Abstract
Due to significant improvement in the pre-hospital treatment of patients with out-of-hospital cardiac arrest (OHCA), an increasing number of initially resuscitated patients are being admitted to hospitals. Because of the limited data available and lack of clear guideline recommendations, experts from the EAPCI and “Stent for Life” (SFL) groups reviewed existing literature and provided practical guidelines on selection of patients for immediate coronary angiography (CAG), PCI strategy, concomitant antiplatelet/anticoagulation treatment, haemodynamic support and use of therapeutic hypothermia. Conscious survivors of OHCA with suspected acute coronary syndrome (ACS) should be treated according to recommendations for ST-segment elevation myocardial infarction (STEMI) and high-risk non-ST-segment elevation -ACS (NSTE-ACS) without OHCA and should undergo immediate (if STEMI) or rapid (less than two hours if NSTE-ACS) coronary invasive strategy. Comatose survivors of OHCA with ECG criteria for STEMI on the post-resuscitation ECG should be admitted directly to the catheterisation laboratory. For patients without STEMI ECG criteria, a short “emergency department or intensive care unit stop” is advised to exclude non-coronary causes. In the absence of an obvious non-coronary cause, CAG should be performed as soon as possible (less than two hours), in particular in haemodynamically unstable patients. Immediate PCI should be mainly directed towards the culprit lesion if identified. Interventional cardiologists should become an essential part of the “survival chain” for patients with OHCA. There is a need to centralise the care of patients with OHCA to experienced centres.
Introduction
Sudden out-of-hospital cardiac arrest (OHCA) remains the leading cause of death in developed countries with an annual incidence of between 36 and 81 events per 100,000 inhabitants1. Pre-hospital treatment is initiated with a sequence of critical events known as the “chain of survival” which includes the early recognition of symptoms, bystander cardiopulmonary resuscitation, early defibrillation and advanced cardiac life support. Re-establishment of spontaneous circulation (ROSC) before hospital arrival is currently achieved in 40-60% of patients in whom advanced cardiac life support is attempted. Due to significant improvement in the pre-hospital “chain of survival” in Europe, an increasing number of initially resuscitated patients are being admitted to hospitals.
According to autopsy and immediate coronary angiography (CAG) data, significant coronary artery disease may be documented in more than 70% of patients with resuscitated OHCA2,3. These data are well known by the physicians caring for these patients. Furthermore, the 2012 European Society of Cardiology (ESC) guidelines on ST-segment elevation myocardial infarction (STEMI) included recommendations on the management of survivors of OHCA4: “In patients with resuscitated cardiac arrest, whose electrocardiogram (ECG) shows STEMI, immediate CAG with a view to primary percutaneous coronary intervention (PCI) is the strategy of choice, provided that the guidelines mandated times can be met (class I, level B). Given the high prevalence of coronary occlusions and potential difficulties in interpreting the ECG in patients after cardiac arrest, immediate CAG should be considered in survivors of cardiac arrest having a high index of suspicion of ongoing infarction (class IIa, level B)”. Interventional cardiologists in “24-7” STEMI centres are therefore being increasingly alerted for immediate CAG and PCI. Because of the limited data available, the 2012 ESC STEMI guidelines could not address several important practical issues while treating patients with resuscitated OHCA:
1) Should a coronary angiogram be performed immediately at admission in all survivors of an OHCA with STEMI or should a selection be performed on the basis of neurological status on admission, prognostic criteria such as the duration of no-flow, initial rhythm, age and pre-arrest comorbidities?
2) Should patients without STEMI be admitted directly to the catheterisation laboratory or should a rapid assessment be performed before to exclude a non-cardiac cause of arrest?
3) Which anticoagulation and antiplatelet treatment should be administered before, during and after PCI?
4) Should PCI be performed only on the culprit artery or on all lesions during the initial procedure or subsequently, and what stents should be used?
5) What are the current indications for cardiac assist devices in OHCA?
6) What are the indications for a coronary artery spasm provocation test in survivors of OHCA?
The aim of this statement paper on CAG and PCI after resuscitated OHCA, designated as invasive coronary strategy, is to provide practical guidelines on the management for interventional cardiologists based on the limited current data and an expert consensus. This paper should therefore be regarded as complementary to the existing ESC guidelines for STEMI and NSTE-ACS.
Literature review on invasive coronary strategy in resuscitated OHCA
It is now well known that, in the presence of STEMI, defined as an ST elevation of >1 mm in two contiguous standard leads or >2 mm in precordial leads on the early post-resuscitation ECG, acute thrombotic lesions, such as those usually apparent in acute coronary syndromes (ACS) without preceding cardiac arrest, may be found in up to 90% of cases5-9. However, the absence of STEMI on the ECG performed in the pre-hospital setting or on admission does not exclude obstructive or even thrombotic “ACS” coronary stenosis, which may be present in 25% to 58% of cases3,7,9,10. These ECG patterns include ischaemic ST-T changes, bundle branch block, non-specific intraventricular delay and non-specific ST-T changes9,10.
There are no randomised trials investigating the possible benefits of an immediate invasive coronary strategy in patients with resuscitated OHCA. In fact, patients with preceding sudden cardiac arrest have been systematically excluded from major randomised trials which unequivocally demonstrated the benefits of primary PCI in STEMI11 and early PCI in non-ST-elevation acute coronary syndrome (NSTE-ACS)12. We therefore performed a “PubMed” search using the key words “cardiac arrest” and “coronary angiography”. Each paper was reviewed and possible additional references within the text identified. If patients undergoing urgent coronary angiography and PCI were identified, they were included in the pooled analysis (Table 1). Forty-two studies with 3,665 patients confirmed the high prevalence of >1 obstructive coronary lesion and the feasibility and safety of an immediate invasive coronary strategy3,5-10,13-46. The fact that seven of these studies with a total of 1,110 patients have been published since 2012 points to the growing utilisation of an invasive coronary strategy in patients with OHCA40-46.
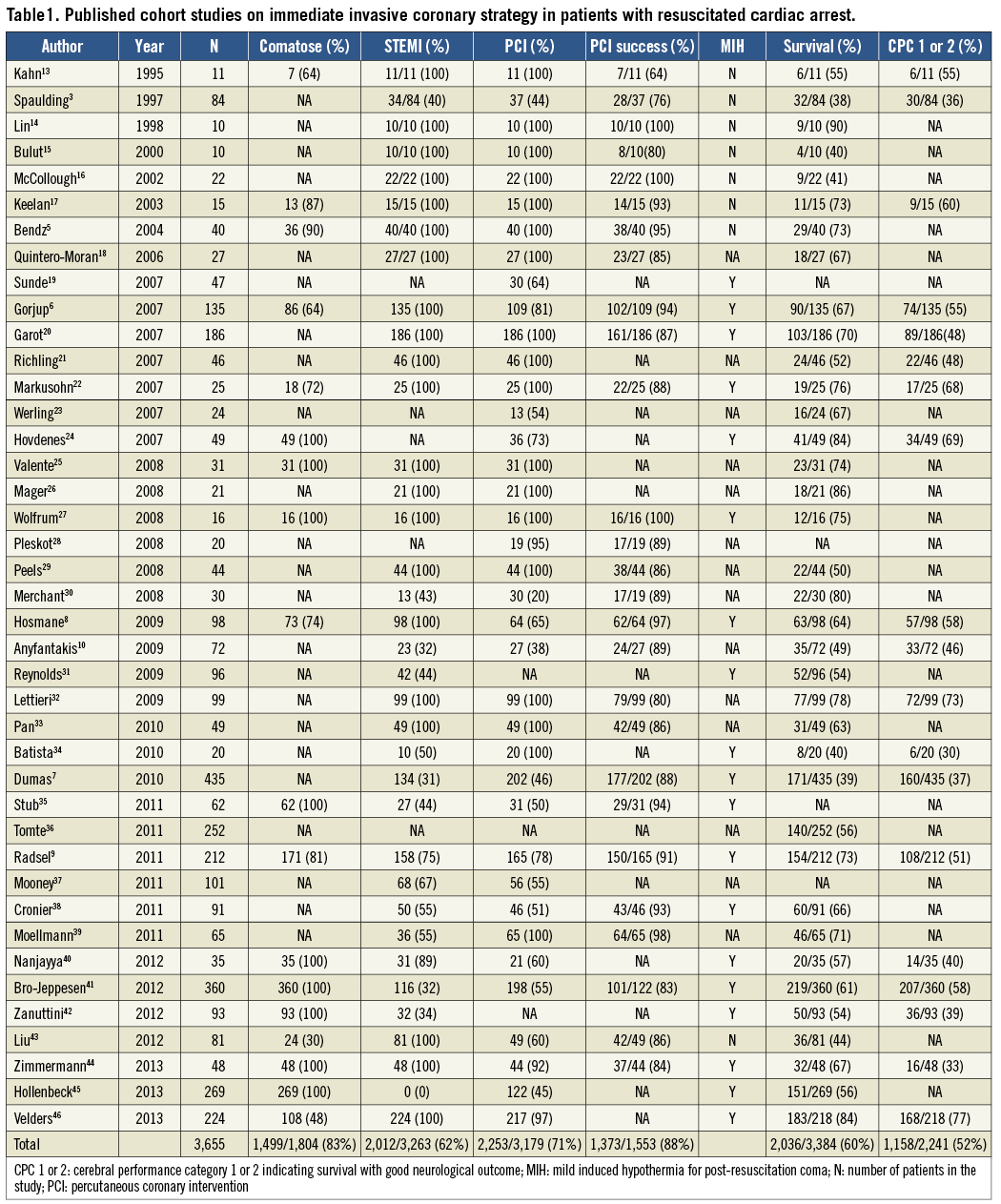
Recently, Larsen and Ravkilde47 performed a meta-analysis of 10 observational studies comparing the survival of patients with resuscitated OHCA in relation to an immediate invasive coronary strategy. In 3,103 patients, an immediate invasive strategy was associated with improved survival (pooled unadjusted OR 2.78; 95% CI: 1.89-4.10; p<0.001). Because of the heterogeneity of studies and lack of data for adjusted analysis, this finding should be interpreted with caution.
More recently, investigators from the United States analysed 3,981 patients with OHCA admitted after ROSC of whom 19% and 17% underwent coronary angiography and PCI within 24 hours, respectively48. There was very wide variability among the 151 admitting hospitals in the frequency of these procedures. However, survival and favourable neurological outcome were independently associated with early coronary angiography (adjusted OR 1.87; 95% CI: 1.15-3.04) and reperfusion (adjusted OR 2.14; 95% CI: 1.46-3.14).
Selection of patients for immediate coronary invasive strategy
Because of the lack of appropriate trials and unequivocal guideline recommendations, there is considerable variability in the selection of patients for immediate invasive coronary strategy among different hospitals and countries. In this context, it is also important to notice that there are two very different populations of resuscitated patients at hospital admission. Because of typical delays in the pre-hospital “chain of survival”, up to 80% of resuscitated patients present in coma despite ROSC, which indicates post-resuscitation brain injury, the severity of which may vary from no or mild disability to permanent vegetative state or brain death. Importantly, this cannot be predicted on hospital admission. Only a minority of fortunate patients regain consciousness immediately after ROSC if chest compression and defibrillation have been initiated immediately, which is usually the case if the emergency medical team is already present at the scene or if the patient has received well performed cardiopulmonary resuscitation immediately before the arrival of the medical team. Conscious survivors of OHCA, in contrast to comatose survivors of cardiac arrest, have little or no post-resuscitation brain injury and represent a less complex subpopulation in terms of post-resuscitation treatment. Pooling published studies on conscious survivors of OHCA triggered by ACS who underwent immediate invasive coronary strategy, 117 out of 119 (98%) patients survived to hospital discharge with good neurological outcome6,8,9,13.
Based on the described observational studies and by extrapolation from “ACS-no cardiac arrest” interventional trials, we suggest recommendations for interventional cardiologists for decision making regarding selection of patients for immediate coronary invasive strategy in a hospital with an immediately available catheterisation laboratory and an intensive care unit (Figure 1). There is little doubt that conscious survivors of OHCA with suspected ACS should be treated according to recommendations for STEMI and high-risk NSTE-ACS which also includes life-threatening ventricular arrhythmias4,12. Accordingly, patients with ST-segment elevation on the post-resuscitation ECG should undergo immediate CAG and patients without STEMI rapid CAG in less than two hours. Comatose survivors of OHCA with ECG criteria for STEMI on the post-resuscitation ECG should follow the “STEMI fast track” and go directly to the catheterisation laboratory4. For patients without STEMI ECG criteria, whilst coronary disease is likely, there are many other possible causes of cardiac arrest. We advise a short “emergency department or intensive care unit stop” to exclude non-coronary causes, such as respiratory failure, shock of non-cardiogenic aetiologies, cerebrovascular event, pulmonary embolism and intoxication, by performing the appropriate diagnostic procedures. These include more detailed information from bystanders regarding possible symptoms before cardiac arrest and review of previous medical history if available. Echocardiography is very useful to document cardiac tamponade, acute “cor pulmonale” due to massive pulmonary embolism and aortic dissection which can be subsequently confirmed by contrast CT scan of the thorax. Head CT should not be routinely performed but, if a cerebrovascular event is suspected as a cause of cardiac arrest, this should be the first diagnostic procedure. Although attractive, we believe that selective diagnostic tests rather than a routine total body CT scan are currently more validated and are a cheaper way to approach the cause of cardiac arrest. In the absence of an obvious non-coronary cause, CAG should be performed as soon as possible, within two hours according to the guidelines for high-risk NSTE-ACS12. This is particularly important in haemodynamically unstable patients and in patients with recurrent malignant ventricular arrhythmias12. Coma on admission should not automatically represent a contraindication for immediate coronary angiography. However, unfavourable pre-hospital settings related to cardiac arrest and initial resuscitation indicating a remote likelihood for neurological recovery should be strongly considered and argue against an invasive coronary strategy regardless of post-resuscitation ECG. These include unwitnessed cardiac arrest, late arrival of a pre-hospital team without lay basic life support (>10 minutes), presence of an initial non-shockable rhythm, and more than 20 minutes of advanced life support without ROSC49. As we already mentioned, there is currently no parameter which can adequately predict neurological outcome at hospital admission when the decision for immediate invasive coronary strategy is taken. Also, severe pre-arrest comorbidities and limited life expectancy should be taken into account. All these factors should be analysed on a per patient basis and the decision-making process individualised.
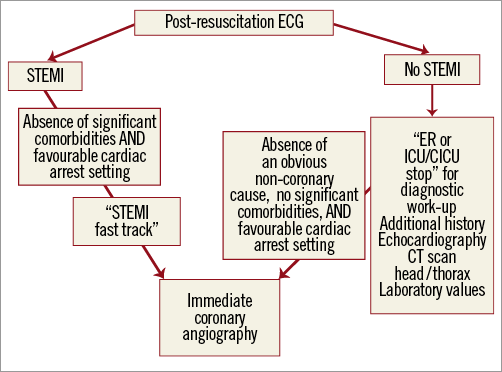
Figure 1. Selection of patients for immediate coronary invasive strategy. CICU: specialised cardiac intensive care unit; CT scan: computed tomography scanner; ECG: electrocardiogram; ER: emergency room; ICU: intensive care unit; STEMI; ST-segment elevation myocardial infarction. See text for the definition of favourable cardiac arrest setting.
Pre-hospital triage and regional networks among hospitals for the management of OHCA should be encouraged. Referral centres for OHCA with at least an intensive care unit and a “24-7” interventional cardiology department should be identified. Direct admission of survivors of OHCA to these centres is recommended. If a survivor of OHCA is admitted to a hospital without an interventional cardiology department, and the expected “time to reperfusion by PCI” is less than two hours4, transfer decisions to a catheterisation laboratory should be taken immediately with the referral centres, based on the same criteria as described previously. If the patient is more than two hours from a referral centre, transfer decisions are taken on a per patient basis. Thrombolytic therapy should only be considered if the patient cannot be transported within the appropriate delays for PCI, presents with clinical evidence suggestive of STEMI within 12 hours of symptom onset, and if there are no contraindications to thrombolytic therapy, such as prolonged and traumatic CPR21. However, one should keep in mind that assessment of contraindications to such therapy is often difficult after an OHCA. If thrombolysis is administered, the patient should then be transported to a centre with PCI capability and a CAG performed three to 24 hours after the administration of thrombolysis4.
Cardiac arrest PCI (CA-PCI)
CAG after resuscitated OHCA may reveal very heterogeneous findings particularly in patients without ST-elevation. These include angiographically normal coronary arteries, non-obstructive disease (<50% diameter stenosis), intermediate disease (50-70%), obstructive disease with angiographically stable appearance, and presence of typical culprit “ACS” lesion (TIMI 0 or 1 flow with an abrupt closure, or TIMI 2 or 3 flow with angiographic images suggesting thrombus or ulcerated plaques). The aim of CA-PCI is to reduce the incidence of recurrent cardiac arrest, to reduce infarct size in case of an acute occlusion and thereby to improve the haemodynamic status. In comatose survivors, who represent a major subgroup of resuscitated OHCA patients, stabilised haemodynamic status may allow enough time for possible neurological recovery during the following days.
There is a lack of studies addressing different revascularisation strategies in relation to angiographic characteristics, completeness of revascularisation and outcome. Again, we are left mainly with extrapolation of interventional “ACS” studies to this much higher-risk population of patients with resuscitated OHCA. We suggest an algorithm which integrates the angiographic characteristics of a lesion, its cause-effect relationship to the cardiac arrest, the haemodynamic status of a patient and the presence of post-resuscitation brain injury to obtain the maximal benefit and avoid futility at the time of the index intervention (Figure 2).
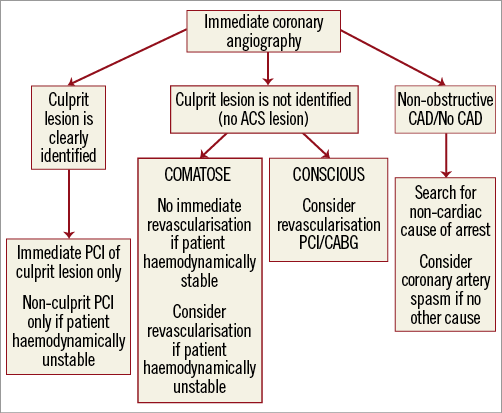
Figure 2. Revascularisation strategies in survivors of out-of-hospital cardiac arrest. ACS: acute coronary syndrome; CABG: coronary artery bypass graft surgery; CAD: coronary artery disease; PCI: percutaneous coronary intervention
Clear “ACS” lesions obviously represent the cause of cardiac arrest, and successful CA-PCI is likely to decrease the incidence of recurrent cardiac arrest, reduce infarct size and improve haemodynamic stability. Immediate CA-PCI should therefore be performed in clear “ACS” lesions and, in the absence of data, non-culprit PCI should probably be postponed despite very recent evidence indicating possible benefit in STEMI without OHCA50. Index CA-PCI of additional non-culprit but obviously obstructive lesions with stable angiographic appearance may be beneficial if the patient is haemodynamically unstable51. Immediate coronary artery bypass grafting (CABG) is a realistic option only in conscious survivors of OHCA on hospital admission with severe multivessel disease and not in comatose patients in whom the ultimate neurological outcome is difficult to predict.
Significant obstructive disease with angiographically stable appearance may also be the cause of cardiac arrest even though the cause-effect relationship is much less certain. Possible mechanisms include transient ischaemia caused by plaque thrombosis - spontaneous reperfusion, coronary spasm or decrease in perfusion pressure across the lesion or collaterals in case of sudden drop in arterial pressure. On the other hand, obstructive lesions may also be “innocent bystanders” without direct cause-effect relationship to the cardiac arrest. While in “conscious survivors” of cardiac arrest revascularisation by either PCI or CABG should be considered, decision making becomes more complex in comatose patients with uncertain neurological prognosis. We suggest that immediate CA-PCI of obviously obstructive “stable” lesion(s) be performed only in haemodynamically unstable patients51. If, on the other hand, a comatose survivor is haemodynamically stable, the decision for revascularisation, either by PCI or CABG, should be postponed and planned if the patient survives with no or minimal neurological sequelae.
Angiographic absence or presence of non-obstructive disease (<70%) is also important because a negative result should immediately trigger a search for alternative causes of OHCA. For example, if an acute cerebrovascular event or a massive pulmonary embolism is suspected, CT of the head and thorax should be performed on the way from the catheterisation laboratory to the intensive care unit. If an alternative cause of OHCA is not identified, coronary artery spasm should be considered, since several studies have shown that spasm may trigger lethal arrhythmias and lead to sudden death52-54. Accordingly, if the index procedure reveals normal coronary arteries, if the patient survives with no or minimal neurological sequelae, and if there is no other obvious cause of arrest, a coronary artery spasm provocation test may be performed during a second coronary angiogram by an experienced operator using either intracoronary acetylcholine or ergonovine54,55.
There are no published data regarding stent selection in the setting of CA-PCI. We believe contemporary drug-eluting stents should be preferred to bare metal stents in patients with a high likelihood of good neurological recovery. This includes conscious and selected comatose survivors of OHCA with favourable predictive factors for survival with no or minimal neurological sequelae (presence of initially shockable rhythm and short delays to advanced cardiac life support and ROSC).
There are no randomised data comparing the radial and femoral approaches for coronary procedures in survivors of OHCA. Radial access reduced mortality in the subgroup of patients with STEMI without OHCA56. We suggest using the radial approach as the default strategy in survivors of OHCA if the operator is experienced and if the radial pulse is present. However, femoral access may have an advantage in haemodynamically compromised patients who may require insertion of a percutaneous assist device. The introducer can be left at the end of the procedure to monitor blood pressure in the intensive care unit. There are no data on the use of femoral arterial closure devices in the setting of CAG or CA-PCI in survivors of OHCA.
Periprocedural anticoagulation and antiplatelet therapy
PCI with stenting is obviously associated with the need for anticoagulation and antiplatelet therapy to prevent stent thrombosis. In conscious survivors of OHCA, this treatment should be used according to the current guidelines4,12. No randomised studies are available for comatose survivors of cardiac arrest. Furthermore, these patients are often at high risk for bleeding complications due to chest compression and intubation before admission and often develop renal failure. Based on expert consensus, the following strategy is suggested for comatose patients:-
1) Intravenous administration of acetylsalicylic acid and anticoagulant therapy, for which we recommend unfractionated heparin (UFH), are generally advised after the assessment of coronary anatomy. However, in patients with clear STEMI on the post-resuscitation ECG who have a high likelihood of thrombotic occlusion and subsequent CA-PCI, this can be done prior to the CAG. UFH should be closely monitored during and after the procedure.
2) While acetylsalicylic acid, UFH and GP IIb/IIIa inhibitors may be administered by an intravenous route, P2Y12 inhibitors are available only in tablets. Since comatose survivors are intubated and mechanically ventilated during and after PCI when hypothermia is also initiated, administration of crushed tablets via a nasogastric tube remains the only option57. Early post-resuscitation state and ongoing hypothermia may significantly affect P2Y12 absorption and metabolism leading to delayed onset of action not only after clopidogrel loading58,59 but also in case of newer agents such as prasugrel and ticagrelor60 which result in no or suboptimal platelet inhibition. In cases with a high thrombotic burden, complex stenting, or “bail-out” situations, GP IIb/IIIa inhibitors may therefore be considered. There is very recent evidence that administration of eptifibatide in these settings results in profound platelet inhibition measured by both the VerifyNow IIb/IIIa and the Multiplate TRAP tests for at least 22 hours59. Even a bolus of GP IIb/IIIa without additional infusion might be sufficient to bridge the delayed effect of P2Y12 inhibitors, as demonstrated in patients with STEMI without cardiac arrest61,62.
Cangrelor may represent an attractive pharmacological solution to inhibit platelet reactivity temporarily in these patients but further studies are needed63. However, profound inhibition in platelet reactivity should always be weighed against the increased risk of bleeding due to possible traumatic injury related to chest compression and endotracheal intubation.
There are few and conflicting data regarding the rate of stent thrombosis after PCI in survivors of OHCA. A recent observational study suggests no increase compared to STEMI patients57, while other investigators found a 10.9% incidence of acute and subacute stent thrombosis in comatose survivors of OHCA, which was five times more than in a comparable group of patients with ACS but without cardiac arrest64.
Invasive haemodynamic support in survivors of OHCA and haemodynamic compromise
Post-resuscitation shock appears in 30 to 40% of survivors of OHCA, most often four to six hours after the arrest. The mechanisms are multifactorial and include vasoplegia and myocardial stunning. Immediate PCI seems to reduce the rate and intensity of post-resuscitation shock65. The role of haemodynamic support in patients with resuscitated OHCA and haemodynamic compromise has not been specifically studied. Feasibility and safety of the intra-aortic balloon pump (IABP) have been demonstrated in comatose survivors of OHCA with cardiogenic shock24. Such support was beneficial only in small and uncontrolled studies, while the SHOCK IABP trial, which included around 40% of patients with resuscitated cardiac arrest and cardiogenic shock66, and the CRISP AMI trial67 did not demonstrate any benefit of IABP. In the absence of proven benefit, routine periprocedural implantation of IABP cannot therefore be recommended in this setting and should be decided on an individual basis. This is true also for haemodynamically more effective devices including Impella™ (Abiomed, Inc., Danvers, MA, USA), TandemHeart™ (CardiacAssist, Inc., Pittsburgh, PA, USA) and venous-arterial extracorporeal membrane oxygenation (VA ECMO) which may be considered in this setting in conscious patients on admission and in comatose patients with a high likelihood for survival with no or minimal neurological sequelae and absence of severe pre-arrest comorbidities.
Refractory cardiac arrest
The number of patients admitted to hospital with refractory OHCA and no ROSC despite prolonged and ongoing advanced cardiac life support by manual or automated chest compression is increasing. Since there are only case reports or small case series of successful PCI performed during ongoing chest compression (which are clearly biased by mainly reporting survivors68), this strategy cannot be recommended as a universal clinical routine. However, it may be used in selected patients by experienced interventional cardiologists with a skilled resuscitation team taking care of the ongoing resuscitation. VA ECMO69,70 or Impella™71 have been successfully implemented in refractory cardiac arrest to restore perfusion promptly, to allow for subsequent CAG and PCI and to bridge until recovery of cardiac function. The potential benefits of such an aggressive strategy are currently being addressed by the controlled Prague OHCA randomised trial72. Another option is the pre-hospital implantation of VA ECMO73. However, due to the lack of data, these strategies should be either assessed in trials or applied to selected cases in experienced centres.
Induced hypothermia
The benefit of mild induced hypothermia between 32 and 34°C in comatose survivors of OHCA was demonstrated with the publication in 2002 of two randomised trials showing a marked increase in survival74,75. A recent randomised trial, however, demonstrated that maintaining a target core temperature at 36°C may be sufficient to facilitate neurological recovery76. While the debate regarding the optimal temperature is still ongoing, we suggest measuring the body temperature of survivors of OHCA when they are admitted to the catheterisation laboratory. In most cases the temperature will be less than 36°C either spontaneously or if cooling has been initiated before the arrival to the hospital or during the “emergency department or intensive care unit stop”. Interventional cardiologists may facilitate induction and maintenance of hypothermia by infusion of cold saline or the use of ice packs. The use of endovascular cooling catheters through the femoral vein into the inferior vena cava with the tip at the entrance of the right atrium has been suggested77. It is important to emphasise that angiographic success of CA-PCI seems not to be compromised by ongoing hypothermia27,57,78.
Call for interventional cardiologists to become members of the “post-resuscitation team”
Interventional cardiologists should become an essential part of the “survival chain” for patients with OHCA which includes bystander cardiopulmonary resuscitation, pre-hospital emergency medical units, emergency departments, acute cardiac care units and electrophysiology departments. Because immediate CAG and PCI are best achieved within existing STEMI networks, this system can also be used to become a “fast track” for patients with OHCA. The history and evolution of primary PCI for STEMI shows that there is a need to centralise the care of patients with OHCA to centres of excellence with a high-volume “24-7” interventional cardiology service, an intensive care unit, on-site cardiac and vascular surgery with a cardiac assist programme, and an integrated electrophysiology department. Indeed, there is evidence that patients with resuscitated OHCA admitted to such specialised cardiac arrest centres have better outcomes79,80.
Organisation of hospital “post-resuscitation team”
Because of the involvement of different specialists, it is very important to decide who will direct the treatment and take responsibility after hospital admission. Because of different hospital size, organisation and resource availability, this varies significantly among hospitals and countries. Our general recommendation is that an intensive care (ICU)/cardiac intensive care (CICU) physician or anaesthesiologist who ultimately admits the patient should take the lead. Since monitoring and treatment of the patient during the CAG and PCI procedure are crucial, ICU/CICU or anaesthesiology teams experienced in managing such patients should manage the patient in the catheterisation laboratory. This team should take care of therapeutic measures including mechanical ventilation, vasopressor/inotropic drug administration, cardiac rhythm management and induction of hypothermia if indicated. Uncontrolled movements of arms and legs (which may occur in some patients) can be managed by increasing sedation. This allows the interventional cardiologist to be focused on decision making based on angiographic findings and patient condition and to perform CA-PCI in optimal conditions. The interventional cardiologist together with ICU/CICU physicians/anaesthesiologists should also decide on the use of an invasive haemodynamic support. After the procedure, the patient should be admitted by the ICU/CICU physician/anaesthesiologist who is already familiar with the patient situation and who will take responsibility for further diagnostic procedures and treatment. After ICU/CICU discharge, the patient can be transferred to a cardiology unit. A “Heart Team” approach is necessary to discuss further revascularisation (PCI or CABG), spasm provocation studies, and diagnostic procedures such as MRI. The electrophysiology specialist is an essential part of the team to discuss electrophysiology testing, arrhythmia ablation or the implantation of an internal cardiac defibrillator.
Further research
Closer involvement of the interventional community is essential to stimulate appropriate randomised studies which will provide definitive answers about optimal patient selection for immediate CAG and guidance for optimal revascularisation strategy. While randomisation of patients with clear STEMI in post-resuscitation ECG does not seem to be ethical, there is undoubtedly a great need for a trial in patients presenting with no-STEMI post-resuscitation ECG patterns. CA-PCI strategy should be explored and the possible benefits of non-culprit PCI during the index procedure versus staged procedure should be addressed. Periprocedural pharmacology including antiplatelet effects of newer P2Y12 inhibitors and cangrelor in these settings is largely unknown. More reliable information is needed regarding stent thrombosis in this setting.
Acknowledgements
The authors are grateful to Peter Radsel, MD, for help in reviewing the published studies presented in Table 1, and to Etienne Puymirat, MD, PhD, Philippe Gabriel Steg, MD, and Stefan James, MD, for their critical review of the manuscript.
Conflict of interest statement
The authors have no conflicts of interest to declare.

