker fees from ZOLL. M. Holzer has received speaker fees from Bard and ZOLL. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

