Abstract
Aims: Stent thrombosis (ST) is a rare but potentially fatal complication of coronary artery stenting. Little is known about the optimal treatment strategy at the time of an ST event. We aimed to identify the incidence and predictors of adverse cardiac events after treatment of a definite ST.
Methods and results: A total of 695 patients with definite ST were included between 1996 and 2017 in two academic medical centres. The primary endpoint was MACE, the composite of cardiac death, myocardial infarction (MI) and target vessel revascularisation (TVR). Mean age was 62.8±12.1 years and 76.3% were male. ST occurred at a median of 22 days (IQR 3-551 days); 50.8% were early and 49.2% were late/very late ST. At 60-month follow-up, the MACE rate was 43.7%, cardiac death 19.5%, MI 17.9%, TVR 24.8%, and repeat definite ST was 12.1% (10.5% in target vessel). Independent predictors of MACE were cardiogenic shock (HR 2.54, 95% CI: 1.75-3.70; p<0.001), ST in the LAD (HR 1.76, 95% CI: 1.32-2.35; p<0.001), prior CVA/TIA (HR 1.68, 95% CI: 1.08-2.62; p=0.020), peripheral vascular disease (HR 1.55, 95% CI: 1.00-2.39; p=0.046), multivessel disease (HR 1.53, 95% CI: 1.12-2.08; p=0.007), and final TIMI flow 2-3 (HR 0.54, 95% CI: 0.34-0.85; p=0.009). No specific treatment of ST influenced MACE; however, new-generation P2Y12 inhibitors reduced the risk of MI (HR 0.56, 95% CI: 0.32-0.99; p=0.049).
Conclusions: The incidence of adverse events remains high after a first episode of ST. New-generation P2Y12 inhibitors reduce the risk of MI. Additional stenting, GP IIb/IIIa inhibitors and thrombectomy did not improve outcomes following ST.
Introduction
Over the years, improvements in stent technology have reduced the incidence of future target lesion failure1. Conversely, stent thrombosis (ST) has emerged as a safety concern associated with high rates of death and myocardial infarction (MI)2. Amongst others, the problem has been linked to stent-related factors such as underexpansion, malapposition, polymer-related hypersensitivity reactions, neoatherosclerosis and incomplete stent coverage, and patient-related factors such as premature discontinuation of antiplatelet therapy3,4,5.
The risk of early or late ST appeared to occur at a rate of 0.6% per year after the implantation of a first-generation drug-eluting stent (DES)6, and up to 0.3% per year in novel-generation DES7,8. The latter triggered the development of more biocompatible and bioresorbable polymers and pushed guideline committees to review dual antiplatelet therapy (DAPT) strategies9. At the same time, exhaustive attempts were made to identify baseline patient and procedural characteristics associated with an increased risk for ST10,11,12,13.
To date, little is known about ST treatment strategies applied in daily clinical practice and their impact on adverse events. Therefore, the purpose of our study was to identify the incidence and predictors of future adverse cardiac events after treatment of a first definite ST.
Methods
POPULATION
This is a retrospective study including two academic hospitals (Erasmus University Medical Center, the Netherlands, and Bern University Hospital, Switzerland). All patients who presented with a first definite ST between 1996 and 2017 were included.
ENDPOINTS AND DEFINITIONS
The primary endpoint was major adverse cardiac events (MACE), a composite of cardiac death, non-fatal MI, and ischaemia-driven target vessel revascularisation (TVR) at 60-month follow-up after the first ST event. Death was classified as cardiac or non-cardiac. Secondary endpoints included the components of MACE and repeat definite ST in the target vessel (ST-TV). Cardiac death was defined as any death due to a clear cardiac cause, unwitnessed death or death of unknown cause, and all procedure-related deaths, including those related to concomitant treatment. Coronary artery bypass grafting (CABG) revascularisation was considered an event if not part of the initial ST treatment. TVR, MI and ST were defined according to the Academic Research Consortium definitions14. Repeat ST-TV was identified as any new definite ST in the target vessel after the successful treatment of the index ST.
CLINICAL FOLLOW-UP
Survival data were obtained from municipal civil registries. A health questionnaire was sent to all living patients with questions on re-admission and MACE. For patients who had an adverse event at another centre, medical records or discharge summaries were systematically reviewed. General practitioners, referring cardiologists, and patients were contacted as necessary for additional information. There was no independent or external monitoring of data entry. We performed censoring at 60 months with 14 patients lost to follow-up. Clinical events were adjudicated by trained study personnel not involved in the specific procedures during the course of the study. All patients provided written informed consent for the procedure and the use of anonymised data sets for research purposes in alignment with the Dutch Medical Research Acts and the appropriate Health Insurance Portability and Accountability Act waiver/authorisation or the appropriate informed consent documentation per institutional policy for the collection of data in Switzerland.
STATISTICAL ANALYSIS
Categorical variables are expressed as numbers and frequencies and compared using the χ² test or Fisher’s exact test when appropriate. Continuous variables are presented as the mean±standard deviation (SD) and tested using the Student’s t-test or as the median and interquartile range (IQR: 25th-75th percentile) and tested with the Mann-Whitney rank-sum test.
Missing values for covariates were present in less than 5%, except for smoking (6.6% missing values), statin prescription (6.8% missing values), index stent type (15.5% missing values), multivessel disease (MVD) (17.6% missing values), and estimated glomerular filtration rate <60 ml/min/1.73 m² (34.2% missing values). Therefore, we applied multiple imputation to handle missing values. Values were imputed using a regression approach based on patients’ clinical data. Results from five imputed data sets were pooled to obtain risk estimates.
Univariate predictors of outcomes were identified using Cox proportional hazards models. Predictors with a p-value <0.1 were introduced into the multivariate Cox proportional hazards model using the “enter” method. In case of outcomes with an insufficient number of events, the most strongly associated covariates were included in the model. Data are presented as hazard ratios (HRs) with 95% confidence intervals (95% CI). All tests were two-tailed and a p-value <0.05 was considered statistically significant. The Kaplan-Meier method was applied to show the cumulative incidence of the primary and secondary endpoints.
SPSS software, Version 24.0 for Windows (IBM Corp., Armonk, NY, USA) was used to perform all the analyses.
Results
CLINICAL PRESENTATION
A total of 695 patients presenting with a first episode of definite ST were included. Mean age was 62.8±12.1 years and 76.3% were male. The first ST occurred at a median of 22 days (25th-75th percentile: 3-551 days; min 0, max 5,859 days) after the index percutaneous coronary intervention (PCI). Early ST (0-30 days) and late/very late ST (>30 days) occurred in 50.8% and 49.2% of the cases, respectively. MI was the presenting symptom in 87.2% of the cases and accompanied by cardiogenic shock in 11.8%. Aspirin was used by 88.9% of the patients at baseline and 53.8% used P2Y12 inhibitors (Table 1, Table 2).
According to the timing of ST (early vs late/very late), patients with early ST were older (64.1±12.1 vs 61.4±11.9 years, respectively, p=0.004), presented more often with MI (93.4% vs 80.6%, respectively, p<0.001) and haemodynamic instability (16.9% vs 6.9%, respectively, p<0.001), had multivessel ST (5.2% vs 1.5%, p=0.007) or left coronary system as culprit (for LAD 58.6% vs 48.5%, p=0.009; for left circumflex artery 21.2% vs 14.1%, respectively, p=0.015) (Supplementary Table 1, Supplementary Table 2).
TREATMENT
Thrombectomy and glycoprotein IIb/IIIa (GP IIb/IIIa) inhibitors were used in 47.9% and 57.6% of the patients, respectively. In 27.6% of the patients, intracoronary imaging was used to assess the mechanism of the ST, with neoatherosclerosis (31.8%), malapposition (25.5%) and underexpansion (17.7%) as the main findings (Table 2). Additional stenting was performed in 59.8% of the patients with the use of DES in 90.8%. Plain old balloon angioplasty (POBA) alone was performed in 34.4% of the cases and CABG in 0.9%. DAPT was prescribed in 95.7% of the patients, 28.1% of whom received either prasugrel or ticagrelor. The remaining patients were treated with a combination of an oral anticoagulant (OAC) and one antiplatelet therapy (APT) (1.1%), one APT (1.8%) or OAC alone (0.3%); in 1.1% of the cases no APT or OAC was prescribed due to concomitant major bleeding (Table 2).
As compared to late/very late ST, patients with early ST more often received treatment with POBA (45.6% vs 24.3%, respectively, p<0.001) and GP IIb/IIIa inhibitors (66.6% vs 47.9%, p<0.001), but fewer patients with early ST underwent intracoronary imaging assessment as compared to those with late/very late ST (24.3% vs 30.8%, respectively, p=0.058) (Supplementary Table 2).
OUTCOMES
At 60 months, the cumulative incidence of the primary composite endpoint was 43.7% (238 cases). Cardiac death occurred in 19.5% (111 cases), MI in 17.9% (82 cases) and TVR in 24.8% (118 cases). Repeat definite ST occurred in 12.1% (58 cases) and repeat definite ST-TV in 10.5% (51 cases) (acute 9.8% [5 cases], subacute 27.5% [14 cases], late 23.5% [12 cases], and very late 39.2% [20 cases]) (Figure 1).
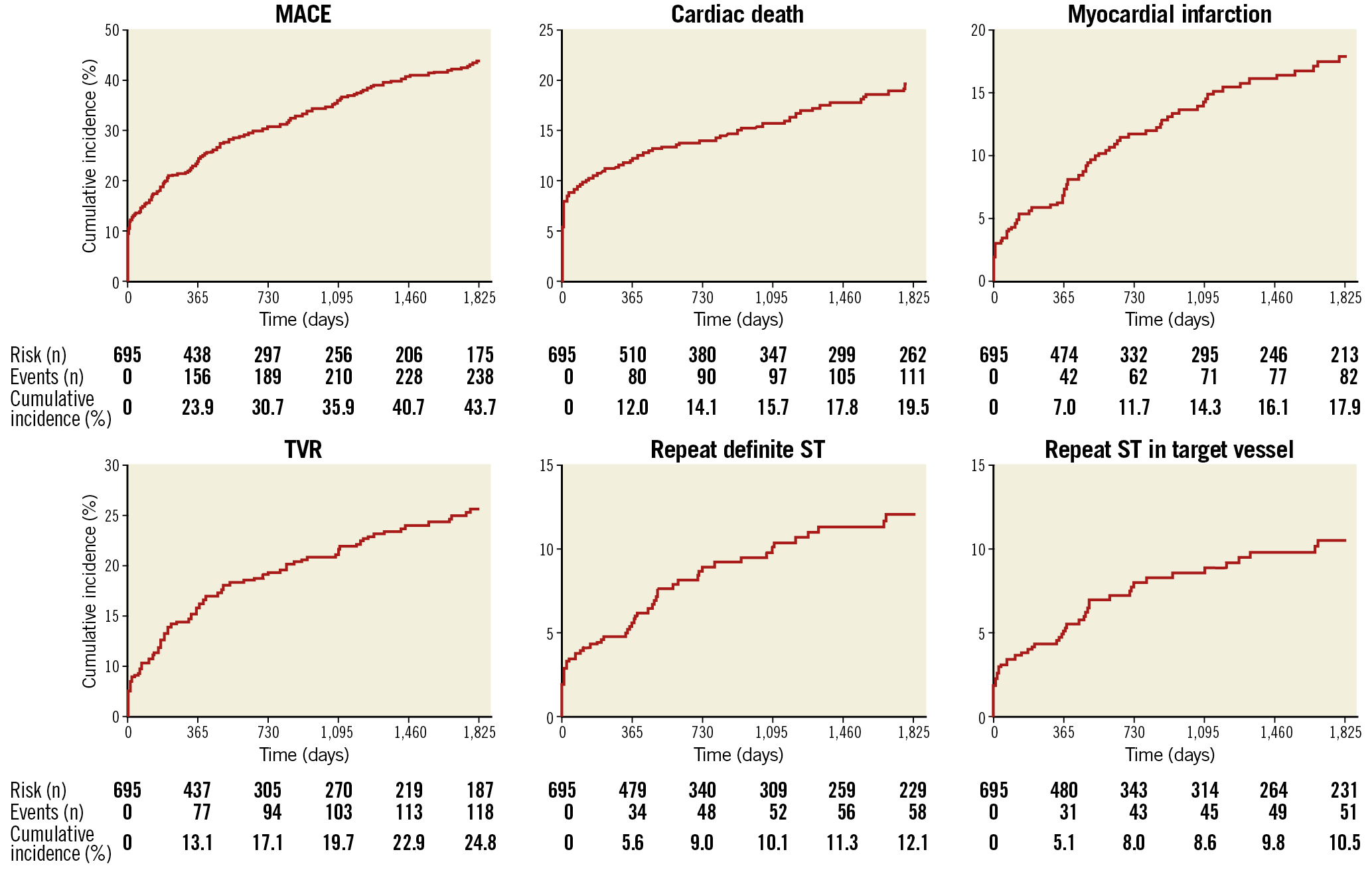
Figure 1. Outcomes at 60-month follow-up. MACE: major adverse cardiac events; ST: stent thrombosis; TVR: target vessel revascularisation
Independent predictors of MACE were cardiogenic shock (HR 2.54, 95% CI: 1.75-3.70; p<0.001), ST in the LAD (HR 1.76, 95% CI: 1.32-2.35; p<0.001), prior cerebrovascular accident (CVA)/transient ischaemic attack (TIA) (HR 1.68, 95% CI: 1.08-2.62; p=0.020), peripheral vascular disease (HR 1.55, 95% CI: 1.00-2.39; p=0.046) and MVD (HR 1.53, 95% CI: 1.12-2.08; p=0.007). Final Thrombolysis In Myocardial Infarction (TIMI) flow 2-3 was inversely associated with MACE (HR 0.54, 95% CI: 0.34-0.85; p=0.009) and cardiac death (HR 0.33, 95% CI: 0.18-0.60; p<0.001) at 60 months. Treatment with new-generation P2Y12 inhibitors was inversely associated with future MI events (HR 0.56, 95% CI: 0.32-0.99; p=0.049), and the use of intracoronary imaging was associated with an increased risk for repeat ST-TV (HR 1.85, 95% CI: 1.06-3.23; p=0.032). No other modifiable procedural characteristics predicted any of the outcomes (Table 3).
According to the timing of the ST, similar predictors were found for MACE following early ST as for the total population. Cardiogenic shock and final TIMI flow 2-3 were the only independent predictors for MACE in patients with late/very late ST. Intracoronary imaging increased the risk for future MI, TVR and ST-TV in patients with early ST, and index stent type DES reduced future MI events in patients with late/very late ST. No procedural characteristics predicted any of the outcomes in patients presenting with late/very late ST (Supplementary Table 3, Supplementary Table 4).
When considering the time point of the index ST (years 1996-2007 and 2008-2017), similar predictors of MACE were found for both groups as for the total population, except for additional stenting which increased the risk of adverse events in the first group (HR 1.82, 95% CI: 1.16-2.86; p=0.008) (Supplementary Table 5).
Discussion
Patients presenting with ST have a significantly increased risk for morbidity and mortality following PCI. While extensive research has been performed on finding predictors of ST10,11,12,13, little to no evidence is available on the optimal treatment strategy for those presenting with the event. Furthermore, the low incidence of ST and the lack of systematic follow-up entail great difficulty in recognising the real incidence of adverse events and their predictors. In the present investigation we assessed the incidence and predictors of future MACE after the treatment of a first definite ST in the largest series of patients thus far.
At first, we quantified the incidence of MACE after the index ST. At 60 months, almost every second ST patient suffered from MACE (43.7%), mainly driven by a high mortality rate (25.8%), 75% of which were cardiac. Furthermore, the incidence of TVR was as high as 24.8%. Interestingly, 51 out of 118 TVR (43.2%) resulted from a repeat ST-TV event, indicating that the applied ST treatment was ineffective in a substantial proportion of patients. Looking for baseline predictors, we found that cardiogenic shock, ST in the LAD and post-procedural TIMI flow were strong predictors of MACE; similar patient and lesion-related factors have been found in previous studies with smaller patient cohorts and shorter follow-up15,16,17,18,19.
With a specific focus on modifiable procedural characteristics, we found that 59.8% of the patients were treated with additional stents (90.8% were DES). Their use, however, did not impact on future MACE. The latter puts the findings of the Dutch Stent Thrombosis registry (DSR), in which the use of additional stents increased cardiac death and repeat ST up to 73% at three years, into perspective17. Merely 26% of the patients in the DSR presented with late or very late ST as compared to 49.2% in our study - an important difference given the substantially higher incidence of neoatherosclerosis in patients with late or very late ST as compared to early ST. Furthermore, the difference in timing between both studies should be taken into account, resulting in a significant difference in the use of bare metal stents (BMS) and new P2Y12 inhibitors (±50% and 0%, respectively, in the DSR).
Thrombus aspiration did not emerge as a protective measure against future MACE. The latter extends the findings of several recent randomised trials in which thrombus aspiration failed to reduce future events in STEMI patients17,20,21.
Significant improvement in the risk of future MI was also found with the use of either prasugrel or ticagrelor in patients presenting with ST, a finding that adds to previous studies including ACS populations22,23,24.
Differences in treatment profiles were found in patients presenting with early versus late/very late ST. Patients with an early ST event were more likely to receive treatment with POBA and GP IIb/IIIa inhibitors, which is in line with the assumption that stent deployment-related issues and an initial impaired response to ADP-receptor antagonist therapy during a prothrombotic state mostly explain an early ST event2,25. Intravascular imaging findings confirmed a higher incidence of procedure-related issues (underexpansion and edge dissections) in this population. Moreover, additional stenting was more frequent in patients with late or very late ST, which could suggest a higher incidence of neoatherosclerosis.
A stratified analysis following either early or late/very late ST revealed one remarkable finding: the risk for future MI, TVR and repeat ST-TV appeared to be significantly increased when intravascular imaging was performed. Intravascular imaging was performed more frequently in younger and male patients, cases where the index stent was bioresorbable, the LAD was the culprit, and the presentation of the ST was “very late”. Furthermore, those patients more often received treatment with GP IIb/IIIa inhibitors, thrombectomy, and direct stenting, with a larger stent number and length. However, we were not able to identify a consistent and significantly higher risk profile of patients receiving imaging versus those who did not (Supplementary Table 6). Finally, a play of chance could not be excluded.
It is essential to remark that including patients over almost 20 years is both our strength and our main limitation. Several important changes have taken place in the coronary field, entailing great difficulty in finding individual predictors of future outcomes. Periprocedural treatment strategies have been influenced by novel insights, the availability of pharmacological and technical resources, and improvements in stent technology; as such, optical coherence tomography was only introduced in 2008, the new-generation P2Y12 inhibitors became available in 2009, and stents have evolved from BMS to DES (first and second generations) to platforms with bioresorbable polymers/backbone. Nevertheless, a sensitivity analysis regarding the time of presentation showed similar predictors as for the whole population; of note, additional stenting was a strong predictor of MACE only in the population 1996-2007, which could be explained by a higher use of earlier stent technologies26.
Limitations
This is a retrospective study including patients over a long period of time; changes in treatment strategies over the years might have influenced our results. An important selection bias might be present concerning the use of intracoronary imaging. Information on lesion complexity was not available. Data on compliance to antiplatelet agents were not accessible. Finally, given the retrospective nature of the study analysis, there were some missing baseline data and a multiple imputation technique was used. Hence, our results are hypothesis-generating only and must be confirmed in larger-scale randomised studies.
Conclusions
The incidence of adverse events remains high after a first episode of ST. Treatment with new-generation P2Y12 inhibitors reduces the risk of future MI. The use of new stents, GP IIb/IIIa inhibitors and thrombectomy was not associated with improved cardiovascular outcomes following ST.
|
Impact on daily practice There is a significantly increased risk for morbidity and mortality following the treatment of a first ST episode. While placing stents and using GP IIb/IIIa inhibitors was not shown to improve outcome, treatment with new-generation P2Y12 inhibitors might be preferable to clopidogrel in order to reduce the risk of myocardial infarction. Larger and randomised studies are needed to compare the effect of procedural and medical treatment strategies for ST in the current era. |
Conflict of interest statement
J. Daemen has received institutional grant/research support from Abbott Vascular, Boston Scientific, ACIST Medical, Medtronic and PulseCath, and consultancy and speaker fees from Pythagoras Medical, ACIST Medical, Medtronic and PulseCath. S. Windecker has received research grants from Amgen, Abbott Vascular, Bayer, Boston Scientific, Biotronik, Medtronic, Edwards Lifesciences, St. Jude and Terumo. L. Räber has received speaker fees from Abbott, Amgen, and Sanofi, and research grants from Abbott, Sanofi, Regeneron and HeartFlow. T. Zanchin has received a grant from the Swiss National Science Foundation (grant no. 323530_171146). The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

