
Abstract
Aims: Hypothermia reduces reperfusion injury and infarct size in animal models of acute myocardial infarction if started before reperfusion. Human studies have not confirmed benefit, probably due to insufficient myocardial cooling and adverse systemic effects. This study sought to assess the safety and feasibility of a novel method for selective, sensor-monitored intracoronary hypothermia.
Methods and results: Ten patients undergoing primary percutaneous coronary intervention (PPCI) were included. Saline at room temperature was administered distal to the culprit lesion through an inflated over-the-wire balloon (OTWB) in order to cool the endangered myocardium for 10 minutes (occlusion phase). Next, the OTWB was deflated and cooling continued with saline at 4°C for another 10 minutes (reperfusion phase). A sensor-tipped temperature wire in the distal coronary artery allowed titration of the infusion rate to achieve the desired coronary temperature (6°C below body temperature). Target coronary temperature was achieved within 27 seconds (median; IQR 21-46). Except for two patients with inferior wall infarction experiencing transient conduction disturbances, no side effects occurred. Systemic temperature remained unchanged. Finally, PPCI was performed as per routine.
Conclusions: Selective hypothermia of the infarct area by intracoronary infusion of saline provides a novel method to reduce coronary temperature quickly and guarantee local myocardial hypothermia. In anterior wall myocardial infarctions, the protocol appeared safe, without serious haemodynamic or systemic side effects. In inferior wall myocardial infarctions, transient conduction abnormalities of short duration occurred. Potentially, selective intracoronary delivery of hypothermia could attenuate reperfusion injury caused by traditional PPCI.
Abbreviations
AMI: acute myocardial infarction
AV: atrioventricular
IQR: interquartile range
LAD: left anterior descending artery
LCx: left circumflex artery
OTWB: over-the-wire balloon
PPCI: primary percutaneous coronary intervention
RCA: right coronary artery
STEMI: ST-elevation myocardial infarction
TIMI: Thrombolysis In Myocardial Infarction
VT: ventricular tachycardia
Introduction
Timely reperfusion is paramount for myocardial salvage in acute myocardial infarction (AMI). The major role of primary percutaneous coronary intervention (PPCI) as first-line treatment is indisputable. Nevertheless, AMI still carries a one-year mortality rate of 7%, and 22% of patients experience prolonged or new hospitalisation for heart failure1. Therefore, additional treatment to limit final infarct size remains important. Besides ischaemic damage, the process of restoring epicardial blood flow itself can injure the myocardium2. This so-called “reperfusion injury” annihilates part of the gain achieved by PPCI, differs from patient to patient, and remains incompletely understood3,4.
Hypothermia may attenuate several pathological mechanisms of reperfusion injury5-7. Reduced infarct area was observed in animal studies but only when hypothermia was started before reperfusion. Studies in humans, however, have not confirmed this effect, probably because the target temperature within the affected myocardium was not reached before reperfusion in the vast majority of patients8-17. These prior studies all employed whole body hypothermia, which requires too much time, may lead to volume overload, and induces systemic reactions such as shivering and an enhanced adrenergic state.
The application of intracoronary hypothermia can potentially overcome these problems by delivering cold saline selectively to the jeopardised myocardium. Otake et al showed that intracoronary infusion of cold saline is safe and reduces infarct size in a pig model of AMI18; however, translational methodology to cool the ischaemic myocardium selectively in humans before reperfusion has not been available so far.
Recently, the feasibility and thermal efficacy of an intracoronary method to apply hypothermia using standard percutaneous coronary intervention (PCI) equipment, monitored by a pressure/temperature sensing guidewire, was demonstrated in a beating pig heart model19. In the present pilot study, we evaluate the safety and feasibility of selective hypothermia in patients with AMI by intracoronary infusion of cold saline before and during reperfusion using standard PCI equipment.
Methods
STUDY POPULATION
The study was approved by the institutional review board of the Catharina Hospital in Eindhoven, the Netherlands. All subjects provided both oral and written informed consent. Online registration was completed before enrolling the first subject (ClinicalTrials.gov NCT02753478). Patients considered for enrolment had to meet the following inclusion criteria: acute ST-elevation myocardial infarction (STEMI) with a total ST-segment deviation of more than 5 mm and presenting within six hours of symptoms, and Thrombolysis In Myocardial Infarction (TIMI) 0-2 flow in the infarct-related artery at baseline angiography.
Patients were excluded for any of the following conditions: age <18 years; cardiogenic shock; poor clinical condition according to the judgement of the treating interventionalist; previous myocardial infarction in the culprit territory; previous coronary artery bypass surgery; tortuous or severely calcified coronary arteries; severe concomitant disease; inability to give informed consent; other known myocardial diseases, such as moderate or severe left ventricular hypertrophy or cardiomyopathy; pregnancy; or pre-existing conduction disturbances.
INSTRUMENTATION
All materials used during the protocol are routinely available in the catheterisation laboratory. No investigational devices were used. Primary PCI was initiated according to routine via the femoral or radial artery. Coronary angiography was performed, and the culprit lesion was located. If the patient met the inclusion criteria, oral consent was obtained. Thereafter, a regular guidewire was advanced beyond the culprit lesion, immediately followed by an over-the-wire balloon (OTWB) (Apex™; Boston Scientific, Marlborough, MA, USA) that was inflated to 4 atm at the location of the occlusion to prevent reperfusion. If necessary, according to the judgement of the treating interventionalist, fentanyl and/or midazolam was administered to reduce chest pain.
Next, a standard 0.014” coronary pressure wire that can also measure temperature (PressureWire™ Certus™; St. Jude Medical, St. Paul, MN, USA) was positioned next to the regular guidewire in the (balloon occluded) distal coronary artery and connected to its console (RadiAnalyzer™ Xpress; St. Jude Medical). The temperature signal measured by the wire was calibrated such that 0°C represented normal body temperature. Thereafter, the regular guidewire in the OTWB was removed and its central lumen was connected to parallel infusion pumps (Rempress 800; Nemoto, Tokyo, Japan), one filled by saline at room temperature and the other filled by saline at 4°C (Figure 1). Tympanic temperature was measured non-invasively before and during hypothermia to monitor systemic body temperature.
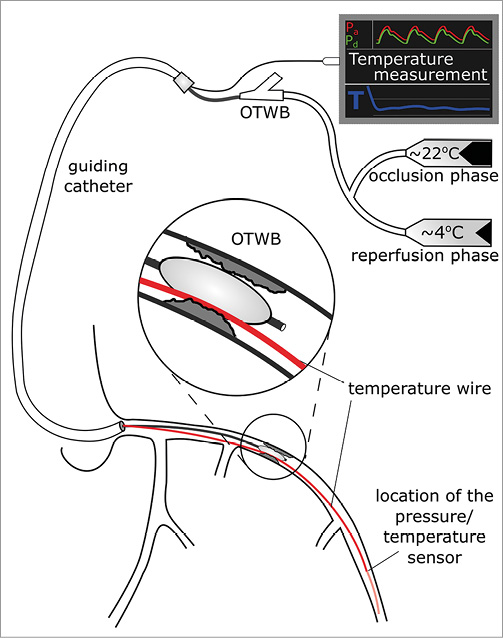
Figure 1. Schematic drawing of the equipment for selective intracoronary hypothermia. A pressure/temperature wire is positioned next to the OTWB. OTWB: over-the-wire balloon
INTRACORONARY HYPOTHERMIA PROTOCOL
Hypothermia was initiated 10 minutes before the onset of reperfusion (the occlusion phase, OTWB inflated) and continued for 10 minutes after reperfusion (the reperfusion phase, OTWB deflated).
In the occlusion phase, saline infusion at room temperature was started at a flow rate of 20 ml/min and was subsequently adjusted based upon distal coronary temperature measurements to achieve a temperature of approximately 6°C below body temperature.
Based on our previous experiments, the target temperature in the distal coronary artery should be maintained at approximately 6°C below body temperature, which corresponds to a myocardial temperature in the infarct area of approximately 4°C below body temperature in the occlusion phase (heat transport by conduction)19. This infusion and temperature was maintained for 10 minutes, whereafter the balloon was deflated and reperfusion started. Just before deflating the balloon, saline temperature was switched to 4°C to maintain the adequate decrease in distal coronary and myocardial temperature during this reperfusion phase. In the isolated beating pig heart model, it was demonstrated that, during the reperfusion phase, coronary and myocardial temperatures are almost equal (heat transport by convection)19. Switching the temperature of the saline from 20°C during the occlusion phase to 4°C during the reperfusion phase is necessary because in the reperfusion phase partial or complete reperfusion occurs and the cooler saline mixes with warmer blood. During both phases, adjustments in the infusion rate could be made to fine-tune distal coronary temperature to the desired level.
After completing intracoronary infusion, the OTWB was removed and PPCI was performed using the pressure wire as guidewire. Medical treatment before and after the intervention was performed as per routine.
ENDPOINTS
The primary safety endpoints were haemodynamic conditions during hypothermia, i.e., systemic and distal coronary blood pressure and heart rate; occurrence of rhythm or conduction disturbances; technical safety; dissipation of cooling after stopping the infusion; and systemic side effects.
The primary feasibility endpoints were: time to adequate induction of hypothermia and adequate depth of hypothermia, defined as a drop in distal coronary temperature by at least 6°C; ability to maintain a stable coronary temperature during the entire 20 minutes of infusion; technical ease of instrumentation and door-to-balloon time (including time to perform the protocol).
STATISTICAL METHODOLOGY
It should be emphasised that this study was a small, first-in-human pilot study to investigate the safety and feasibility of a new technique.
Analyses were performed using R version 3.3.2 (R Foundation for Statistical Computing, Vienna, Austria). We employed standard statistical techniques: mean±standard deviation or median with interquartile range (IQR), count (percentage), and paired t-test or Wilcoxon signed-rank test as appropriate. Applicable tests were two-tailed, and p<0.05 was considered statistically significant.
We chose a sample size of 10 patients, given the twin goals of demonstrating feasibility of the technique and collecting safety data before moving forward with a larger trial. While this number is arbitrary, a modest sample size was intentionally selected given the highly novel and experimental nature of our pilot study, in line with prior novel trials in this field14. In this way, we tried to find a reasonable balance between ethical considerations of subjecting patients to a new, unproven method and a potentially beneficial treatment for future patients.
Results
PATIENTS
Between June and November 2016, a total of 10 patients were included. Baseline characteristics can be found in Table 1. Six patients had anterior wall infarctions (left anterior descending artery [LAD]) and four patients had inferior wall infarctions (three right coronary artery [RCA], one left circumflex artery [LCx]).

After completing the study protocol, all patients were stented according to normal routine with TIMI flow 3 and had an uneventful recovery, with the exception of one patient who developed stent thrombosis one hour after the index procedure due to stent underexpansion. The vessel was opened again with high-pressure inflation and the further course was uneventful.
SAFETY
Safety parameters for each patient are shown in Table 2. No patient died, and no patient experienced shivering. The median volume of infused saline was 336 ml (IQR 215-392) comprising 177 ml in the occlusion phase and 159 ml in the reperfusion phase. Due to ongoing angina from prolonging the ischaemic period by 10 minutes before reperfusion, six patients received fentanyl 100 μg for pain relief during the occlusion phase. No effect on systemic haemodynamics was observed.
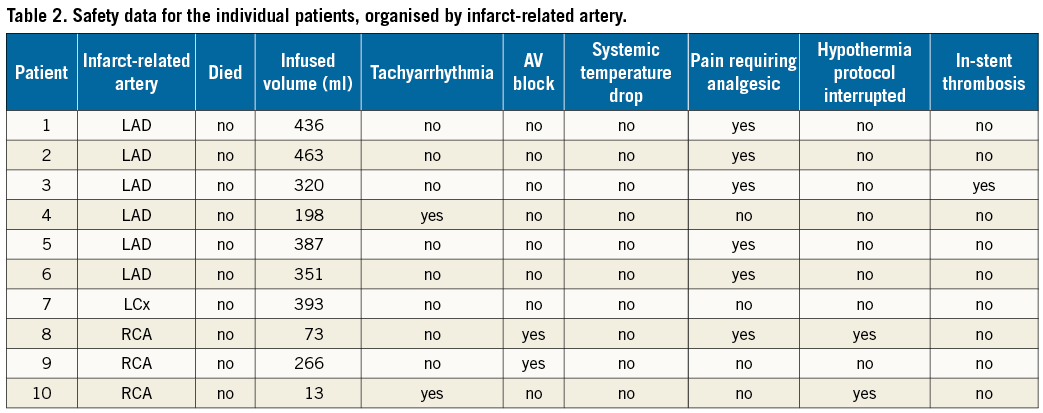
In one patient with inferior myocardial infarction, recurrent ventricular fibrillation occurred due to ongoing ischaemia, requiring repeated defibrillation. After deflating the balloon, no further ventricular fibrillation occurred, not even after restarting saline infusion. In a different patient with inferior wall infarction due to a right coronary occlusion, the hypothermia protocol was interrupted because of symptomatic second-degree atrioventricular (AV) block at a distal coronary temperature of 4.7°C below body temperature. After administration of 1 mg of atropine, infusion was resumed without problems. In a third patient with inferior myocardial infarction, also due to a right coronary occlusion, a temporary pacemaker was necessary due to persistent third-degree AV block. In that patient, no relationship was observed between the cooling protocol and the AV block.
In one patient with anterior wall infarction with multiple short runs of ventricular tachycardia (VT) already present before starting the study protocol, the occlusion phase was interrupted after only three minutes, whereafter the VT did not return even when infusion was continued without occlusion of the coronary artery.
Finally, as mentioned above, one patient developed stent thrombosis one hour after the index procedure, probably due to stent underexpansion as confirmed by stent boosting. The stent was further inflated, and the subsequent course was uneventful. No coagulopathy or electrolyte disturbances were documented.
In summary, except for the occurrence of transient conduction disturbances in two patients with inferior wall myocardial infarctions, all observed events were most probably related to the infarction itself or to the occlusion of the coronary artery, rather than to the cooling. No further haemodynamic abnormalities were observed during cooling in any patient. After cooling, temperature returned to normal within 43 seconds (IQR 17-59) and clinically relevant systemic side effects were not observed in any patient. Body temperature during the procedure decreased by 0.2°C (IQR 0.1-0.4), which was non-significant (p=0.237).
FEASIBILITY
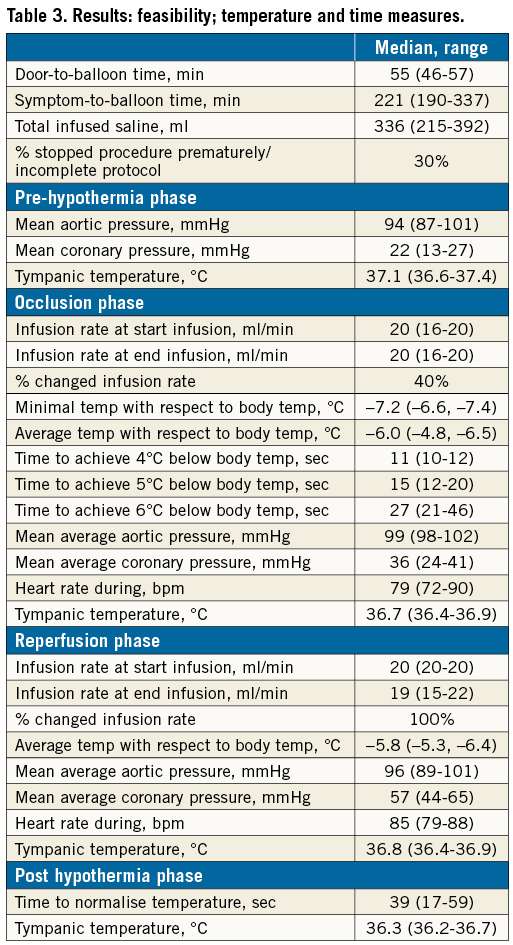
No technical problems with the instrumentation or the study protocol occurred in any of the patients. Median door-to-balloon time was 55 minutes (IQR 46-57 minutes) and symptom-to-balloon time was 221 minutes (IQR 190-337). The total ischaemic time was prolonged by 19.2 minutes (IQR 18.4-19.6 minutes), representing the time necessary for extra instrumentation plus the occlusion phase.
After starting the saline infusion in the occlusion phase, hypothermia of at least 6°C below body temperature in the distal coronary artery was achieved within 27 seconds (IQR 21-46 seconds). In nine out of the ten patients, this temperature was achieved within one minute. In all patients, target distal coronary temperature could be maintained during the complete course of the infusion. An example of such a recording is shown in Figure 2.

Figure 2. Selective intracoronary hypothermia in a 56-year-old man with anterior wall myocardial infarction and an occluded proximal LAD artery. The figure illustrates systemic (red) and distal coronary blood pressure (green) and distal coronary temperature (blue, with reference to body temperature which is set at 0°C) during the occlusion and reperfusion phases. The distal coronary target temperature is reached within 30 seconds and remains stable throughout the protocol. After stopping the infusion at 20 minutes, temperature returns to normal within 20 seconds. *marks the switch from saline at room temperature to saline at 4°C.
The total volume of saline infused was 336 ml (IQR 215-392). An overview of all the feasibility parameters is given in Table 3.
IN-HOSPITAL FOLLOW-UP
With the exception of the one patient with acute stent thrombosis related to underexpansion, no further events occurred during the hospital stay. The average CKMB peak was 119 u/l (IQR 83-202). Echocardiography, performed before discharge from the hospital, showed a preserved left ventricular ejection fraction in eight patients.
Discussion
This pilot study demonstrates the safety and feasibility of selective intracoronary hypothermia in patients undergoing primary percutaneous intervention. The protocol could be executed in all patients with an acceptable prolongation of the procedure. The target temperature was achieved quickly and could be maintained easily during both the occlusion and reperfusion phases. Continuous monitoring of distal coronary temperature enabled instantaneous feedback and optimisation of the infusion rate, while continuous distal coronary pressure measurement enhanced safety.
All three patients with inferior wall infarction due to a right coronary artery occlusion developed transient conduction disturbances necessitating modification of the protocol. In contrast, none of the seven patients with the LCA as culprit artery experienced any conduction abnormality, not even when cooled to -7°C compared to blood temperature.
Although infarct size in animals has been decreased by hypothermia5,18,20, translational studies to humans have been disappointing8-17. These prior negative results were due to a number of major shortcomings inherent to any systemic cooling technique, now overcome or circumvented by the protocol as applied in this study with the following six key advantages.
First, our method can rapidly achieve the target temperature in the jeopardised myocardium within one minute, much faster than in any previous study. Because reperfusion damage in animal models occurs within a few minutes, an essential requirement for therapeutic hypothermia is reaching the target temperature before re-establishing significant epicardial flow. Second, the technique cools the infarcted myocardium selectively and effectively without spillover to the adjacent myocardium or systemic side effects. In our study no patient was aware of the cooling. Third, the use of a coronary pressure/temperature wire enables precise monitoring and control of the infusion rate and guarantees effective and stable cooling. This is important because in this study all patients needed small adjustments of the infusion rate. Fourth, intracoronary hypothermia requires significantly less infused volume compared to systemic cooling. In previous studies, up to two litres of cold saline were needed to achieve sufficient systemic hypothermia16, which poses a risk for volume overload and pulmonary oedema. In contrast, in our study only a few hundred cc of saline was needed. Fifth, the decision to start hypothermia can be based on coronary angiography observations such as TIMI flow, in contrast to systemic cooling when coronary angiography only follows implantation of a cooling catheter. Sixth, our protocol can be applied easily using standard catheterisation laboratory equipment.
In Table 4 the procedural characteristics of previous studies on systemic hypothermia in patients with AMI are compared to the present study, emphasising the potential advantages of the intracoronary technique.

Limitations
The present study has several limitations. First, it should be noted that this is a first-in-human pilot study with a sample size of 10 patients without a control group. Therefore, we cannot say anything regarding its efficacy to reduce infarct size. Second, the optimal duration of hypothermia before and after the onset of reperfusion is not known. We chose an arbitrary duration of 10 minutes before and 10 minutes after reperfusion, which is based upon the knowledge that reperfusion injury occurs within a few minutes after opening the occluded coronary artery4. We do not know if a longer time window is desirable or if a shorter time window is possible.
Additionally, our target temperature of 6°C below blood temperature was obtained by extrapolation from animal models18,20,21-28 and studies of cooling other organs29. In this human study we were not able to measure myocardial temperature directly, but recorded coronary temperature continuously and assumed a similar relationship between coronary and myocardial temperature as was investigated by infrared mapping in the beating pig heart with a similar instrumentation19.
Third, although this target temperature seems to be feasible and safe in anterior wall myocardial infarction, it might be too low in inferior wall infarction.
Fourth, our method prolongs the occlusion of the coronary artery by approximately 20 minutes. Clinical trials are necessary to determine if the negative effects of a slightly prolonged occlusion are counterbalanced by reduced reperfusion injury. Therefore, such trials might be limited to patients presenting with TIMI 0 or 1 flow.
Fifth, we used two guidewires and an OTWB. Presently we are testing a monorail infusion balloon catheter that enables occlusion of the artery and infusion of saline simultaneously while just using the pressure wire as a single guidewire (C catheter; Hexacath Inc., Paris, France). This will further simplify the procedure by not requiring a regular guidewire and OTWB anymore.
Finally, with respect to other side effects, the stent thrombosis in one patient after the procedure was probably related to stent underdeployment as confirmed by the stent boost, and not related to the cooling. In one patient, VF occurred during the occlusion phase. It is speculative to what extent this was related to our protocol but, when looking at comparable patients in our SEMPER FI trial (ClinicalTrials.gov NCT02125526), VF has occurred in seven out of 93 patients so far. The AV conduction disturbances in the RCA patients were all transient, although these necessitated small adaptations in the protocol. Based upon these experiences, our planned, randomised, controlled, proof-of-principle trial will only include large anterior wall infarctions with a proximal or mid LAD occlusion.
Conclusions
Selective intracoronary hypothermia, as applied in this study, is a feasible method to cool the myocardium quickly and effectively before the onset of reperfusion. In anterior wall myocardial infarctions, the protocol appeared safe, without serious haemodynamic or systemic side effects. In inferior wall infarctions due to an occlusion of the right coronary artery we observed quickly transient conduction abnormalities.
Compared to systemic hypothermia methods, the intracoronary technique reaches the target temperature within minutes, uses significantly less infused volume, and causes no systemic side effects.
These results justify a randomised controlled proof-of-principle study to assess the ability of intracoronary hypothermia to limit reperfusion injury and to reduce infarct size.
| Impact on daily practice During PPCI, the application of selective intracoronary hypothermia may provide a tool to attenuate reperfusion injury and therefore to reduce infarct size. |
Funding
This study was supported in part by a grant of the “Stichting Vrienden Van Het Hart” (Friends of The Heart Foundation) and was part of a service project of the Rotary Soeterbeek (member of Rotary International) in Eindhoven, the Netherlands.
Conflict of interest statement
N. Pijls receives institutional research grants from and is a consultant for St. Jude Medical. The other authors have no conflicts of interest to declare.

