Abstract
Background: There are limited data on the outcomes of percutaneous coronary intervention (PCI) following stent thrombosis (ST) and differences exist based on timing.
Aims: Our aim was to study the rates of PCI procedures for an ST indication among all patients admitted for PCI at a national level and to compare their characteristics and procedural outcomes based on ST timing.
Methods: All PCI procedures in England and Wales (2014-2020) were retrospectively analysed and stratified by the presence of ST into four groups: non-ST, early ST (0-30 days), late ST (>30-360 days), very late ST (>360 days). Multivariable logistic regression models were performed to assess the odds ratios (OR) of in-hospital MACCE (major adverse cardiovascular and cerebrovascular events, a composite of mortality, acute stroke and reinfarction) and mortality.
Results: Overall, 7,923 (1.4%) procedures were for ST indication, most commonly for early ST (n=4,171; 52.6%), followed by very late ST (n=2,801; 35.4%) and late ST (n=951; 12.0%). The rate of PCI for ST declined between 2014 and 2020 (1.7 to 1.4%; p<0.001). Early ST was the only subgroup associated with increased odds of MACCE (OR 1.22, 95% CI: 1.05-1.41), all-cause mortality (OR 1.21, 95% CI: 1.07-1.36) and reinfarction (OR 2.48, 95% CI: 1.48-4.14), compared with non-ST indication. The odds of mortality were significantly reduced in ST patients with the use of intravascular imaging (OR 0.66, 95% CI: 0.48-0.92) and newer P2Y12 inhibitors (ticagrelor: OR 0.69, 95% CI: 0.49-0.95; prasugrel: OR 0.54, 95% CI: 0.30-0.96).
Conclusions: PCI for ST has declined in frequency over a 7-year period, with most procedures performed for early ST. Among the different times of ST onset, only early ST is associated with worse clinical outcomes after PCI. Routine use of intravascular imaging and newer P2Y12 inhibitors could further improve outcomes in this high-risk procedural group.
Introduction
Percutaneous coronary intervention (PCI) is the most common means of coronary revascularisation12. A rare, yet serious complication of PCI is stent thrombosis (ST), which can occur at different time points post-procedure, ranging in onset from acute (0-24h) to subacute (24h-30 days), late (31-360 days) and very late (>360 days)3. The incidence of ST is estimated to be up to 1% within the first year, declining to rates of between 0.2% and 0.4% thereafter4567. Risk factors for ST differ according to time of onset, as does the overall prognosis8.
ST often presents with an acute myocardial infarction (AMI) and requires urgent PCI9, although there are limited data on the characteristics, management and outcomes of these patients when undergoing PCI, and whether this varies according to the timing of ST in relation to the index PCI. Furthermore, whilst patients suffering from ST have been shown to be at a very high risk of mortality, little is known about the predictors of in-hospital adverse outcomes1011. Finally, there are limited data on the frequency of PCI for ST from a national perspective and whether this has changed in recent years, commensurate with advances in stent platforms, procedural techniques (e.g., the use of intracoronary imaging), and the greater use of newer P2Y12 inhibitors121314.
The present study sought to examine the rates, characteristics and in-hospital outcomes in patients presenting with ST undergoing PCI, compared with PCI for non-ST indication, with further stratification according to the timing of ST onset, in a nationwide cohort of PCI procedures in England and Wales between April 2014 and March 2020.
Methods
Data source, study design and population
All adult (aged ≥18 years) PCI procedures for acute coronary syndrome (ACS) between 1 April 2014 and 31 March 2020 in England and Wales from the British Cardiovascular Intervention Society (BCIS) registry were retrospectively analysed and stratified by indication for PCI into two groups: ST and non-ST. Further stratification was performed based on the timing of ST from the index PCI: early (0-30 days), late (>30 days to 360 days) and very late (>360 days). The BCIS registry comprises clinical and procedural data, and in-hospital outcomes (death, bleeding, arterial complications) for all procedures undertaken in England and Wales1516. There were no specified exclusion criteria except missing data for death (n=14,150; 2.4% of the original cohort) and stent thrombosis (n=18,008; 3.0% of the original cohort) (Supplementary Figure 1).
Outcomes
The main outcomes were in-hospital major adverse cardiovascular and cerebrovascular events (MACCE; a composite of death, acute stroke/transient ischaemic attack and reinfarction), all-cause mortality and Bleeding Academic Research Consortium (BARC) stage 3-5 bleeding, as per the standard definition17.
Statistical analysis
Patient and procedural characteristics of patients undergoing PCI for ACS were compared according to indication (ST and non-ST) and by the timing of ST (early ST, late ST and very late ST) as part of the exploratory analysis. Continuous variables were normally distributed and, therefore, presented as mean values with standard deviation (SD), and compared using the Student’s t-test. Categorical variables are summarised as percentages and analysed using the chi-squared test. Multivariable logistic regression models were performed to 1) assess the association between ST (overall and different onsets) and in-hospital adverse outcomes, namely MACCE, mortality, BARC 3-5 bleeding and acute stroke, and 2) examine predictors of in-hospital adverse outcomes among patients undergoing PCI for ST indication. All associations are reported as odds ratios (OR) with corresponding 95% confidence intervals (CI).
Variables adjusted for in the models included: age, sex, race, clinical syndrome (ST-elevation myocardial infarction [STEMI] vs non-STEMI), previous AMI, previous PCI, previous coronary artery bypass graft surgery (CABG), previous non-coronary surgery, diabetes mellitus, renal failure (creatinine >200 µmol/l and/or dialysis), cardiac transplant, family history of ischaemic heart disease (IHD), left ventricular (LV) function, hypercholesterolaemia, peripheral vascular disease (PVD), previous cerebrovascular accident (CVA, stroke or transient ischaemic attack), hypertension, smoking, valvular heart disease (VHD), out-of-hospital cardiac arrest (OHCA), mechanical ventilation, circulatory support (intra-aortic balloon pump or LV assist device), vascular access (radial vs femoral), number of vessels and lesions attempted, number of stents, drug-eluting stent (DES), DES stent generation (1st vs 2nd/3rd generation), use of fractional flow reserve (FFR), intravascular imaging (intravascular ultrasound [IVUS] or optimal coherence tomography [OCT]), calcium modification (rotablation, laser angioplasty), vessel attempted (prognostic targets: left main stem [LMS], proximal left anterior descending [LAD], graft), and in-hospital pharmacotherapy (aspirin, clopidogrel, ticagrelor, prasugrel, warfarin, glycoprotein IIb/IIIa inhibitor [GP-2b3a], bivalirudin).
Multiple imputation with chained equations was performed for variables with missing data (except ST and death) prior to model fitting, with a total of 10 imputations. Model estimates were later combined using Rubin’s rules18. All statistical analyses were performed using Stata 16 MP (Stata Corp).
Results
A total of 571,949 procedures were performed in England and Wales between 1 April 2014 and 31 March 2020, of which 351,735 (61.5%) were for an ACS indication. Among those with ACS, 7,923 cases (~1.4% of the total cohort, 2.3% of the ACS cohort) were for ST indication including 4,171 (52.6%) cases for early ST, 951 (12.0%) cases for late ST, and 2,801 (35.4%) cases for very late ST. Both the rate and frequency of ST among all PCI indications decreased over the study period in the overall cohort (2014 to 2020; rate: 1.7% to 1.4%, frequency: 2.4 to 2.2 per 100,000 population; ptrend <0.01 for both) (Figure 1). Similar findings were observed in both sexes, although ST indication was more frequent in males than females throughout the study period (2014 to 2020; rate: 1.8% to 1.5% for males, 1.3% to 1.0% for females; frequency: 3.8 to 3.6 per 100,000 population for males and 1.0 to 0.8 per 100,000 population for females; ptrend ≤0.01 for all). The frequency of ST per 100,000 population was also higher among those aged >60 years throughout the study period.
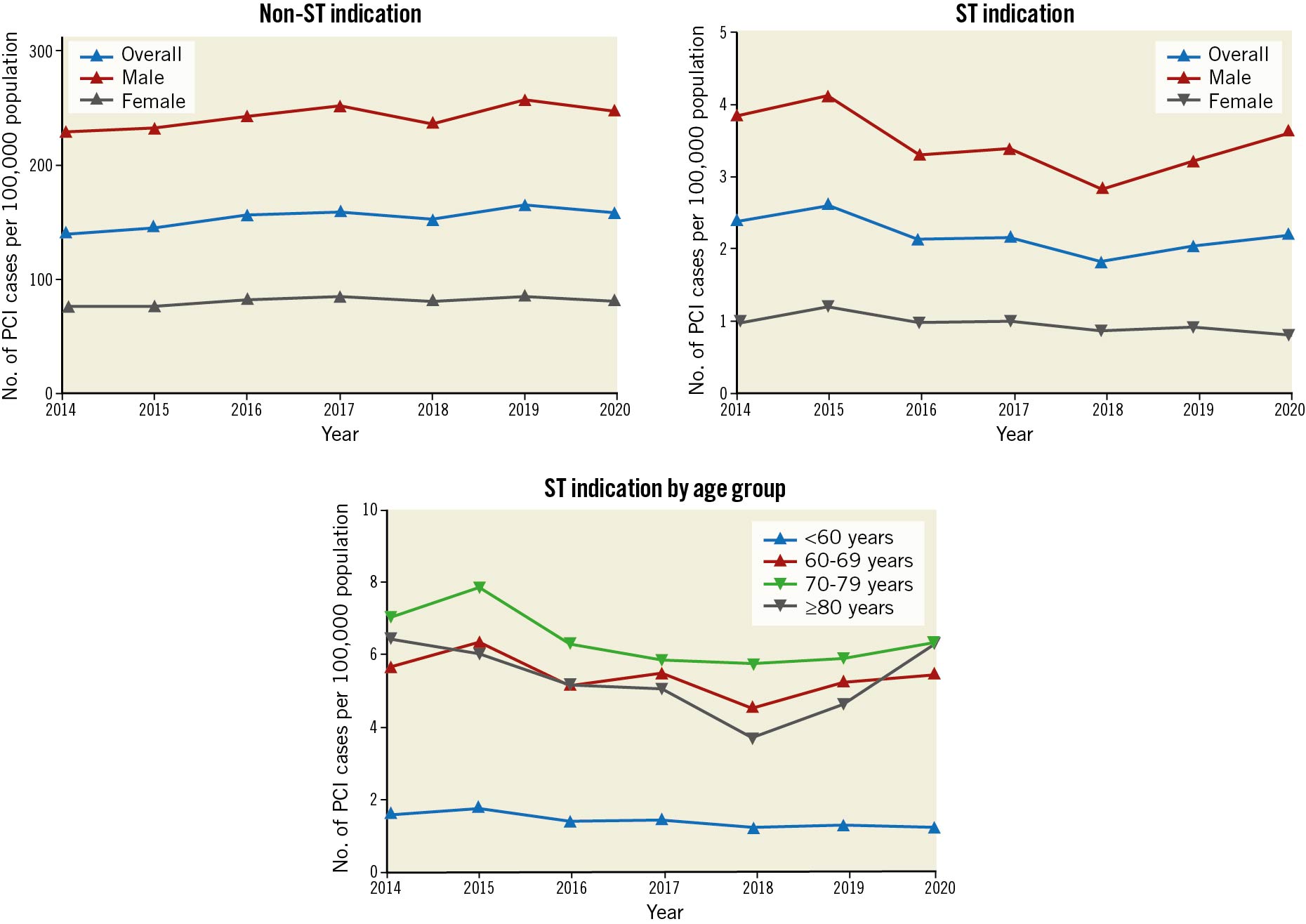
Figure 1. Frequency of PCI procedures per 100,000 population according to indication (2014 to 2020). PCI: percutaneous coronary intervention; ST: stent thrombosis
Patient characteristics
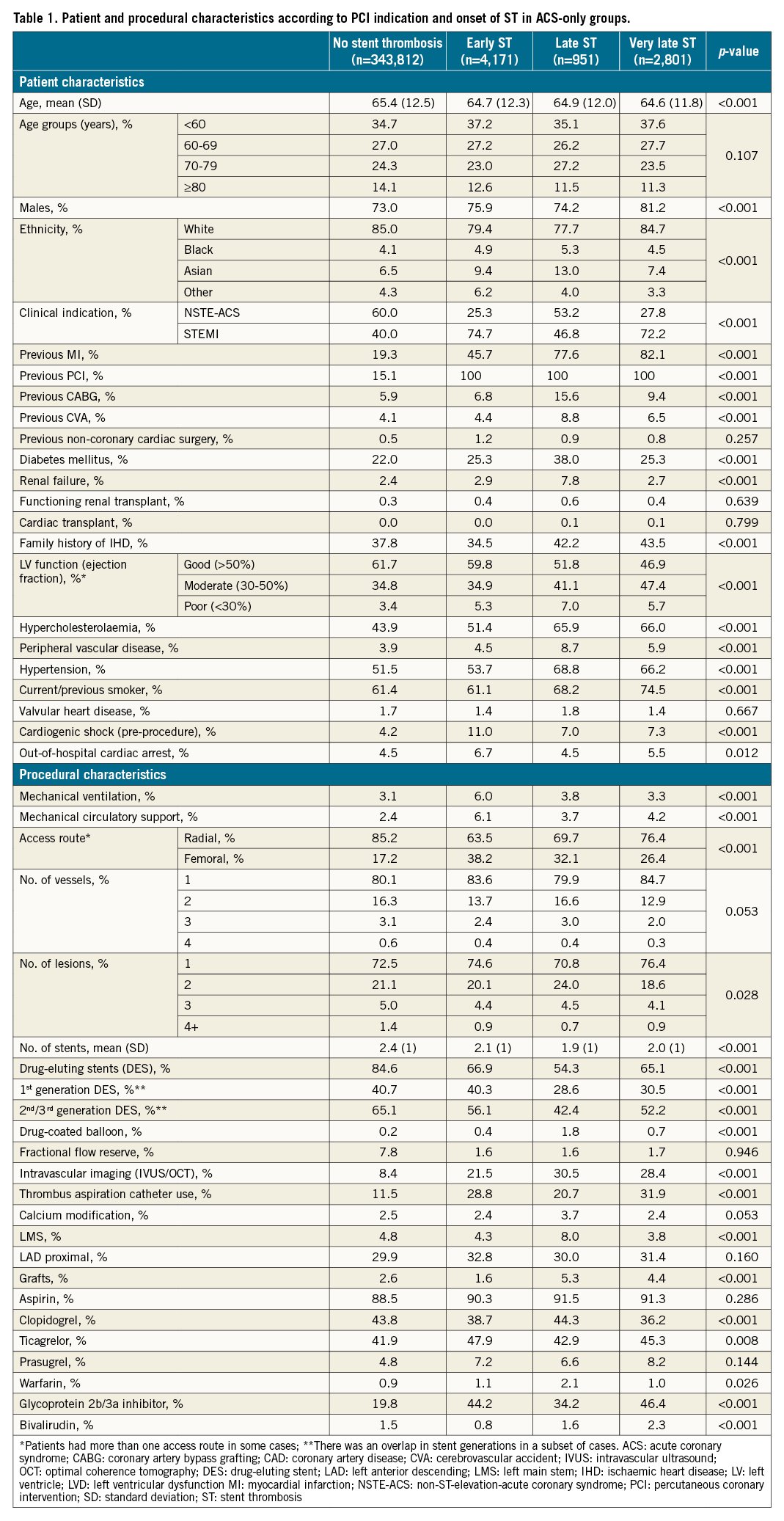
Several differences in patient characteristics were observed between patients undergoing PCI for a non-ST indication, and patients undergoing PCI for an ST indication at different times of onset (Table 1). Patients with an ST indication were generally younger (mean age 64.6 to 64.9 years vs 65.4 years), were more likely to be male (74.2 to 81.2% vs 73.0%), from a non-white background (white: 77.7 to 84.7% vs 85.0%) and to present with STEMI (46.8 to 74.7% vs 40.0%). ST patients had a higher prevalence of renal failure (2.7 to 7.8% vs 2.4%), especially in the late ST group (7.8%), as well as diabetes mellitus (25.3 to 38.0% vs 22.0%), hypertension (53.7 to 68.8% vs 51.5%), PVD (4.5 to 8.7% vs 3.9%), hypercholesterolaemia (51.4 to 66.0% vs 43.9%), previous CVA (4.4 to 8.8% vs 4.1%) and MI (45.7 to 82.1% vs 19.3%). ST patients were also more likely to have LV impairment (good LV function: 46.9 to 59.8% vs 61.7%), experience out-of-hospital cardiac arrest (early ST: 6.7% and very late ST 5.5% vs 4.5% for non-ST) as well as preprocedural cardiogenic shock (7.0 to 11.0% vs 4.2%).
Procedural characteristics
Patients with an ST indication for PCI were more likely to require mechanical ventilation (3.3 to 6.0% vs 3.1%), circulatory support (4.2 to 6.1% vs 2.4%), and femoral access for intervention (26.4 to 38.2% vs 17.2%) compared with the non-ST indication group. Procedures for patients with early ST and very late ST more commonly involved the proximal LAD (31.4 and 32.8%, respectively vs 29.9%) while those with late ST were more likely to undergo an LMS intervention (8.0%). The utilisation of IVUS/OCT was significantly higher in patients with an ST indication (21.5 to 30.5% vs 8.4%). Patients with an ST indication were less likely to receive DES (54.3 to 66.9% vs 84.6%) and more likely to be managed with drug-coated balloons (0.4 to 1.8% vs 0.2%) compared with those with a non-ST indication. Patients with an ST indication were also more likely to receive intraprocedural GP-2b3a inhibitors (34.2 to 46.4% vs 19.8%) as well as newer P2Y12 agents such as prasugrel (6.6 to 8.2% vs 4.8%) and ticagrelor (42.9 to 47.9% vs 41.9%) as part of their antithrombotic regime.
Similar patterns in patient and procedural characteristics were observed between the overall ST indication and non-ST indication groups (Supplementary Table 1).
In-hospital outcomes
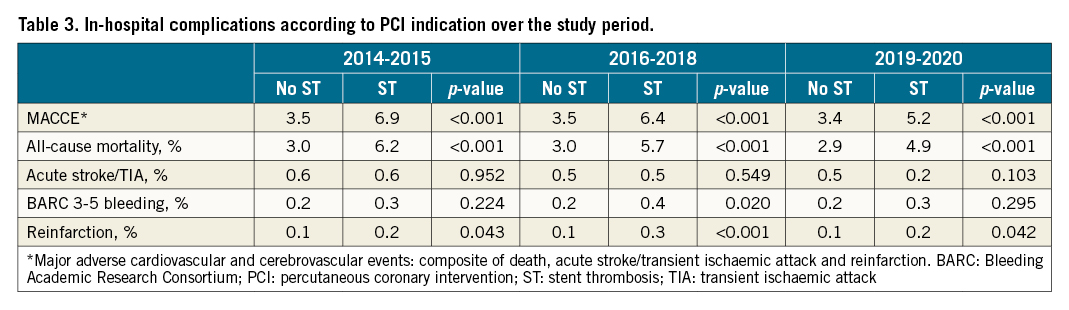
Overall, patients undergoing PCI for an ST indication experienced higher crude rates of MACCE (6.4% vs 3.5%) compared with non-ST indication PCI (Supplementary Table 2), a pattern that was consistent across all ST time onset groups and highest in the early ST group (early ST: 8.1%, late ST: 4.8%, very late ST: 4.0%) (Table 2, Figure 2). This was primarily driven by higher all-cause mortality (overall ST: 5.7%, early ST: 7.2%, late ST: 4.6%, very late ST: 3.7% vs non-ST: 3.0%). All other adverse outcomes were higher in the ST onset groups compared with the non-ST group, including BARC 3-5 bleeding and reinfarction. However, no difference in acute stroke/TIA was observed between the non-ST indication groups and the overall ST group (p=0.784) or any of the three ST onset groups (p=0.326). While the rates of MACCE, all-cause mortality and reinfarction were stable in the non-ST group during the study years (from 2014-2015 to 2019-2020), they declined in the ST group (Table 3).
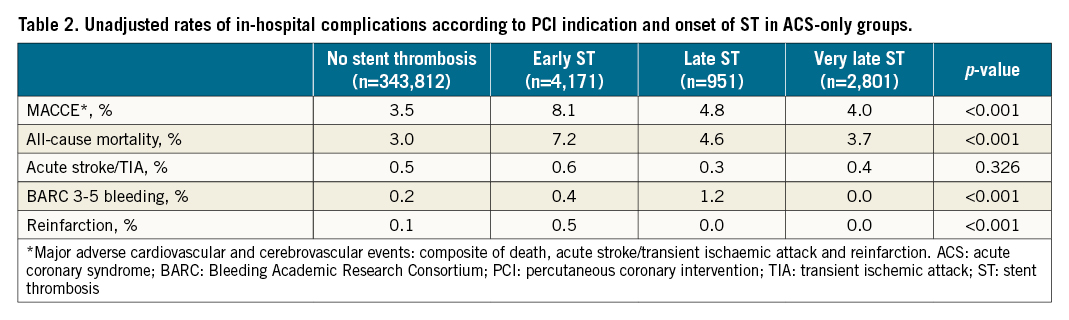
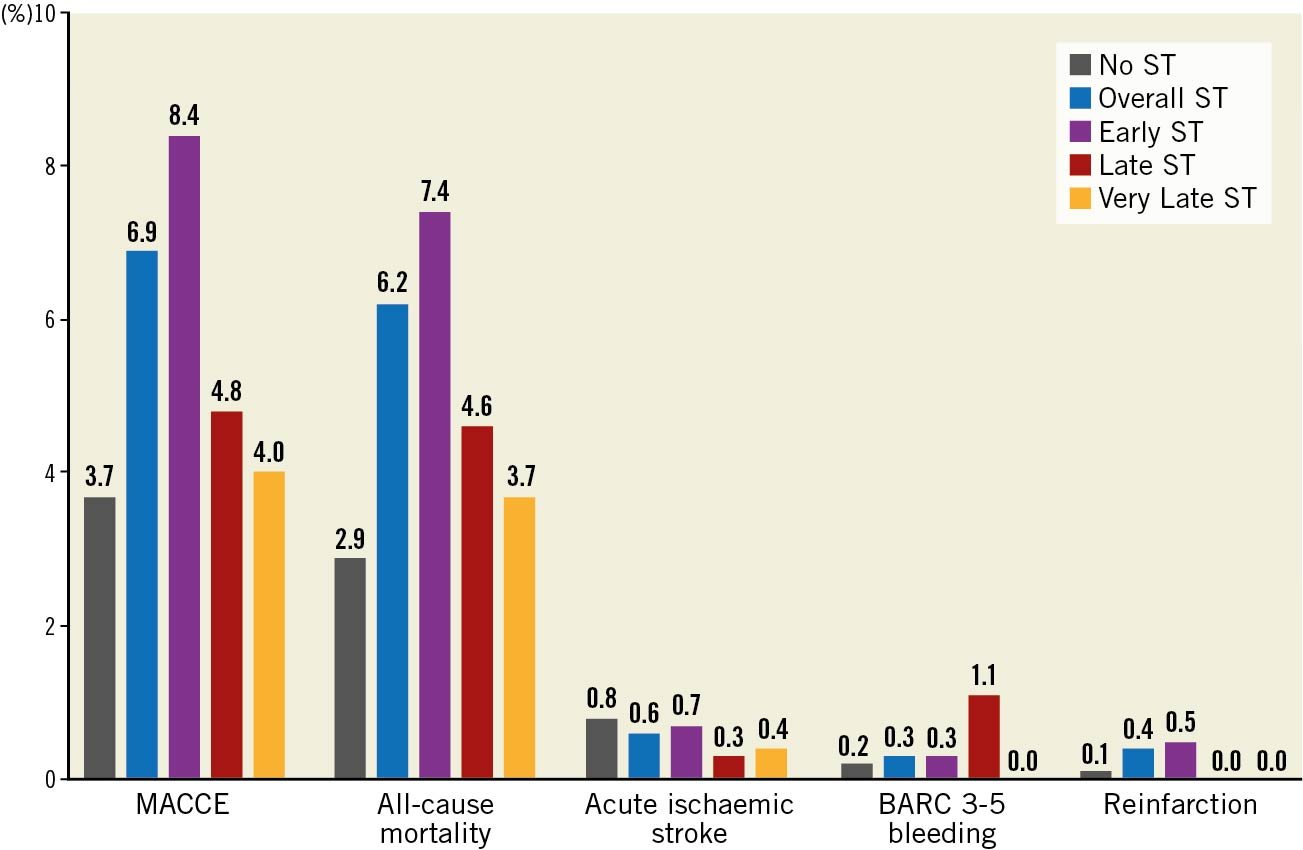
Figure 2. In-hospital post-procedural adverse outcomes according to indication for PCI. p<0.01 for all except acute ischaemic stroke (p=0.256 for overall ST vs non-ST groups, p=0.784 for ST onset vs non-ST groups). BARC: Bleeding Academic Research Consortium; MACCE: major adverse cardiac and cerebrovascular events; ST: stent thrombosis
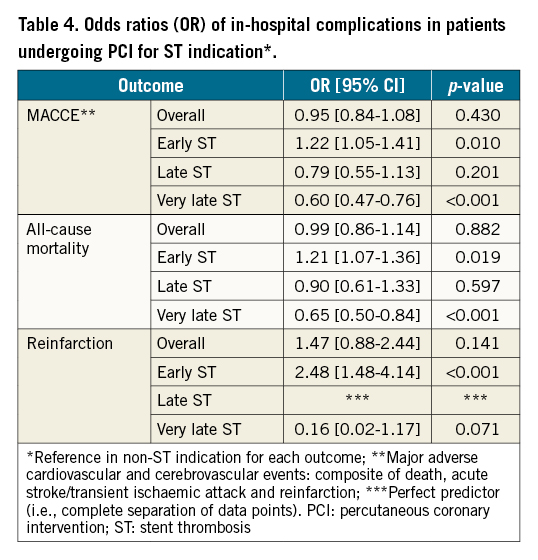
After adjustment for baseline differences, only patients with early ST were associated with increased odds of MACCE (OR 1.22, 95% CI: 1.05-1.41), all-cause mortality (OR 1.21, 95% CI: 1.07-1.36) and reinfarction (OR 2.48, 95% CI: 1.48-4.14) compared with the non-ST indication groups while the other ST onset groups (late and very late) were not associated with increased odds of adverse outcomes (Figure 3, Table 4). Similarly, the overall ST indication group was not associated with increased odds of MACCE, mortality or reinfarction compared with the non-ST indication group.
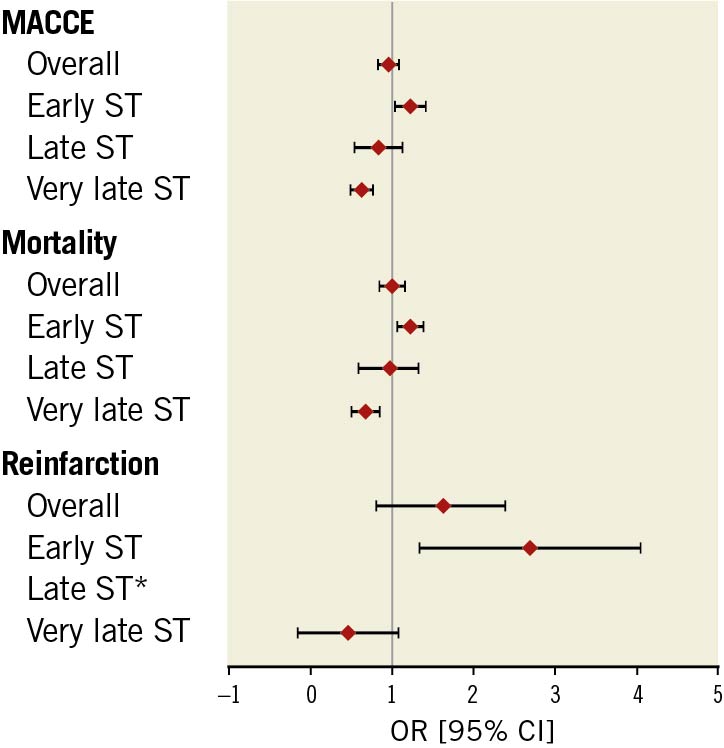
Figure 3. Odds ratios (OR) of adverse events in ST group (vs non-ST). * perfect predictor. CI: confidence interval; MACCE: major adverse cardiac and cerebrovascular events; OR: odds ratio; ST: stent thrombosis
Predictors of adverse outcomes in ST indication PCI
In multivariable analysis, several factors were predictive of MACCE and mortality in the ST group (Table 5). The strongest correlates of all-cause mortality included STEMI presentation (OR 3.07, 95% CI: 2.07-4.56), renal failure (OR 3.49, 95% CI: 2.19-5.55), moderate-poor LV function (moderate: OR 1.56, 95% CI: 1.14-2.13; poor: OR 3.98, 95% CI: 2.64-6.00), OHCA (OR 1.78, 95% CI: 1.20-2.64), circulatory support (OR 2.19, 95% CI: 1.57-3.05), as well as procedure-related variables including femoral access (OR 3.14, 95% CI: 1.75-5.64) and proximal LAD PCI (OR 1.62 95% CI: 1.19-2.21).
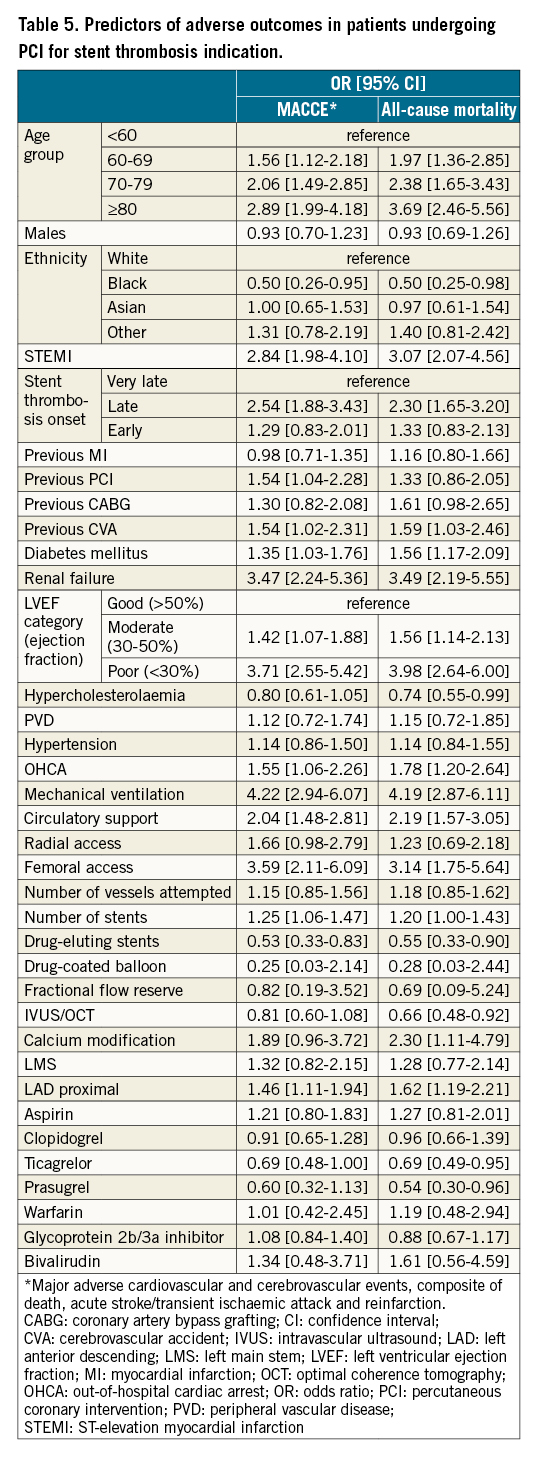
In contrast, the odds of mortality were reduced with the use of newer P2Y12 inhibitors (ticagrelor: OR 0.69, 95% CI: 0.49-0.95; prasugrel: OR 0.54, 95% CI: 0.30-0.96) and the use of intravascular imaging (IVUS or OCT; OR 0.66, 95% CI: 0.48-0.92).
Discussion
This national analysis is by far the largest to examine the frequency, characteristics and outcomes of patients presenting with stent thrombosis undergoing PCI over a 7-year period. Our key findings can be summarised as follows (Central illustration): first, we find that ST represents 1.4% of all indications for PCI, but its frequency has decreased over the study period, from 1.7% in 2014 to 1.4% in 2020. The most common form of ST among those undergoing PCI was early ST (0-30 days; 52.6%), followed by very late ST (>1 year; 35.4%) and late ST (>30 days to 1 year; 12.0%). Second, we show that ST patients have a worse risk profile compared with those undergoing PCI for a non-ST indication, with a higher prevalence of cardiovascular risk factors, and are more critically ill during admission with greater need for mechanical ventilation and circulatory support. Importantly, we show that intravascular imaging is underutilised in this group of patients, with less than a third of procedures using either IVUS or OCT. We report worse prognosis among patients with early ST whose odds of in-hospital mortality and reinfarction were ~20% and 150% higher, respectively, compared to those without ST, despite adjustment for baseline risk profile, while those with late and very late ST were not associated with an increased risk of adverse outcomes. Finally, our study reports several important patient and procedure-related predictors of mortality and adverse outcomes in patients with ST undergoing PCI and find that use of intravascular imaging and newer P2Y12 agents is independently associated with reduced odds of mortality.
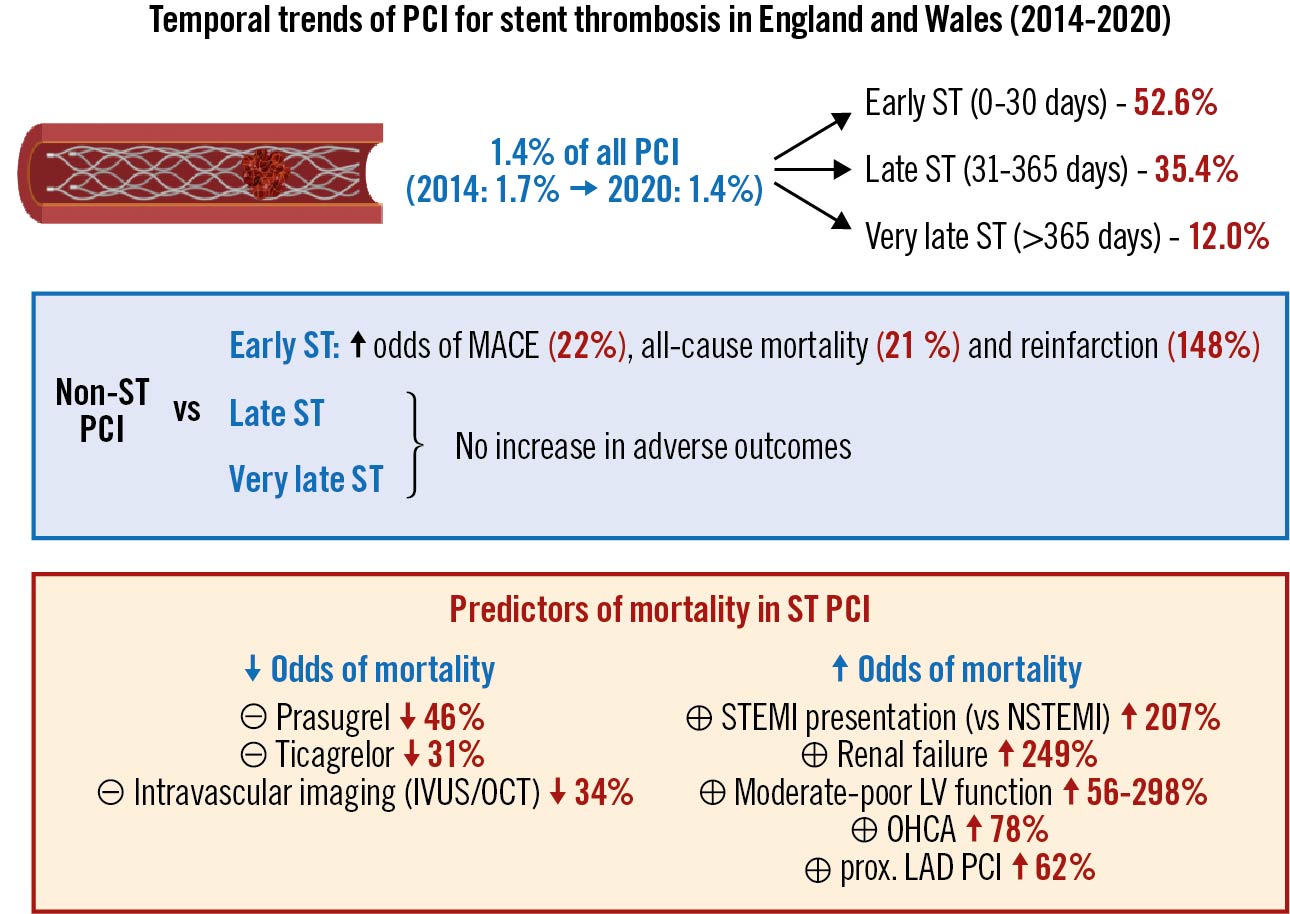
Central illustration. Summary of study findings. IVUS: intravascular ultrasound; LAD: left anterior descending; LV: left ventricular; MACE: major adverse cardiovascular events; NSTEMI: non-ST-elevation myocardial infarction; OCT: optical coherence tomography; OHCA: out-of-hospital cardiac arrest; PCI: percutaneous coronary intervention; ST: stent thrombosis; STEMI: ST-elevation myocardial infarction
Stent thrombosis is a rare complication of PCI, which is known to be associated with a high rate of mortality and morbidity4567. Patients presenting with ST are often critically unwell and some may die prior to PCI1119. Therefore, the characteristics and outcomes of patients with ST undergoing PCI will differ from those who are not invasively managed due to survivorship bias. While there are many reports on the incidence of ST, limited data exists for the frequency and characteristics of ST patients undergoing PCI and how they differ from those undergoing PCI for other indications. Furthermore, there are no data on how the frequency of PCI for ST has changed in recent years. A study of 415,306 DES procedures (2013; from Medicare claims data in the US, which was linked to the NCDR [National Cardiovascular Data Registry] CathPCI Registry) reported only 1,346 PCI procedures for stent thrombosis (i.e., ~0.3%)20. This is significantly lower than our reported frequency (~1.4%), although this could be explained by two reasons. First, the authors were only able to link ~86% of procedures to the NCDR CathPCI Registry. Second, their cohort was aged exclusively >65 years, when more than a third of stent thrombosis cases are in patients <60 years, as shown in our analysis. Since age is an important predictor of mortality among patients with stent thrombosis, it is possible that many elderly patients will have died before an intervention and, thus, would not have been captured in their analysis.
In the absence of any previously published temporal data, our study is the first to report a decline in the frequency/rate of ST as an indication for PCI over a 7-year period. This is likely attributed to advances in stent technologies and deployment techniques, and antithrombotic strategies (e.g., newer P2Y12 inhibitors, low-dose rivaroxaban) in the past decade, all of which are associated with a reduced incidence of stent thrombosis121421.
We find that patients undergoing PCI for ST indication are more critically unwell, with a higher proportion presenting with OHCA, preprocedural cardiogenic shock, or requiring circulatory support, which may account for the poorer outcomes associated with ST particularly in the early and late groups.
Differences in procedural management were also observed between PCI indication groups where there was a higher rate of utilisation of IVUS or OCT for patients presenting with ST. The routine use of intravascular imaging has been recommended by major interventional societies for the management of stent thrombosis due to its ability to define the mechanism of stent thrombosis (e.g., stent malapposition, underexpansion, or neoatherosclerosis), as well as to ensure that adequate stent expansion and apposition is achieved during PCI222324. Interestingly, only ~30% of ST patients were managed with intravascular imaging, more frequently in the late and very late ST groups, and this was associated with reduced odds of mortality in our analysis.
ST has been previously attributed to clopidogrel resistance in previous studies and is associated with a high risk of mortality and ischaemic complications, warranting more potent antithrombotic therapy2225. We find that ST patients were indeed more likely to be offered GP-2b3a inhibitors and newer P2Y12 inhibitors (ticagrelor and prasugrel) instead of clopidogrel. In addition, while GP-2b3a inhibitor therapy was not associated with a reduction of in-hospital mortality in our analysis, ticagrelor and prasugrel were associated with a 31% and 46% reduction in odds of all-cause mortality, respectively. These findings reaffirm those observed in an observational study by Ferero et al where GP-2b3a inhibitor use was not associated with better survival or reduced MACE at 60 months among 695 patients with definite ST, while newer P2Y12 inhibitor use was associated with a reduced risk of reinfarction at 60 months (hazard ratio 0.56, 95% CI: 0.32-0.99)26.
In totality, ST was not associated with an increased risk of MACCE, mortality or reinfarction following PCI after adjustment for differences in baseline characteristics (when compared to PCI undertaken for ACS indications). A study by Ohno et al from the Japanese PCI (J-PCI) registry that compared in-hospital outcomes between PCI for ACS cases with and without ST found no difference in mortality between either group but higher reinfarction rates in the ST group in a 1:1 matched cohort of 1,898 patients, which is consistent with our findings27. However, they reported higher rates of recurrent ST, unlike in our analysis, which may be related to their sample size or their balancing of covariates, as differences in certain characteristics remained unbalanced after matching in their analysis (e.g., higher rates of LMS/LAD culprit vessel in the ST group).
Importantly, we observed differences in prognosis between different times of ST onset. Early ST was the only onset group associated with increased odds of mortality and reinfarction (21% and 148% increase in odds, respectively). It is difficult to place our findings within the context of other studies since none have previously compared procedural outcomes between non-ST PCI and ST PCI stratified by time of ST onset. However, the increased risk of post-procedural adverse outcomes among specific ST onset groups, such as early ST has been demonstrated in previous studies. In a meta-analysis by Yang et al, patients with early ST were associated with an increased risk of in-hospital and 30-day mortality (risk ratio [RR] 1.67, 95% CI: 1.17-2.37 and RR 2.05, 95% CI; 1.58-2.67, respectively)28. The increased risk of complications among this specific ST onset group, despite adjustment for their higher risk profile at baseline, could be possibly attributed to differences in the aetiology and vascular bed status at different times of ST onset. Early ST is more commonly caused by stent underexpansion and acute malapposition as well as early discontinuation of antiplatelet therapy especially at a time when re-endothelialisation has not occurred, while late and very late ST have been previously attributed to late stent malapposition, delayed endothelialisation and neoatherosclerosis82930. Coronary collateral circulation, which can limit myocardial damage, is less likely to be established in the acute phase, as is myocardial healing, possibly contributing to their higher risk of mortality and reinfarction3132. In contrast, in cases of very late ST, which are driven by neoatherosclerosis, coronary collaterals are more likely to develop, contributing to their relatively better outcomes in comparison to those with a non-ST indication. Previous studies have demonstrated a higher thrombotic burden among those with early ST, which has a higher risk of no-reflow that is known to portend worse outcomes, thereby necessitating a greater need for more aggressive antithrombotic therapy as seen in our analysis, with concomitant increased periprocedural bleeding risk3334.
Previous studies have examined predictors of ST after PCI, such as heart failure, diabetes mellitus, type of stent implanted, indication for the index PCI and antithrombotic therapy10113536. However, it is unclear what the predictors are for adverse outcomes among patients undergoing PCI for ST. We find that several patient and procedure-related factors are associated with increased odds of any in-hospital complication (MACCE) as well as mortality, including patient age (>60 years), STEMI presentation, renal failure, diabetes mellitus, moderate-poor LV function, OHCA, circulatory support, femoral (vs radial) access as well as proximal LAD PCI. While many of these factors are non-modifiable, increased uptake of radial access, intravascular imaging during PCI and newer P2Y12 inhibitor use in patients with ST may help further improve their post-procedural outcomes.
Limitations
There are several limitations to the present study. First, whilst the BCIS dataset captures cardiovascular risk factors and procedural characteristics, it does not capture measures of comorbidity or frailty, which are important determinants of mortality post-PCI3738. Second, the observational nature of our analysis means that residual unmeasured confounders may not be accounted for. Several factors, such as the type of stent used in the index PCI, indication of index PCI, and differences in antithrombotic regime prior to admission for ST PCI, were not captured in our dataset. Third, specific information on ST management (e.g., balloon angioplasty or drug-eluting balloon/additional stent use) was not captured in our dataset. Finally, our findings were based on the study of in-hospital outcomes, and more significant differences in outcomes may become evident on longer follow-up. For example, the use of overlapping or two-layer stents to manage ST could increase the risk of target vessel failure and/or revascularisation39.
Conclusions
Our temporal analysis of all PCI procedures in England and Wales shows a decline in the frequency of procedures for ST indication over a 7-year period. The prognosis of ST patients undergoing PCI, compared with non-ST indication procedures, differs between different times of onset, with early ST being the only onset group associated with an increased risk of mortality and reinfarction. Our analysis demonstrates that intravascular imaging is underutilised in this high-risk group, with less than a third of cases receiving IVUS or OCT, even though its use is associated with better outcomes.
Impact on daily practice
The present study provides operators with contemporary data on the frequency of ST PCI according to time of onset. Our findings suggest that, while the rate of ST PCI has declined, early ST is the most common subtype and is associated with an increased risk of in-hospital mortality and reinfarction. Furthermore, our findings remind operators of the benefits of intravascular imaging use in ST cases, which was shown to correlate with improved in-hospital survival and lower rates of reinfarction in our analysis and yet remains significantly underutilised in this high-risk patient group.
Conflict of interest statement
The authors report no conflicts of interest relevant to the current study.
Supplementary data
To read the full content of this article, please download the PDF.

