Abstract
Background: With the improvements of percutaneous coronary intervention (PCI) technology and post-PCI patient management, several registry studies reported temporal trends in post-PCI clinical outcomes. However, their results are inconclusive, potentially reflecting region-specific trends, based on site-reported events without external validity.
Aims: This study aimed to investigate temporal trends in post-PCI clinical outcomes in all-comers randomised controlled trials (RCTs) involving coronary stents.
Methods: We performed a systematic review identifying RCTs comparing a clinical outcome as a primary endpoint among different coronary stents with an all-comers design and independent clinical event adjudication, extracting the study start year, patient baseline characteristics, and one- and five-year clinical outcomes. Temporal trends in clinical outcomes (cardiac death, myocardial infarction [MI], target lesion revascularisation [TLR], stent thrombosis [ST]) were assessed using random-effects meta-regression analyses, estimating the relationship between clinical outcomes and study start year.
Results: Overall, 25 all-comers trials (51 device arms, 66,327 patients) conducted between 2003 and 2018 fulfilled the eligibility criteria. Random-effects meta-regression analysis revealed significant decreasing trends in one- and five-year cardiac death, one-year TLR, and five-year ST incidences (relative risk per 10-year increase: 0.69 [0.51-0.92], 0.66 [0.44-0.98], 0.60 [0.41-0.88], and 0.18 [0.07-0.44], respectively). There was no significant trend in myocardial infarction incidences.
Conclusions: This is the first attempt to clarify and quantify the temporal trends of post-PCI outcome incidence. The 15-year improvements in PCI therapy and post-therapeutic patient management are associated with reduced incidences of cardiac death and PCI-related adverse events.
Introduction
Percutaneous coronary intervention (PCI) technology has advanced since the first human coronary balloon angioplasty in 1977, with coronary stents (including drug-eluting stents [DES]) ensuring better safety and efficacy after PCI1. Furthermore, patient management strategies for acute coronary syndrome (ACS) and adjunct pharmacological therapy (e.g., antiplatelet and lipid-lowering therapy) have improved. Considering this combination of developments, the degree of improvement in post-PCI clinical outcomes is of paramount interest. While several registries have reported temporal trends in post-PCI clinical outcomes, their generalisability is limited because the majority of the included patients are from a particular region or institution. Nationwide registries have also reported temporal trends in post-PCI clinical outcomes. However, they did not sufficiently measure the impact of therapeutic developments per se, as they reported only crude outcomes such as all-cause mortality, including deaths from non-cardiac causes2. Furthermore, these studies were limited by a relatively low patient follow-up rate and lack of objective adjudication of site-reported clinical outcomes. Recent prospective randomised controlled trials (RCTs) have commonly applied an independent clinical events committee (CEC) for objective event adjudication. Considering the limitations of registry reports, the current study aimed to clarify and quantify the temporal trends in post-PCI clinical outcomes using historical data from all-comers RCTs evaluating the efficacy and safety of coronary stents, with independent clinical event adjudication based on a comprehensive systematic review and meta-analytic approach.
Methods
SEARCH STRATEGY AND SELECTION CRITERIA
Eligible studies for the systematic review and current meta--analysis were RCTs comparing different coronary stents with an all-comers design based on minimal patient exclusion criteria, presumably reflecting routine clinical practices. Eligible studies included RCTs (1) investigating the safety and efficacy of a DES compared to other stent(s), designed with clinical outcomes as primary endpoints rather than being surrogate markers (e.g., angiographic parameters); (2) with the studied coronary stents being approved by CE marking; (3) with clinical event adjudication by independent CECs; and (4) with an all-comers design. An all-comers trial was defined as a trial without the major exclusion criteria listed in the Supplementary Table 1. As there is no clear definition of an all-comers trial in the literature, our selection was based on consensus among the authors. In the current analysis, trials investigating bifurcation-dedicated, covered, self-expandable stents or bioresorbable scaffolds were excluded for consistency, although we imposed no language, publication date, or publication status restrictions. We searched for relevant trials through Medline, Embase, the Cochrane database, and abstracts and presentations from major cardiovascular meetings, using the keywords “randomized controlled trial”, “coronary artery disease”, and “stent” (Supplementary Table 2). The systematic review was conducted in accordance with PRISMA guidelines3. Two independent investigator teams (M. Ono/A. Saito and T. Kanie/Y. Takaoka) reviewed the titles, abstracts, and texts for trial eligibility. Disagreements were resolved by consulting another investigator (T. Asano). To identify all-comers trials, we first restricted the target RCTs to those conducted in non-specific populations by excluding specific population trials with particular patient characteristics, clinical settings, or anatomical conditions (e.g., diabetes, high bleeding risk, ST-segment elevation myocardial infarction [STEMI], chronic total occlusion, or left main disease). We then assessed the inclusion and exclusion criteria of these trials and determined their eligibility. Study quality was assessed using the Cochrane Collaboration’s tool for assessing bias risk. The current systematic review was registered and published in PROSPERO (CRD42020108188).
ENDPOINTS AND DEFINITIONS
The investigated clinical outcomes included cardiac death, myocardial infarction (MI), target lesion revascularisation (TLR), and stent thrombosis (ST) one year after the index procedure. In the included trials, the relevant outcomes at five years were also collected, if applicable. The clinical outcomes were defined as applied in each trial. To reduce bias, we included only TLR incidences reported as repeat revascularisation with objective clinical indication, such as clinically indicated TLR defined by the Academic Research Consortium (ARC)4. Clinically indicated TLR was defined as a reintervention for clinically significant stenosis, confirmed by quantitative coronary angiography, with functional or clinical factors justifying the indication of the reintervention. ST was defined as definite ST according to the ARC definition or relevant definitions in trials before the ARC definition was published4. The initial iterations of DES (i.e., CYPHER® sirolimus-eluting stent [Cordis, Santa Clara, CA, USA] and TAXUS™ paclitaxel-eluting stent [Boston Scientific, Marlborough, MA, USA]) and later iterations of DES were categorised as early and new DES, respectively.
STATISTICAL ANALYSIS
Inclusion and exclusion criteria, study start year, participating countries, patient demographics, comorbidities, and incidences of the clinical outcomes in each study arm were extracted from the publications. Temporal trends in post-PCI clinical outcomes were examined using random-effects meta-regression analysis with a restricted maximum likelihood (REML) estimation method and time (i.e., study start year) as a moderator. Log-transformed incidences were applied in each meta-regression model. Relative risk (RR) per 10 years was calculated based on an exponentiated beta coefficient in the regression model. Results were exponentiated for interpretation and visualisation in figures. The pooled incidence rates were calculated for clinical outcomes, along with 95% confidence intervals (CIs) using the random effects model with REML. The pooled values were also calculated for subgroups stratified by every five years of the first patient enrolment and were presented in forest plots.
The pooled patient follow-up rates in the included trials (i.e., the rate of patients with available clinical status at the one- and five-year follow-ups) were calculated. The homogeneity assumption between treatment effects in different trials was tested using the Q test and further quantified using the τ2 and I2 statistics. The amount of heterogeneity accounted for by the moderator was given under the R2 statistic. For the sensitivity analysis, we repeated the analyses in a more consistent population by including only trials conducted in European countries. Incidences were calculated based on an intention-to-treat analysis and p<0.05 was considered statistically significant. All statistical analyses were conducted using R version 3.62 (R Foundation for Statistical Computing, Vienna, Austria). The meta-regression model fitting and pooled outcome measure calculations were performed using the metafor package, and the results were visualised with the ggplot2 package.
Results
Of 10,139 citations identified by the initial database search performed in September 2020, there were 49 eligible RCTs (95 device arms, 97,465 non-specific patients). After assessing the inclusion and exclusion criteria, we identified 25 all-comers trials (51 device arms) enrolling 66,327 patients from 2003-2018 (publication year: 2005-2020) (Supplementary Figure 1). The included trials were conducted in Europe, North and South America, East Asia and the Middle East, and Oceania. Twenty trials with 44,943 patients were conducted in European countries alone. The included trials (Table 1) had 9 study arms with 12,510 patients receiving early DES and 42 study arms with 53,817 patients receiving new DES. The inclusion and exclusion criteria of the included trials, along with the 49 eligible trials enrolling non-specific patients, are presented in the Supplementary Table 3. All 25 all-comers trials reported one-year clinical outcomes at nine or 12 months, whereas 13 with 34,463 patients reported five-year clinical outcome incidences. The pooled patient follow-up rates of the included trials were 99.0% (95% CI: 98.6-99.3) at one year and 98.4% (95% CI: 97.6-99.2) at five years. Detailed information regarding the included trials with the applied definitions for clinical outcomes and the results of the bias assessment are reported in Supplementary Table 4-Supplementary Table 7.
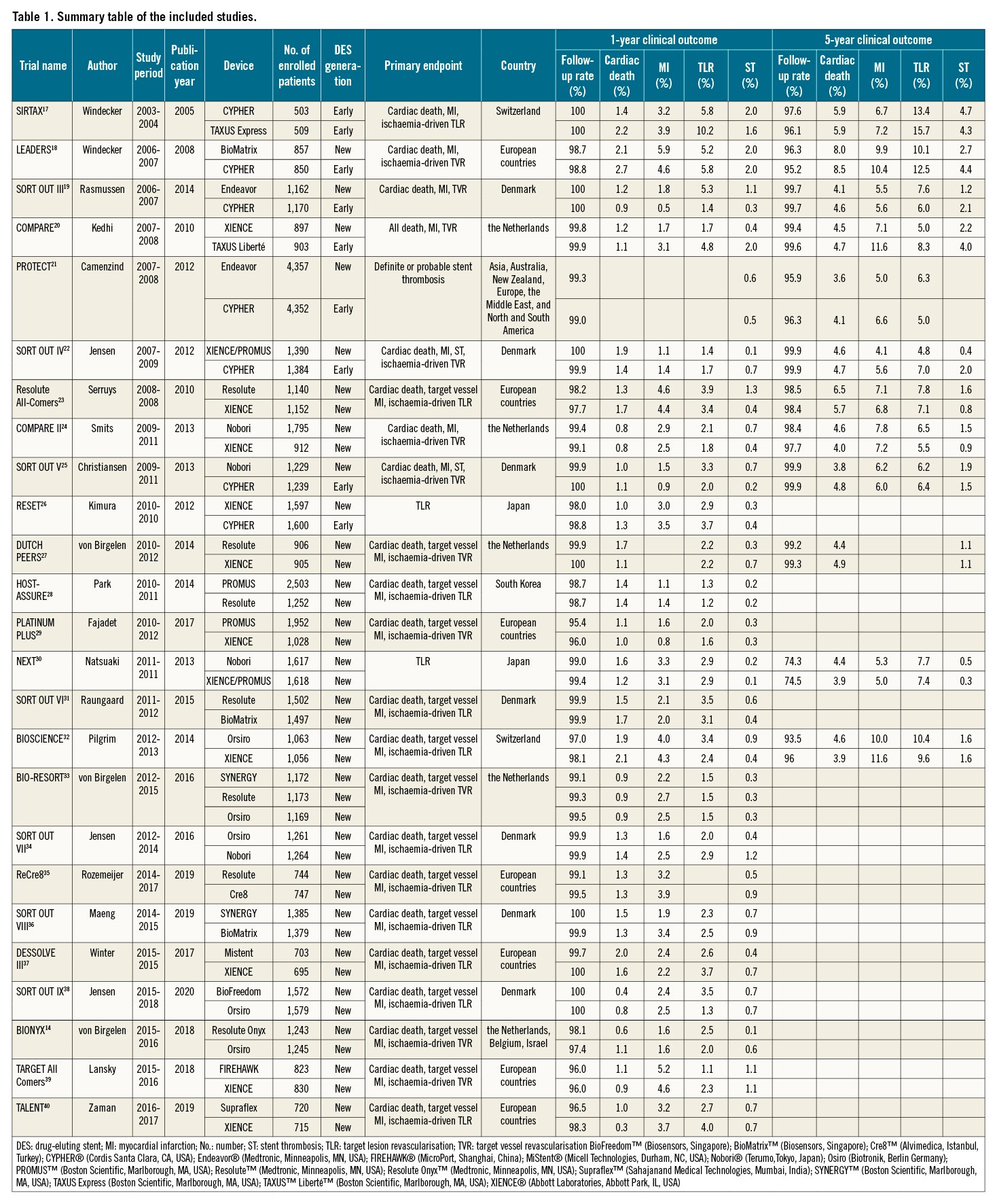
Among the one-year clinical outcomes, we observed a decreasing trend over time in cardiac death (RR per 10 years: 0.69 [95% CI: 0.51-0.92]; R2=0.23, p=0.01) and TLR (RR per 10 years: 0.60 [0.41-0.88]; R2=0.15, p=0.01), whereas we observed no significant trend in MI incidence (RR per 10 years: 1.15 [0.76-1.74]; R2=0.00, p=0.50) (Figure 1). Our analysis also revealed a decreasing trend in five-year cardiac death incidence (RR per 10 years: 0.66 [0.44-0.98]; R2=0.15, p=0.04) but not in five-year MI or TLR incidence (Figure 2). The forest plots in Figure 1 and Figure 2 summarise the pooled incidences of one- and five-year clinical outcomes stratified by each period (study start year: 2003-2007, 2008-2012, and 2013-2016). The pooled incidence of one-year cardiac death was 1.63% (95% CI: 1.27-1.99) in 2003-2007, subsequently decreasing to 1.09% (95% CI: 0.85-1.34) in 2013-2016. The five-year incidence of cardiac death also decreased from 5.27% (95% CI: 4.39-6.15) to 4.64% (95% CI: 4.20-5.09) over 10 years. The incidence of one-year TLR decreased from 4.32% (95% CI: 2.57-6.08) to 2.55% (95% CI: 2.05-3.04) between 2003-2007 and 2013-2016.
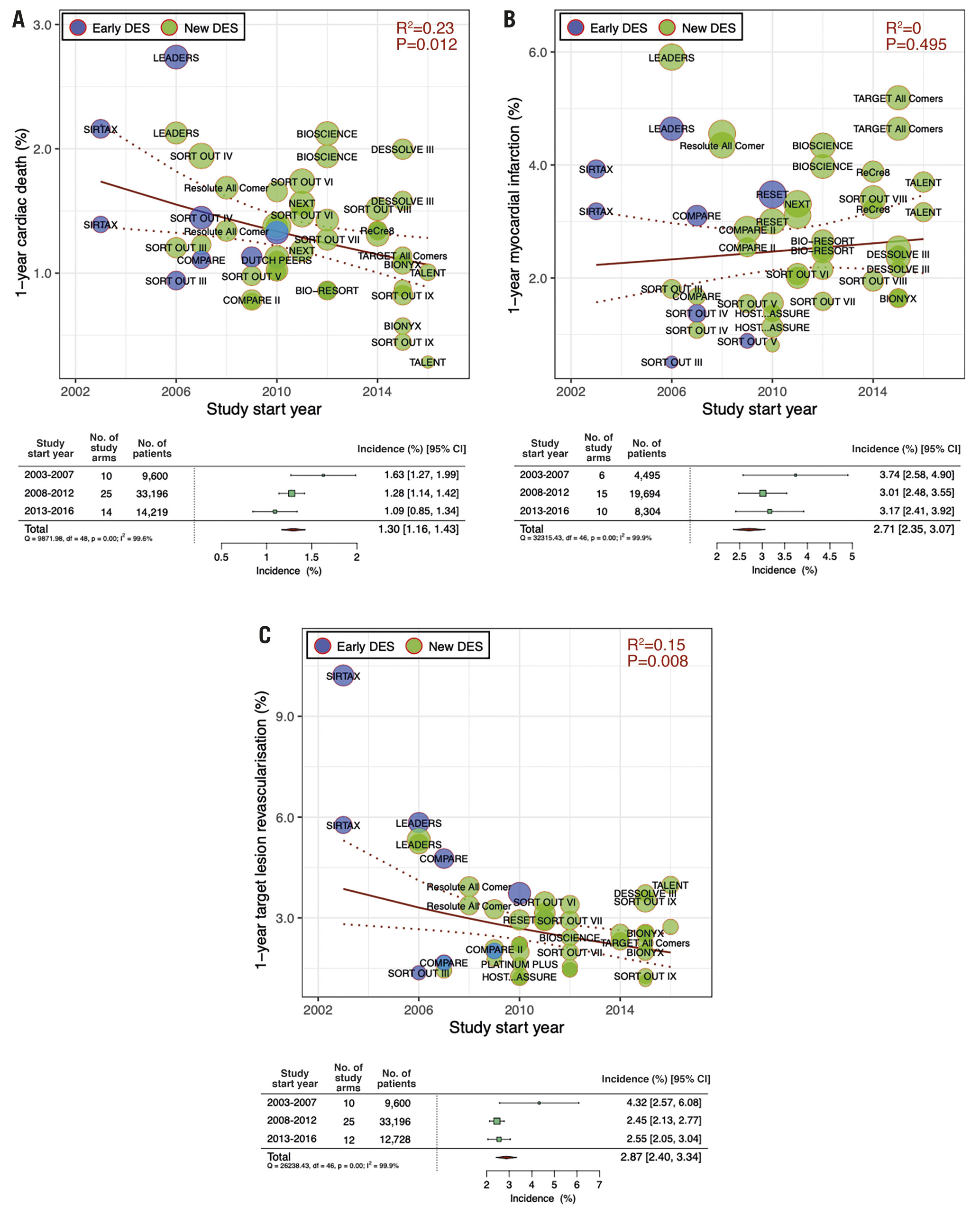
Figure 1. Meta-regression analysis for temporal trends trends at one year. One-year incidences of A) cardiac death, B) myocardial infarction and C) target lesion revascularisation. Bubbles are plotted in accordance with the study start year and incidences of clinical outcomes for each study (device) arm. The size of each bubble is correlated with a weight in the analysis. Forest plots summarise the pooled estimated incidences of each clinical outcome divided into three periods (2003-2007, 2008-2012, and 2013-2016). CI: confidence interval; DES: drug-eluting stents; No.: number
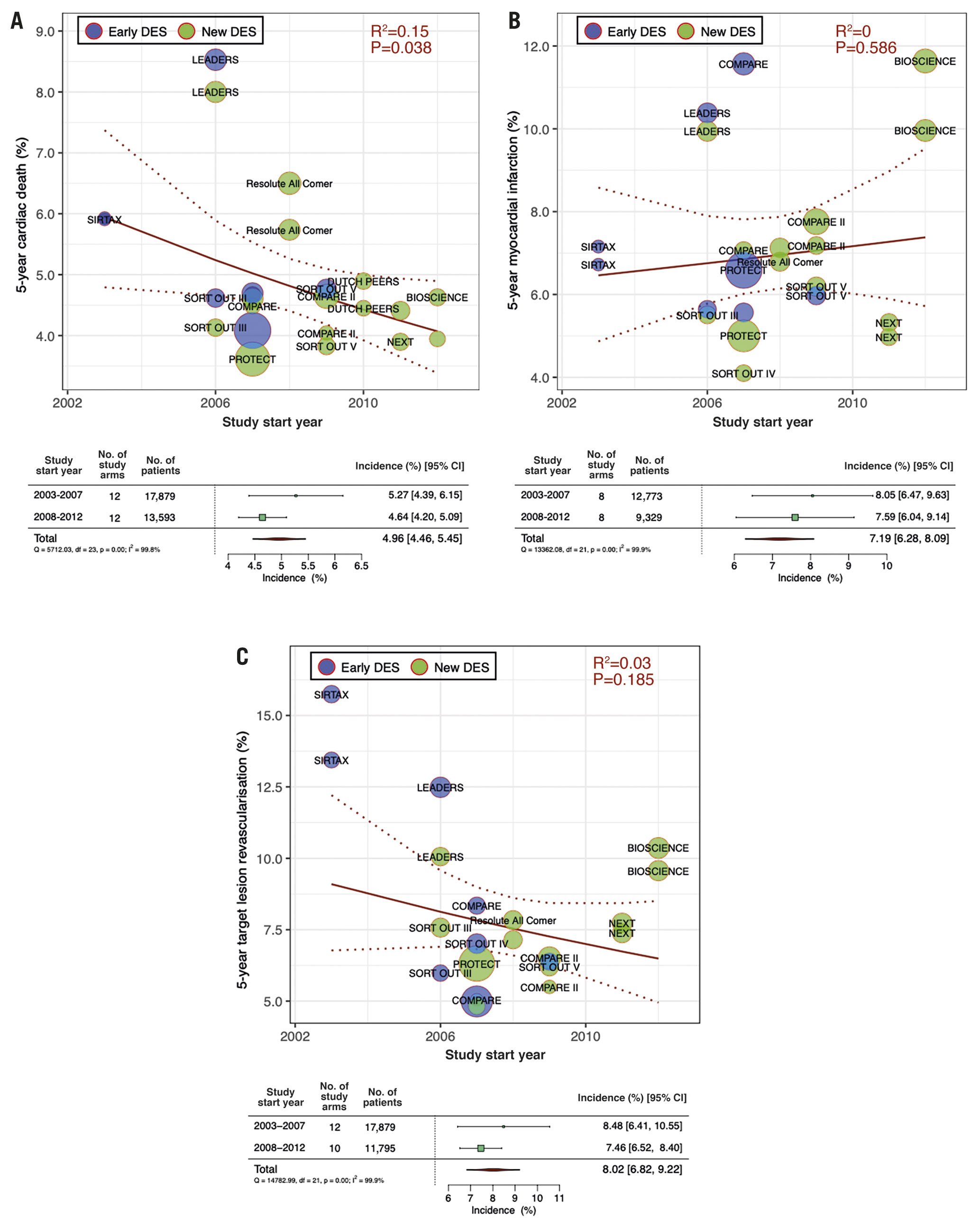
Figure 2. Meta-regression analysis for temporal trends at five years. Five-year incidences of A) cardiac death, B) myocardial infarction and C) target lesion revascularisation. Bubbles are plotted in accordance with the study start year and incidences of clinical outcomes for each study (device) arm. The size of bubble is correlated with a weight in the analysis. Forest plots summarise the pooled estimated incidences of each clinical outcome divided into two periods (2003-2007 and 2008-2012). CI: confidence interval; DES: drug-eluting stents; No.: number
Figure 3 presents temporal trends in one- and five-year ST incidences. There was a significant declining tendency in five-year ST incidence (RR per 10 years: 0.18 [0.07-0.44]; R2=0.50, p<0.01), while there were no significant trends in one-year ST incidence (RR per 10 years: 0.66 [0.39-1.11]; R2=0.07, p=0.12). The pooled five-year ST incidence in 2003-2007 was 2.80% (95% CI: 1.89-3.72), decreasing to 1.20% (95% CI: 0.92-1.47) in 2008-2012.
Figure 3. Meta-regression analysis for temporal at one and five years. Incidence of A) one-year and B) five-year stent thrombosis. Bubbles are plotted in accordance with the study start year and incidences of clinical outcomes for each study (device) arm. The size of each bubble is correlated with a weight in the analysis. Forest plots summarise the pooled estimated incidences of each clinical outcome divided into three periods (2003-2007, 2008-2012, and 2013-2016). CI: confidence interval; DES: drug-eluting stents; No.: number
The sensitivity analysis of the 20 Europe-only trials conducted between 2003 and 2018 revealed similar trends in clinical outcomes. Supplementary Figure 2 includes the detailed results of the sensitivity analysis.
Discussion
In the current analysis, we aimed to clarify and quantify the temporal trends in the incidence of post-PCI clinical outcomes. Our analysis revealed significant decreasing trends in the incidences of one- and five-year cardiac death (31% and 34% decrease per 10 years, respectively) and TLR (40% decrease per 10 years) and five-year ST (82% decrease per 10 years) over time. However, we observed no significant trends in the MI incidence (Central illustration).
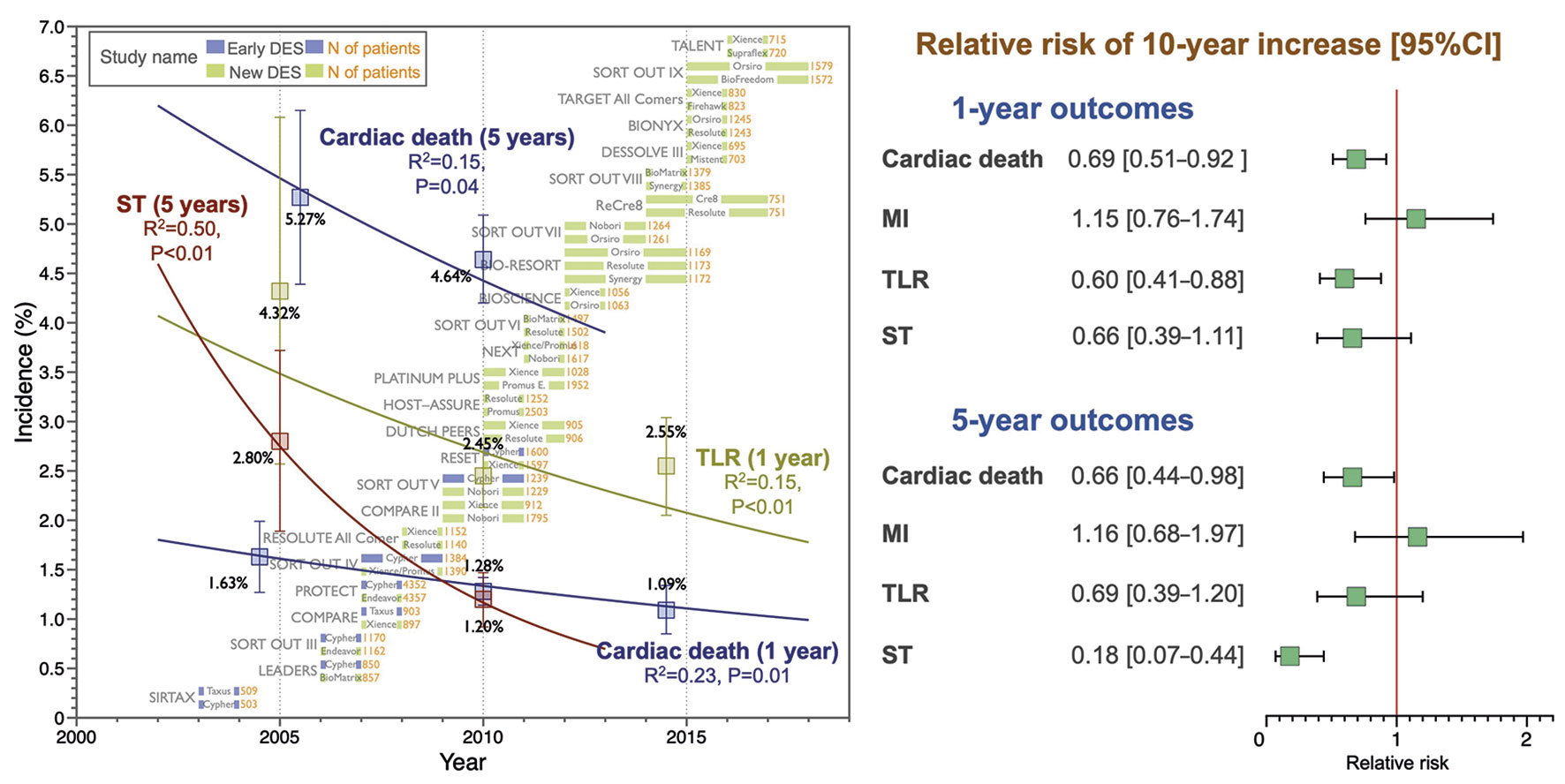
Central illustration. Temporal trends in the incidence of post-PCI clinical outcomes. In the meta-regression analysis including 25 all-comers RCTs, there were significant decreasing trends in the incidence of one- and five-year cardiac death, one-year TLR, and five-year ST whereas there were no trends in MI incidences. CI: confidence interval; DES: drug-eluting stent; MI: myocardial infarction; RCT: randomised controlled trial; RR: relative risk; ST: stent thrombosis; TLR: target lesion revascularisation
To our knowledge, this is the first analysis to investigate temporal trends in clinical outcomes after PCI quantitatively using data from all-comers RCTs (presumably reflecting routine practice) with a substantial patient follow-up rate and objective event adjudication. Indeed, there are considerable discrepancies between objective event adjudication and site reports on which registry studies are generally based. A discordance between investigator-reported and CEC-adjudicated events was reported in a prospective substudy (GLASSY: GLOBAL LEADERS Adjudication Sub-StudY) of the GLOBAL LEADERS trial for the detection of clinical events—especially MI, target vessel revascularisation, and major bleeding—which investigated the safety and efficacy of ticagrelor monotherapy one month after DES implantation5.
TEMPORAL TRENDS IN CARDIAC DEATH AFTER PCI
In a recent study investigating the temporal trend of mortality after PCI using British Cardiovascular Intervention Society PCI Registry data collected between 2007 and 2014, the mean age of patients receiving PCI increased from 63.8 to 65.1 years, with more recent years being associated with higher mortality2. Mortality was also affected by other comorbidities such as cancer, even after age and sex adjustment. Similarly, in the current analysis, the mean age of the patients gradually increased over time (2.30 years per 10 years [95% CI: 0.82-3.77], p<0.001 for trend). However, the cardiac death incidence following PCI tended to decrease, whereas all-cause death did not have decreasing trends at one and five years (RR per 10 years: 0.97 [0.78-1.22] at one year and 1.08 [0.76-1.54] at five years) (Supplementary Figure 3). Interestingly, the incidences of non-cardiac death had increasing tendencies (RR per 10 years: 1.53 [1.08-2.17] at one year and 1.63 [0.94-2.85] at five years) (Supplementary Figure 3). In a more aged population, all-cause death may counterbalance the incidence with other increased comorbidities due to ageing.
Improving the prognosis of coronary artery disease is one of the major goals of PCI therapy. To date, a great deal of effort has been made in various approaches for achieving this goal. Patient management for acute coronary syndrome (ACS) (timing and completeness of revascularisation, mechanical circulatory support device, and acute cardiac care after PCI), general cardiovascular risk control (medical treatment and lifestyle modification), PCI device (DES, intravascular imaging, and guidewire), invasive functional assessment, and antiplatelet therapy after PCI have improved over the years. The observed decreasing trend in the incidence of cardiac death may be attributed to the integration of these developments, suggesting that measures taken thus far have been in the proper direction.
Major contributing factors to the observed decreasing trend in cardiac death incidence might be the improvement of the patient management including cardiovascular risk control and medical treatment rather than improvements in PCI technology and technique. This is because no major trials have demonstrated a significant benefit of revascularisation in mortality for patients with chronic coronary syndrome, who were mainly enrolled in the included trials6. However, it is likely that additional technological and technical improvements in PCI will lead to further decreases in the incidence of TLR and ST, which may in turn improve prognosis among affected patients. In a large patient-level meta-analysis including 32,524 patients, TLR after PCI was an independent predictor of long-term mortality (hazard ratio: 1.23 [1.04-1.45])7. In a recent report from a large registry in the USA, functional fractional flow reserve (FFR) prior to PCI gradually increased from 44% to 75% between 2009 and 2017, and FFR-guided PCI was associated with a lower risk of one-year mortality than angiography-guided PCI8.
TEMPORAL TRENDS IN PCI-RELATED OUTCOMES
The observed improvement in the incidence of PCI-related outcomes (i.e., TLR and ST) was potentially associated with improvements in PCI technologies and techniques, and medical therapies represented by P2Y12 inhibitors and lipid-lowering therapy. The SYNTAX II trial investigated the impact of the integration of new developments in PCI practice (usage of a contemporary DES, intravascular ultrasound, and pressure wire for the physiological assessment) on clinical outcomes in patients with multivessel disease. The results were compared with those of the SYNTAX I trial conducted nine years earlier9. The SYNTAX II study revealed significant decreases in the incidence of repeat revascularisation and ST, in accordance with our findings.
Second-generation DES with modified drug elution, thinner struts, and biocompatible polymers have been associated with improved efficacy and reduced incidence of repeat revascularisation when compared with first-generation DES1. However, no additional positive impact of the thinner strut of the contemporary DES on TLR incidence has been reported10. The results of the current analysis are consistent with those of these previous findings. The pooled one-year incidence of TLR decreased from the first to the second period (2003-2007, 2008-2012, respectively), while the incidences were comparable between the second and latest periods (2008-2012, 2013-2016, respectively) (Figure 1). Furthermore, the significant trend in TLR incidence observed at one year became non-significant at five years (adjusted RR: 0.65 [0.38-1.12]). This suggests that the impact of difference in DES generations on long-term TLR incidence became less obvious at five years. Several RCTs reported their extended results beyond five years (7-10 years), in which differences in clinical outcomes (TLR and ST) between different DES became less significant in the very long term11.
Potential technical factors influencing TLR incidence include the utilisation of intravascular imaging during PCI12. Nevertheless, the penetration rate of intravascular imaging is still low at 5-15% in the USA and European countries, in which the majority of the included trials were conducted13. Although the included trials rarely reported the prevalence of intravascular imaging-guided PCI, the BIONYX trial conducted in European countries and Israel between 2015 and 2016 reported a 1.2% utilisation rate of intracoronary imaging14.
In the current analysis, the five-year ST incidence decreased significantly over time (RR per 10 years: 0.18 [0.07-0.44], p<0.01). This is potentially attributed to the development of coronary stents with thinner struts and biocompatible polymers with or without biodegradation, the advent of potent P2Y12 inhibitors, and improvement in lipid lowering therapy presumably inhibiting the progression of neoatherosclerosis15.
IMPLICATIONS OF THE CURRENT RESULTS AND STUDY LIMITATIONS
The current analysis observed several significant trends in clinical outcomes after PCI in the all-comers RCTs. The observed trends were ascribed to the composite of miscellaneous factors mainly associated with time course, which included developments in PCI technologies and techniques, invasive functional assessment, medical therapies (P2Y12 inhibitors, lipid-lowering therapy, etc.), and cardiovascular prevention measures. The current analysis has a limitation in discriminating specific factors attributed to each endpoint because of the lack of individual data especially for medical treatment including antiplatelet therapy. Furthermore, in the across-study comparison, between-study heterogeneity is hardly eliminated, even if multiple-covariate adjustment is applied. The results should be interpreted with caution considering the potential existence of spurious or collinear effects. Other limitations to the current analysis are as follows. First, the included population was not necessarily representative of the real-world population, although they were selected from all-comers trials with minimal exclusion criteria. In their study, de Boer et al reported that applying the all-comers design did not result in the inclusion of all consecutive patients in the post hoc analysis of the LEADERS and RESOLUTE all-comers trials16. The current analysis may have included selected populations with undetected biases. Second, the definitions of clinical outcomes, especially MI, varied as shown in Supplementary Table 5 and Supplementary Table 6. This may have precluded an unbiased assessment of temporal trends in clinical outcomes. Recent MI definitions include an assessment using sensitive cardiac markers (i.e., cardiac troponins). Consequently, the events which were adjudicated as unstable angina according to the old definitions were adjudicated as MI with the recent definitions, potentially resulting in underestimation of improvements in terms of MI prevention. Third, the performance of TLR is susceptible to the bias of investigators. The early trials tended to include a subgroup with scheduled angiographic follow-up (Supplementary Table 8). It should be noted that in the SIRTAX trial which reported a high TLR incidence, nearly 50% of the patients underwent follow-up angiography at both one and five years. In those trials, the oculostenotic reflex potentially triggered the performance of TLR, although we applied only clinically indicated TLR adjudicated by an independent CEC based on the objective definition. Fourth, our study lacked investigation regarding bleeding events after PCI. PCI therapy has been developed by balancing thrombotic events with bleeding events besides the development of antiplatelet therapy including modification of the duration of dual antiplatelet therapy. Novel P2Y12 inhibitors potentially reduce thrombotic events represented by MI and ST, whereas more potent antiplatelet therapy might introduce an increased incidence of bleeding events. A small number of the included trials (six out of 25 trials) reported the bleeding event incidence with various definitions across the trials. This imposed a limitation on demonstrating the temporal trend of the bleeding event rate. We tabulated the summary of the reported bleeding event incidences and antiplatelet regimen of the included trials in Supplementary Table 9. Fifth, the current analysis was conducted by splitting each trial into individual arms to clarify the impact of the device difference. However, this methodology eventually disbanded a within-trial integrity based on the randomisation.
Conclusions
Our meta-regression analysis, which included all-comers RCTs for DES use between 2003 and 2018, revealed significant decreasing trends in one-year cardiac death (31% decrease per 10 years) and TLR (40% decrease per 10 years) incidences, and five-year cardiac death (34% decrease per 10 years) and ST (82% decrease per 10 years) incidences but no significant trends in the MI incidence over time. Our analysis indicated that 15-year developments in PCI therapy and post-therapeutic patient care were associated with decreased incidence. PCI procedures and meticulous patient management such as post-PCI MI prevention strategies may be critical for ensuring better clinical outcomes.
Impact on daily practice
Our analysis revealed a decreasing trend in one- and five-year cardiac death, one-year TLR, and five-year ST incidences after PCI in all-comers stent trials conducted over the last 15 years; however, no such trends for MI incidence were observed. The integration of miscellaneous therapeutic developments might have contributed to these decreases. Further improvements in PCI-related outcomes, post-PCI MI prevention strategies, and therapeutic approaches may clarify the effects of PCI on patient prognosis.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

