
Introduction
Antiplatelet therapy was first introduced almost 30 years ago and remains the cornerstone of percutaneous coronary interventions (PCI). During 2019, the results of several pivotal trials seemed to herald a new era of antiplatelet therapy – the era of the aspirin-free strategy. Also in 2019, the debates on the optimal revascularisation strategy for patients with left main or multivessel disease were reignited, attempting to reduce microvascular obstruction after coronary reperfusion by early infusion of alteplase was disproven, and the result of the ISCHEMIA trial showed no advantage of routine invasive treatment in patients with chronic coronary syndrome (CCS).
New publications and research related to coronary interventional practice have emerged and will be highlighted in this review. These include prominent interventional cardiology publications from the high-impact journals: New England Journal of Medicine, The Lancet, JAMA (including JAMA Cardiology), European Heart Journal, Journal of the American College of Cardiology, Circulation, JACC Cardiovascular Interventions, Circulation Cardiovascular Interventions, and EuroIntervention. The focus of this article is to summarise the findings of the pivotal trials (Figure 1) and their impact on clinical practice.
Figure 1. A few of the important trials reported in 2019.
DRUG-ELUTING STENTS
Numerous trials have been conducted comparing the outcomes among second-generation drug-eluting stents (DES), but few have exhibited superiority of one over the other. In 2017, the BIOFLOW V study1 demonstrated that the ultra-thin strut (60 um) Orsiro stent (Biotronik, Bülach, Switzerland) was superior to the XIENCE stent (Abbott Vascular, Santa Clara, CA, USA) in terms of target lesion failure (TLF) in an all-comers population. This year, the BIOSTEMI trial2 further demonstrated the superiority of Orsiro over XIENCE in STEMI patients. At 12 months, the primary endpoint of TLF occurred in 25 (4%) of 649 patients treated with the Orsiro and 36 (6%) of 651 patients treated with the XIENCE (RR 0.59, 95% Bayesian credibility interval 0.37-0.94; posterior probability of superiority 0.986). Another trial comparing the efficacy of ultra-thin strut DES is the TALENT trial3. In an all-comer population, the Supraflex™ (Sahajanand Medical Technologies Pvt. Ltd., Mumbai, India), also a 60 um ultra-thin strut stent, was non-inferior compared to XIENCE at 12 months in terms of the device-oriented composite endpoint (DoCE), a composite of cardiac death, target vessel myocardial infarction (MI) or clinically indicated target lesion revascularisation (TLR). DoCE occurred in 35 (4.9%) of 720 patients in the Supraflex arm and 37 (5.3%) of 715 patients in the XIENCE arm (pnon-inferiority<0.0001). With aggregated results of the ultra-thin strut stents, it seems that these DES might serve as the new “standard of care” in the near future. However, we still need long-term results showing that the efficacy is preserved before we change our practice.
Other non-inferiority trials published this year include the ReCre8 trial4, which demonstrated that the Cre8™ stent (Alvimedica, Istanbul, Turkey) is non-inferior to the Resolute Integrity® stent (Medtronic, Minneapolis, MN, USA), and the SORT-OUT VIII trial5, which showed that the BioMatrix NeoFlex™ (Biosensors, Morges, Switzerland) was non-inferior to the SYNERGY™ (Boston Scientific, Marlborough, MA, USA). Both trials were performed in an all-comers population and used TLF at 12 months as the primary endpoint. The MASTER trial6 and BIOFLOW-IV trial7, which both used the target vessel failure (TVF) rate at 12 months as the primary endpoint, showed that the Ultimaster® DES was non-inferior to the Kaname® bare metal stent (BMS) (both Terumo Corp., Tokyo, Japan) for PCI treatment of STEMI patients, and that the Orsiro was non-inferior to XIENCE P®/Xpedition® (Abbott Vascular), respectively. These trials are summarised in Table 1.
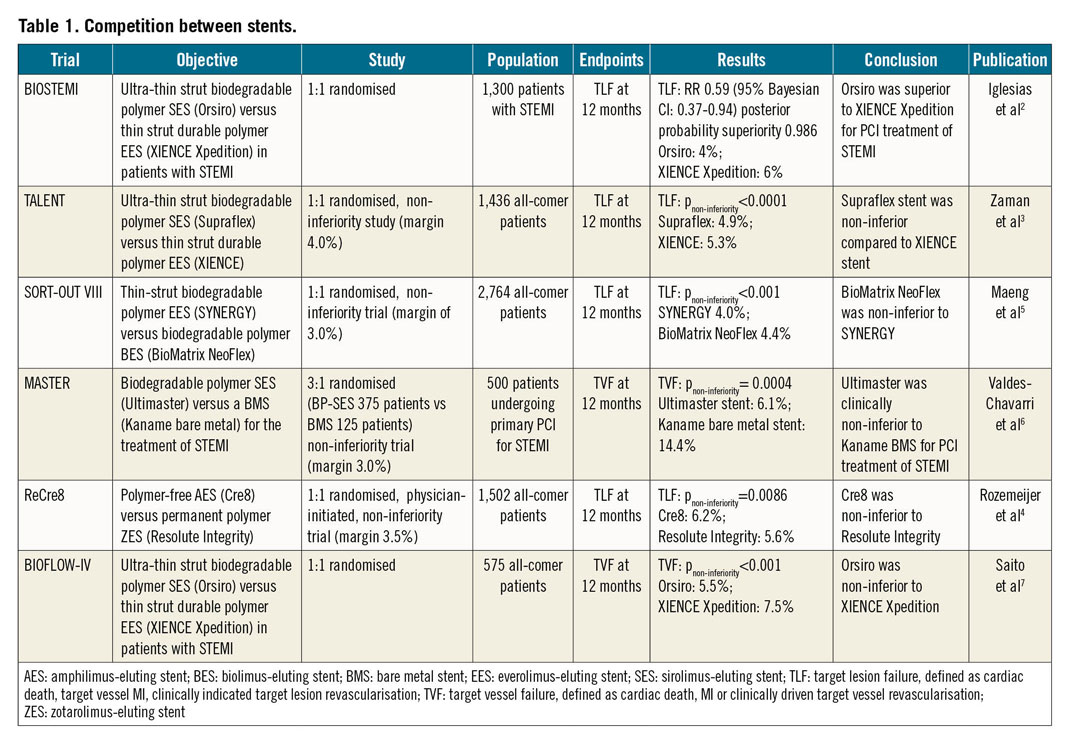
The assessment of extended long-term outcomes for DES is limited, especially regarding the comparison among second-generation DES. This year, the 10-year results of the ISAR-TEST 4 trial were published8. The study showed that second-generation DES, with either a permanent (XIENCE) or biodegradable polymer (Yukon Choice PC; Translumina Therapeutics LLP, New Delhi, India), were associated with better outcomes at 10 years, as compared with an early-generation DES with a permanent polymer (CYPHER®; Cordis, Cardinal Health, Milpitas, CA, USA). The 10-year incidence of major adverse cardiac events (MACE), which consisted of death, MI, or TLR, was 47.7% in the Yukon Choice PC arm, 46.0% in the XIENCE arm, and 54.9% in the CYPHER arm (p=0.003).
For DES versus BMS, the five-year results of the COMFORTABLE AMI study9 strengthened the more favourable earlier results of DES over BMS. In addition, two patient-level meta-analyses, one including 10,979 STEMI patients from 15 studies with a mean follow-up of three years10, the other including 26,616 patients from 20 randomised trials with a mean follow-up of 3.2 years11, both showed that the risk of the composite endpoint, cardiac death or MI, was reduced in DES compared with BMS recipients.
DRUG-COATED BALLOONS
The BASKET-SMALL 2 trial12 published in 2018 has shown that, in small native coronary artery disease (CAD), a paclitaxel-based drug-coated balloon (DCB) was non-inferior to DES regarding TVF up to 12 months. The DEBUT trial13, published in 2019, aimed to compare the efficacy of DCB with BMS among patients with de novo lesions (reference vessel diameter of 2.5-4.0 mm) and at high bleeding risk. After nine months, the primary outcome (cardiovascular death, MI, TLR) occurred in one of 102 (1%) patients in the DCB arm and 15 of 106 (14%) patients in the BMS arm (psuperiority=0.00034). Five of the 102 patients assigned to the DCB group received additional treatment for another lesion (3 with DES and 2 with BMS) and 23 of 106 patients in the BMS group received additional treatment for another lesion (22 with DCB and 1 with DES). There were also three lesions (2%) in two patients requiring bail-out stenting in the DCB group. Notably, in both arms, DAPT was used for only one month in this trial.
The DEBUT trial is so far the first RCT investigating the DCB-only strategy in large de novo coronary artery lesions. The results indicate that the use of DCB was superior to BMS among patients undergoing de novo PCI with a high bleeding risk. Nevertheless, the reference device of that study, a BMS, is not the optimal comparator, since the most recent European Society of Cardiology guidelines14 recommend DES over BMS, even in high bleeding risk patients. The short duration of DAPT (three months) seems to be effective in high bleeding risk patients. Moreover, trials regarding bioabsorbable polymer DES, such as LEADERS FREE15, have shown the safety of one-month DAPT with those stents. Thus, the optimal control of the DEBUT study might be a bioabsorbable polymer DES with a shorter duration of DAPT. However, based on the low MACE rate shown in the study, DCB might be a reasonable option for high bleeding risk patients.
Another interesting trial regarding DCB published in 2019 is the REVELATION trial16, which aimed to compare DCB versus DES in PCI for STEMI patients. Patients with a de novo, non-severely calcified culprit lesion and a residual stenosis of <50% after predilatation were enrolled. The primary endpoint was the fractional flow reserve (FFR) value of the infarct-related lesion. At nine months after enrolment, the mean FFR was 0.92±0.05 in the DCB group (n=35) and 0.91±0.06 in the DES group (n=38) (p=0.27). There were no cardiac deaths or recurrent MI events in any treatment group. Two of 58 patients (3%) in the DCB group and one of 54 patients (2%) in the DES group had TLR.
Although it is still too early to conclude that DCB and DES have comparable clinical outcomes in treating STEMI patients, the results presented are one step forward towards showing the safety and feasibility of the DCB-only strategy.
In-stent restenosis (ISR) represents the most common cause of treatment failure after PCI and the current practice for treating ISR is angioplasty with DCB or repeat stenting with DES. The DAEDALUS study17 published in 2019 is a patient-level meta-analysis, which included 1,976 patients from 10 RCT trials, comparing angioplasty with DCB and repeat stenting with DES in patients undergoing treatment for ISR. The results showed that, at three-year follow-up, DCB was associated with a significant increase in the risk of TLR compared with DES (hazard ratio [HR] 1.32, 95% CI: 1.02-1.70, p=0.035). The number needed to treat to prevent a TLR event was 28.5 in the DES group compared to DCB. The primary safety endpoint of all-cause death, MI, or target lesion thrombosis was comparable between treatments (HR 0.80, 95% CI: 0.58-1.09, p=0.152).
REVASCULARISATION STRATEGY
LEFT MAIN AND MULTIVESSEL DISEASE
The long-term results of several landmark trials comparing the revascularisation strategy of PCI versus CABG were reported in 2019 (Figure 2).
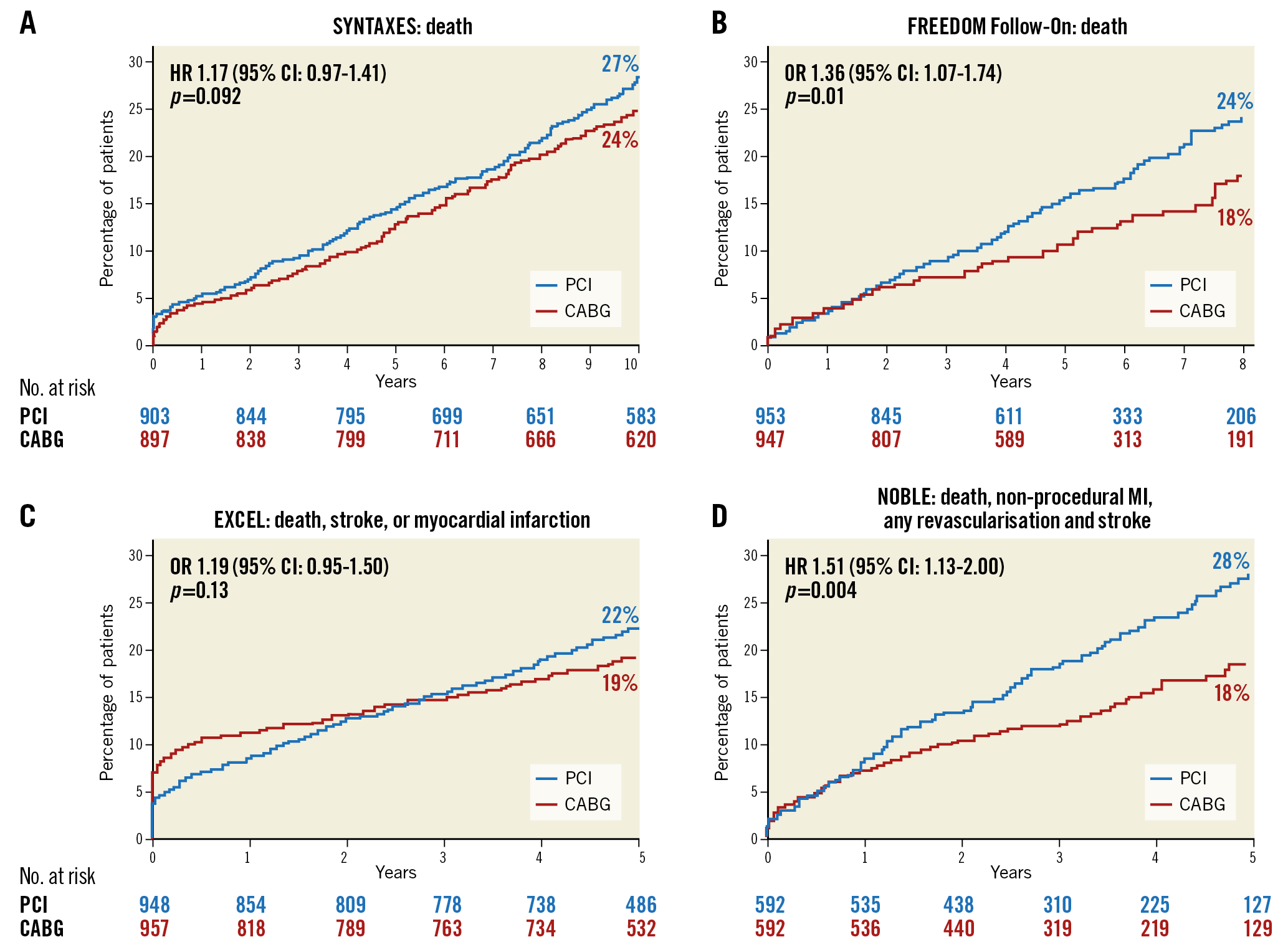
Figure 2. Primary endpoints of the SYNTAXES, FREEDOM Follow-On, EXCEL and NOBLE trials. A) The SYNTAXES study. B) The FREEDOM Follow-On study. C) The EXCEL study. D) The NOBLE study.
The SYNTAX Extended Survival Trial (SYNTAXES)18 reported the 10-year results of the SYNTAX trial, in which investigators randomised 1,800 patients with de novo three-vessel or left main (LM) CAD to receive PCI or CABG during 2005-2007. At 10 years, 244 (27%) of 841 patients had died after PCI and 211 (24%) of 848 had died after CABG (psuperiority=0.092). Among patients with three-vessel disease (3VD), 151 (28%) of 546 patients died after PCI; 113 (21%) of 549 died after CABG (HR 1.41, 95% CI: 1.10–1.80) and, among patients with LM CAD, 93 (26%) of 357 died after PCI versus 98 (28%) of 348 who died after CABG (0.90 [0.68-1.20]; pinteraction for 3VD vs LM=0.019). Patients with a higher SYNTAX score (≥33) benefited more from CABG than from PCI, whereas patients with lower or intermediate scores had similar results with either revascularisation strategy.
The FREEDOM Follow-On study19 showed the seven-year results of the FREEDOM trial20, which compared the outcomes of PCI with CABG in patients with diabetes mellitus (DM) and multivessel coronary disease. The results showed that the all-cause mortality rate was significantly higher in the PCI group than in the CABG group (24.3% [159 deaths] vs 18.3% [112 deaths], psuperiority<0.01). Another finding was that younger patients benefit more from CABG than older patients. For patients who were 63.3 years or younger, all-cause mortality at 7.5 years was 10.2% with CABG surgery and 20.7% with PCI. Comparatively, for those who were 63.3 years or older, the rate of all-cause mortality was 27.6% with CABG and 26.3% with PCI (p=0.01 for interaction by age). These long-term follow-up results support the revascularisation strategy of CABG in diabetic patients with multivessel CAD, regardless of SYNTAX score.
The most contested result in 2019 is the five-year result of the EXCEL trial. The trial enrolled patients with LM CAD of low or intermediate anatomical SYNTAX score (≤32) to undergo PCI or CABG. At five years, the primary endpoint (all-cause death, stroke, or MI) occurred in 22.0% of the patients in the PCI group and 19.2% of the patients in the CABG group (psuperiority=0.13). However, the secondary endpoint, death from any cause, occurred more frequently in the PCI group than in the CABG group (13.0% vs 9.9%; difference, 3.1%; 95% CI: 0.2-6.1). Based on the primary findings, the authors concluded that, in patients with LM CAD of low or intermediate anatomical complexity, there was no significant difference between the outcomes of PCI and CABG with respect to the rate of a composite outcome of all-cause death, stroke, or MI at five years. However, such a conclusion has raised heated debate, such as whether the periprocedural MIs should be included and contribute to the composite primary endpoint – the EXCEL trial included periprocedural MIs, whereas the NOBLE trial21 did not. The NOBLE trial, which enrolled patients regardless of their SYNTAX score, showed that CABG outperformed PCI in treating patients with LM stem CAD. The debate regarding the optimal revascularisation strategy for these patients will continue. For now, in light of EXCEL and NOBLE, a more cautious recommendation for PCI should be adopted in this setting.
OMT VERSUS PCI IN STABLE CAD PATIENTS
One of the greatest highlights of 2019 in the field of coronary intervention, or at least the one that gained the greatest attention, was the presentation of the ISCHEMIA trial. Ever since the controversial results of the COURAGE22 and the ORBITA23 trials, practitioners have been hoping for more conclusive and contemporary results from the large ISCHEMIA trial (basic information on the ISCHEMIA trial, including sponsorship and coordinating centre, can be found at https://www.ischemiatrial.org). In this study, investigators randomised 5,179 patients with stable CAD and moderate to severe myocardial ischaemia on non-invasive stress testing, to test whether routine invasive therapy was associated with a reduction in ischaemic events compared with optimal medical therapy (OMT). Results showed that, at 3.3 years, the primary outcome (cardiovascular death, MI, resuscitated cardiac arrest, or hospitalisation for unstable angina or heart failure) occurred in 13.3% of the routine invasive group compared with 15.5% of the OMT group (psuperiority=0.34). Invasive therapy was associated with harm (2% absolute increase) within the first six months and benefit within four years (2% absolute decrease). The rate of all-cause death was 6.4% in the routine invasive group as compared with 6.5% in the OMT group (psuperiority=0.67). Regarding the quality of life after PCI, improvement in symptoms was observed among those with daily/weekly/monthly angina, but not in those without angina.
The result of the ISCHEMIA trial showing that revascularisation does not lower the rates of death, heart failure, or cardiac arrest in the enrolled patients was not astonishing, since previous studies have indicated that PCI offered little advantage over OMT for these endpoints. However, the study demonstrated that early intervention is safe for patients who prefer to minimise the burden of medical therapies, for those who have limited tolerance to medications, or for those who have persistent symptoms despite medical therapy. This study also helps interventional cardiologists to provide more accurate information to patients regarding the benefits of PCI. Once again it underscores the importance of shared medical decision making between physicians and patients24. It is noteworthy that this study did not include patients with LM stenosis, left ventricular ejection fraction <35%, accelerating anginal symptoms, or an acute coronary syndrome. For these high-risk patients, intervention with OMT remains the recommended course of treatment.
MRI VERSUS FFR IN PLANNING OF REVASCULARISATION
Myocardial perfusion cardiovascular magnetic resonance imaging (MRI) is a non-invasive test for the detection of CAD that has a high concordance with FFR for ischaemia detection25 and can be used to guide revascularisation. The MR-INFORM study26 investigated whether an MRI-guided revascularisation is non-inferior to an FFR-based strategy in CCS patients. Patients with either ≥2 risk factors (smoking, diabetes, hypertension, hyperlipidaemia, positive family history) or positive exercise treadmill test were included in the trial. Revascularisation was recommended for patients in the cardiovascular MRI group with ischaemia in at least 6% of the myocardium or the FFR group with an FFR of 0.8 or less. The primary outcome occurred in 15 of 421 patients (3.6%) in the MRI group and 16 of 430 patients (3.7%) in the FFR group (risk difference, −0.2 percentage points; 95% CI: −2.7 to 2.4), findings that met the non-inferiority threshold. The percentage of patients free from angina at 12 months also did not differ significantly between the two groups (49.2% in the cardiovascular MRI group and 43.8% in the FFR group, psuperiority=0.21). MR-INFORM is the first trial to show that MR perfusion imaging could guide patient management in a high-risk population with the same effectiveness as invasive angiography with FFR. In a broader perspective, it is unclear but would be appealing to know whether other functional imaging, such as single photon emission computed tomography or dobutamine stress, or even computed tomography combined with FFR, would have provided similar results, since patients with angina are likely to undergo one of these tests in routine clinical practice before being referred for invasive angiography. Furthermore, the cost-effectiveness of this strategy needs to be investigated.
COMPLETE OR CULPRIT-ONLY REVASCULARISATION STRATEGY IN ACUTE CORONARY SYNDROMES
For ACS patients with multivessel disease, would a complete revascularisation strategy be superior to a culprit-only strategy? The debate has leaned towards the affirmative in recent analyses, with the caveat that most studies in this field have been small or retrospective. Another question is whether non-culprit lesions need to be treated immediately or whether the operators can wait and revascularise them in a staged procedure. The COMPLETE trial27 aimed to unravel these puzzling questions. Investigators randomised 4,041 STEMI patients with multivessel CAD who had successful culprit-lesion PCI either to undergo further complete revascularisation or be managed on medical therapy alone. All patients had non-culprit lesions with at least 70% diameter stenosis or an FFR measurement of 0.80 or less; the timing of complete PCI was left to operator discretion. At three years, cardiovascular death (CD) or new MI occurred in 158 of the 2,016 patients (7.8%, CD: 2.9%; MI: 5.4%) in the complete revascularisation group as compared with 213 of the 2,025 patients (10.5%, CD: 3.2%; MI: 7.9%) in the culprit-lesion-only PCI group (psuperiority=0.004). Additionally, the benefit of complete revascularisation was consistently observed regardless of the intended timing of non-culprit-lesion PCI (pinteraction=0.62). The optical coherence tomography substudy revealed a large proportion of thin-cap fibroatheroma in the non-culprit lesions. This may help to explain the benefit observed from multivessel revascularisation.
BIFURCATION LESIONS
Conceptually, there is a tenet among interventional cardiologists that “the simpler the better”, i.e., the provisional stent (PS) technique is preferred over the two-stent technique whenever possible28. However, the results of the DKCRUSH-V study reignited the debate in 201729, indicating that, in true distal LM bifurcation lesions (Medina 1,1,1 or 0,1,1), a planned DK crush two-stent strategy resulted in a lower rate of TLF at one year than a PS strategy. In 2019, this conclusion is further supported by its three-year results30. At three years, among the 482 patients enrolled, TLF occurred in 41 (16.9%) patients in the PS group and in 20 (8.3%) patients in the DK group (psuperiority=0.005), mainly driven by increased target vessel MI (5.8% vs 1.7%; psuperiority=0.017) and TLR (10.3% vs 5.0%; p=0.029). The definite or probable ST rate at three years was 4.1% in the PS group and 0.4% in the DK group (psuperiority=0.006). These results should be interpreted cautiously since the sample size was small and the selection of bifurcation Medina 1,1,1 could have affected the result towards a two-stent technique. Additional confirmatory studies by other investigators, such as the ongoing EBC MAIN trial (NCT02497014), will further enhance the evidence in this area.
REVASCULARISATION OF CTO LESIONS
Procedural results of chronic total occlusion (CTO) PCI have improved in recent years, and PCI strategies have moved towards complete revascularisation with more liberal use of CTO PCI. However, evidence in evaluating the efficacy of CTO PCI is still limited. The DECISION-CTO31 trial is one landmark trial in the field. It showed that there was no significant difference between the CTO-PCI and the OMT strategies regarding the incidence of the composite endpoint of death, MI, stroke, or any revascularisation (22.3% [n=93] versus 22.4% [n=89], psuperiority=0.86), indicating that routine CTO-PCI+OMT is not superior to OMT alone in reducing cardiovascular outcomes among patients with at least one CTO. The main limitation of the study is the high rate of crossover: 78 (19.6%) patients crossed over to receive staged CTO PCI within three days of randomisation. Nevertheless, DECISION-CTO is one of the first trials to compare the two therapies in a systematic fashion. CTO lesions are often referred to as the “final frontier” of PCI, but should we put our efforts into revascularising all CTO lesions? So far we believe the answer is still inconclusive. Further analyses will be vital to see if there are certain scenarios, such as high ischaemic burden, where CTO PCI might be beneficial compared with OMT.
ANTITHROMBOTIC THERAPIES AND PCI
THE TWILIGHT OF ASPIRIN?
Starting from 2013, a new series of studies has started to investigate “short DAPT/aspirin-free” antiplatelet strategies32. Following the GLOBAL LEADERS trial33,34, several additional studies of short DAPT/aspirin-free strategies have been published, with more positive findings (Figure 3).
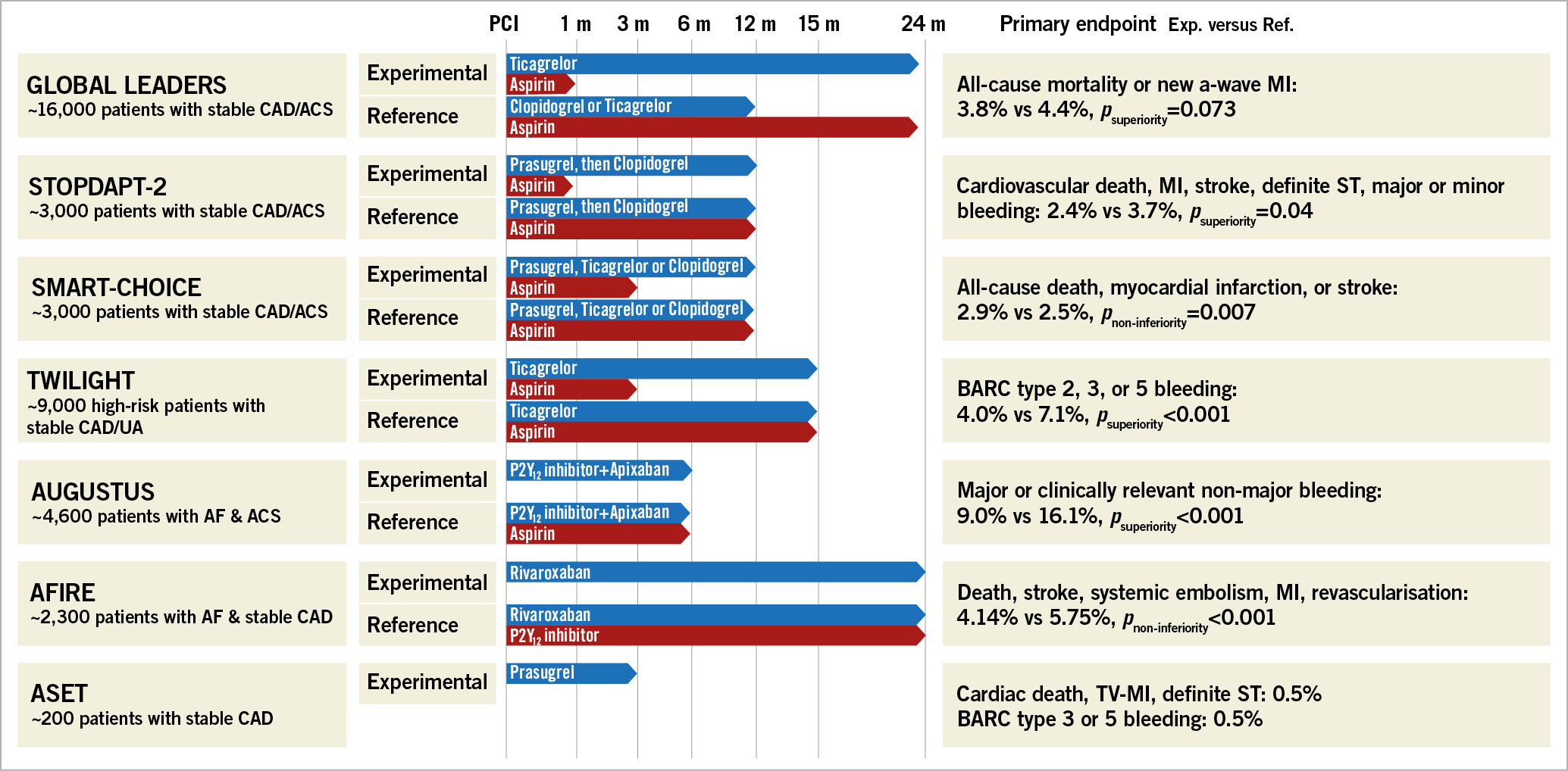
Figure 3. The duration of DAPT and the outcomes of seven studies.
The STOPDAPT-2 trial35 randomised 3,045 Japanese patients to receive either one month of DAPT followed by clopidogrel monotherapy or a 12-month DAPT with aspirin and clopidogrel regimen. Results showed that, at 12 months, the primary endpoint (cardiovascular death, MI, stroke, definite ST, or major or minor bleeding) occurred in 2.36% of patients in the one-month DAPT group and 3.70% in the 12-month DAPT group, meeting the criteria for superiority (p=0.04). Thombolysis In Myocardial Infarction (TIMI) major or minor bleeding occurred in 0.41% of participants with a one-month DAPT and 1.54% with a 12-month DAPT regimen (psuperiority=0.004).
In line with the STOPDAPT-2 trial, the simultaneously published SMART-CHOICE trial, which randomised 2,993 Korean patients to receive either a P2Y12 inhibitor monotherapy after three months of DAPT or 12 months of DAPT, showed that the primary endpoint (all-cause death, MI, or stroke) occurred in 42 patients in the P2Y12 inhibitor monotherapy group and 36 patients in the DAPT group (2.9% vs 2.5%; pnon-inferiority=0.007). The rate of Bleeding Academic Research Consortium (BARC) type 2 to 5 bleeding was significantly lower in the P2Y12 inhibitor monotherapy group than in the DAPT group (2.0% vs 3.4%; psuperiority=0.02).
Moving forward, the TWILIGHT trial, which randomised 7,119 patients, compared ticagrelor monotherapy (3-month DAPT + 12-month ticagrelor monotherapy) with the ticagrelor plus aspirin group (15-month DAPT). The former resulted in a significantly higher incidence of the primary endpoint, BARC type 2, 3, or 5 bleeding events (4.0% vs 7.1%, psuperiority<0.001). Regarding the incidence of the ischaemic endpoint (a composite of death, non-fatal MI, or non-fatal stroke), both groups reached non-inferiority (3.9% vs 3.9%, pnon-inferiority<0.001). It is noteworthy that the TWILIGHT trial enrolled high ischaemic risk patients, i.e., age of at least 65 years, female sex, troponin-positive ACS, DM, chronic kidney disease, multivessel CAD, bifurcation lesion treated with two stents, etc. The exciting findings of these three trials are that long-term DAPT may not be necessary after PCI with contemporary stents and that dropping aspirin may be a better approach than discontinuing the P2Y12 antiplatelet agent. The results were also supported by the subgroup analyses of the GLOBAL LEADERS trial, indicating that, in patients with multivessel PCI36, complex PCI37, with long stent implantation38 or bifurcation PCI39, age older than 7540, prolonged ticagrelor monotherapy for 23 months after one-month DAPT is associated with fewer ischaemic events (all-cause mortality and new Q-wave MI) without increasing the major bleeding events (BARC type 3 or 5), compared with standard 12-month DAPT.
ANTICOAGULANT AND ANTIPLATELET THERAPY IN PATIENTS WITH ATRIAL FIBRILLATION AND CAD
The previous PIONEER AF-PCI41 and RE-DUAL PCI42 trials both showed that, in patients with atrial fibrillation (AF) and requiring antiplatelet treatment, a new oral anticoagulant (NOAC) plus clopidogrel regimen was associated with a lower incidence of bleeding events as compared with a warfarin-based triple antithrombotic strategy. Therefore, the current expert opinions and consensus of North American Societies recommend a NOAC plus a P2Y12 inhibitor in patients with AF and ACS treated by PCI43. However, the European guidance still recommends triple antithrombotic therapy in these patients44. This year, the ENTRUST-AF PCI, AUGUSTUS and AFIRE trials brought us more evidence regarding the optimal antiplatelet therapy for AF patients.
The ENTRUST-AF PCI45 trial enrolled 1,506 patients with AF and who had a successful PCI for stable CAD or ACS to receive either edoxaban (60 mg once daily) plus a P2Y12 inhibitor or a vitamin K antagonist (VKA) in combination with a P2Y12 inhibitor and aspirin. The primary endpoint, 12-month major or clinically relevant non-major bleeding events, occurred in 128 (17%) of 751 patients with the edoxaban regimen and 152 (20%) of 755 patients with the VKA regimen (pnon-inferiority=0.001).
The AUGUSTUS trial46 is a two-by-two factorial design trial. The investigators assigned 4,614 patients with AF (who had an ACS or needed to take a P2Y12 inhibitor) to receive apixaban or a vitamin K antagonist (1st factorial) and to receive aspirin or matching placebo (2nd factorial). Results after six months of follow-up showed that major or clinically relevant non-major bleeding was noted in 10.5% of the patients receiving apixaban, as compared with 14.7% of those receiving a vitamin K antagonist (psuperiority<0.001), and in 16.1% of the patients receiving aspirin, as compared with 9.0% of those receiving placebo (psuperiority<0.001). Patients in the aspirin group (26.2%) and the placebo group (24.7%) had a similar incidence of death or hospitalisation. Based on the totality of the previous and present evidence, a NOAC plus a P2Y12 inhibitor might be considered as the new standard of care for AF patients presenting with ACS, who were previously prescribed triple antithrombotic therapy47.
While AUGUSTUS and other pivotal trials focused mainly on the antithrombotic treatment of AF patients with ACS, the AFIRE trial studied the antithrombotic strategy in patients with AF and CCS. The AFIRE trial48 enrolled 2,236 patients with AF who had undergone PCI or CABG more than one year earlier, or had stable CAD. These patients were randomly assigned to receive either rivaroxaban monotherapy (15 mg) or rivaroxaban (15 mg) plus a platelet inhibitor (approximately 25% of the patients received clopidogrel). The rate of the primary endpoint (death, stroke, systemic embolism, MI, or unstable angina requiring revascularisation) was 4.14% in the monotherapy group and 5.75% in the combination therapy group (pnon-inferiority<0.001). Monotherapy was also found superior to combination therapy for major bleeding, according to the criteria of the International Society on Thrombosis and Hemostasis, with rates of 1.62% and 2.76%, respectively (psuperiority=0.01). The current guidelines recommend mono-antiplatelet therapy with a NOAC for the type of population recruited in the AFIRE trial49. However, this approach was not supported by evidence from randomised, controlled trials. The AFIRE trial has now added an element for future guidelines, namely that rivaroxaban monotherapy without antiplatelet therapy might be a better approach in patients with AF and CCS.
TICAGRELOR, PRASUGREL, AND CLOPIDOGREL
ISAR-REACT 550 is the first head-to-head trial comparing ticagrelor to prasugrel in ACS patients. At one year, the primary endpoint (death, MI, or stroke) occurred in 184 (9.3%) of the 2,012 patients in the ticagrelor group and 137 (6.9%) of the 2,006 patients in the prasugrel group (psuperiority=0.006). BARC-defined type 3, 4 or 5 bleeding was observed in 5.4% of patients in the ticagrelor group and 4.8% of patients in the prasugrel group (psuperiority=0.46). The benefit of prasugrel in reducing ischaemic events was not penalised by a trade-off of increased bleeding events, which were numerically higher among ticagrelor-treated patients.
It is noteworthy that the delivery strategies between the two treatment regimens were different. For STEMI patients, both study drugs were initiated at the time of randomisation whereas, in patients with unstable angina and NSTEMI, ticagrelor was given as a loading dose at the time of randomisation and prasugrel was given at the time of angiography, with both arms receiving maintenance doses following PCI. Therefore, in addition to comparing the two drugs, this trial was also comparing two strategies among the NSTEMI patients, namely pre-treatment with ticagrelor and delayed treatment with prasugrel. Although there are some concerns regarding the trial, including the open-label design, the lack of tight oversight for drug adherence and discontinuation, and the different drug delivery strategies, ISAR-REACT 5 is a landmark study and will have an impact on our practice and future guidelines.
Current guidelines14 favour the more potent platelet inhibitors ticagrelor and prasugrel over clopidogrel because these drugs are more effective for the prevention of thrombotic events. However, this greater efficacy comes with a higher risk of bleeding. Reports have suggested that approximately 30% of Caucasian patients have an inadequate response to clopidogrel as measured with platelet function tests; the variation in response can be partially explained by genetic variations. In patients without these loss-of-function alleles, clopidogrel has shown similar efficacy to ticagrelor and prasugrel51.
Therefore, the investigators conducted the POPular Genetics trial52, in which patients who required antiplatelet therapy were randomised either to a genotype-guided treatment or to standard treatment; the choice of antiplatelet drug was left to investigator discretion. However, patients carrying the CYP2C19*2 or CYP2C19*3 loss-of-function alleles in the genotype-guided group received ticagrelor or prasugrel, and non-carriers received clopidogrel. At 12 months, the primary outcome (death, MI, definite ST, stroke or major bleeding) occurred in 63 of the 1,242 patients (5.1%) in the genotype-guided group and 73 of the 1,246 patients (5.9%) in the standard treatment group (pnon-inferiority<0.001). The bleeding outcome occurred in 122 patients (9.8%) in the genotype-guided group and 156 patients (12.5%) in the standard treatment group (psuperiority=0.04). These data suggest that the CYP2C19 genetic testing to guide the selection of oral P2Y12 inhibitor therapy in patients undergoing PCI was non-inferior to standard treatment with ticagrelor or prasugrel at 12 months in terms of thrombotic events and resulted in a lower incidence of bleeding. A similar large-scale RCT, the 5,300-patient TAILOR-PCI trial (NCT01742117) is awaited to add valuable information on this subject. The trial is in its final phase of recruitment; if it shows the same results, current practice will be modified.
PCI FOR ACS AND CARDIOGENIC SHOCK
MICROVASCULAR OBSTRUCTION
Microvascular obstruction is common, affecting half of the patients with STEMI, and is associated with adverse outcomes, but there remains no therapy to prevent or treat this comorbidity. The T-TIME trial53 aimed to determine whether a low-dose intracoronary fibrinolytic therapy with alteplase infused early after coronary reperfusion would reduce microvascular obstruction. A total of 440 patients who presented with STEMI were randomised 1:1:1 to receive either a placebo, alteplase 10 mg, or alteplase 20 mg. The primary outcome was the amount of microvascular obstruction (expressed as % left ventricular mass) demonstrated by contrast-enhanced cardiac MRI conducted from day 2 up to day 7 after enrolment. Results showed that the mean microvascular obstruction did not differ between the 20 mg alteplase and placebo groups (3.5% vs 2.3%; psuperiority=0.32) or in the analysis of the 10 mg alteplase versus placebo groups (2.6% vs 2.3%; psuperiority=0.74). The study findings disprove the treatment of low-dose intracoronary alteplase given during primary PCI to reduce microvascular obstruction.
TIMING OF RECANALISATION IN PATIENTS WITH CARDIAC ARREST
Although clinically significant CAD is commonly observed in patients who have a cardiac arrest, the generally accepted consensus is that, after resuscitation, the comatose patients who presented with STEMI should undergo immediate coronary angiography (CAG)/PCI. Apart from in these patients, the role of immediate CAG/PCI after successful resuscitation is still uncertain54. The COACT trial55 investigated this issue. A total of 552 patients who had cardiac arrest without signs of STEMI were randomly assigned to undergo immediate or delayed CAG/PCI until after neurologic recovery. At 90 days, 176 of 273 patients (64.5%) in the immediate angiography group and 178 of 265 patients (67.2%) in the delayed angiography group were alive (psuperiority=0.51). These results suggested that immediate angiography was not superior to a strategy of delayed angiography with respect to overall survival. One noteworthy limitation in the COACT trial is that after CAG only less than 20% of overall participants were found to have presented ACS, and only 33% of the patients in the trial had undergone PCI. Thus, only a small fraction of participants in the trial would be affected by the timing of the PCI or the performance of the PCI. In the subgroup analyses, patients older than 70 or with a history of CAD appeared to benefit from immediate CAG/PCI (pinteraction<0.05, respectively). Therefore, the COACT trial should be interpreted cautiously; further work is needed to guide the tailored treatment strategies for selected patients after cardiac arrest.
Patients with ACS who present initially with ST-elevation on the electrocardiogram but who subsequently show complete normalisation of the ST-segment and relief of symptoms before reperfusion therapy are referred to as having transient STEMI and pose a therapeutic challenge. It is unclear what the optimal timing of revascularisation is for these patients and whether they should be treated with a STEMI-like or an NSTEMI-like invasive approach. The TRANSITENT trial56 enrolled 142 patients to determine the effect of an immediate versus a delayed invasive strategy. Overall, infarct size in transient STEMI is small and is not influenced by an immediate or delayed invasive strategy. Infarct size of the left ventricular myocardial mass measured by cardiac MRI at day 4 was 1.3% in the immediate group and 1.5% in the delayed invasive group (psuperiority=0.48). There was no difference in MACE, defined as death, reinfarction, or TVR at 30 days (2.9% vs 2.8%, psuperiority=1.00). These results might indicate that, among patients with transient STEMI, immediate invasive therapy does not have additional benefits in reducing infarct size over delayed invasive therapy. Although the trial was negative, these findings may help to refine guidelines in this patient population.
DEVICES FOR CARDIOGENIC SHOCK AND STEMI
The role of intra-aortic balloon counterpulsation (IABP) in cardiogenic shock is still a subject of debate despite the neutral 30-day results of the IABP-SHOCK II trial57. The six-year results of the IABP-SHOCK II trial58 have now been presented: they show that mortality was not different between the IABP (197/297, 66.3%) and the control (197/294, 67.0%) groups (psuperiority=0.98). Together with the negative short-term results, we can conclude that the use of IABP did not reduce early or late mortality, supporting current guideline recommendations of no routine use of IABP in cardiogenic shock.
The haemodynamic improvement from IABP may be too modest to affect mortality. It is unknown if a stronger mechanical support device such as the Impella® (Abiomed, Danvers, MA, USA) would have resulted in different outcomes. However, there are two retrospective analyses published in 2019 which investigated this question. One analysis showed that, in patients with STEMI with cardiogenic shock, the use of an Impella was not associated with lower 30-day mortality compared with matched patients from the IABP-SHOCK II trial treated with an IABP or medical therapy59; the other analysis, which included 48,306 patients, suggested that the use of the Impella was associated with higher rates of adverse events and costs60.
The cardioprotective remote ischaemic conditioning stimulus can be applied using serial inflations and deflations of a pneumatic cuff placed on the upper arm or thigh to induce brief cycles of ischaemia and reperfusion. Some clinical studies in patients with STEMI have shown that the remote ischaemic conditioning increased myocardial salvage and reduced MI size by 20-30% when applied before or during reperfusion. The CONDI-2/ERIC-PPCI trial61, with an RCT design, aimed to determine whether remote ischaemic conditioning could reduce the incidence of cardiac death and hospitalisation for heart failure at 12 months; however, the results are disappointing. At 12 months post PCI, the primary endpoint (cardiac death or hospitalisation for heart failure) occurred in 220 of 2,569 (8.6%) patients in the control group and 239 of 2,546 (9.4%) in the remote ischaemic conditioning group (psuperiority=0.32), suggesting that remote ischaemic conditioning does not improve clinical outcomes.
INTRACORONARY IMAGING
VULNERABLE ATHEROMATOUS PLAQUE DETECTION
So far, there are no prospective cohort data showing whether the cholesterol content within the coronary artery wall is predictive of future events. Lipid-rich plaque (LRP) is believed to be associated with ACS and can be detected by near-infrared spectroscopy (NIRS). In the LRP study62, investigators aimed to establish the relationship between LRPs detected by combined NIRS-intravascular ultrasound imaging (IVUS) at unstented sites and subsequent coronary events from new culprit lesions. Patients with CAD who underwent cardiac catheterisation were enrolled in the study, and their non-culprit segments were scanned by using NIRS-IVUS imaging. At the two-year follow-up, the cumulative incidence of MACE (cardiac death or arrest, non-fatal MI, ACS, revascularisation, and re-admission to hospital for angina) was 9% (n=103). On a patient level, the hazard ratio for MACE was 1.21 (95% CI: 1.09-1.35; psuperiority=0.0004) for each 100-unit increase in maxLCBI (lipid core burden index) 4 mm. In patients with a maxLCBI 4 mm more than 400, the HR for MACE was 2.18 (1.48-3.22; psuperiority<0.0001). At the plaque level, the HR was 1.45 (1.30-1.60; psuperiority<0.0001) for each 100-unit increase in maxLCBI 4 mm. For segments with a maxLCBI 4 mm more than 400, the HR for non-culprit lesion-related (NC)-MACE was 4.22 (2.39-7.45; psuperiority<0.0001). The results might possibly suggest that a combined NIRS-IVUS approach now adds an important tool to the diagnostic armamentarium in relation to vulnerable plaques and vulnerable patients in clinical practice.
IVUS GUIDANCE OF STENT IMPLANTATION
The IVUS-XPL study was the first demonstration of the clinical benefit of IVUS-guided PCI in second-generation DES implantation. The five-year result of the trial was recently presented63, showing that, when IVUS is used to guide PCI in long lesions, the clinical benefits over angiographic guidance extend up to five years. The primary endpoint (cardiac death, TLR-MI, ischaemia-driven TLR) occurred in 36 patients (5.6%) receiving IVUS guidance and in 70 patients (10.7%) receiving angiography guidance (p=0.001). The difference was driven mainly by a lower risk of TLR (31 [4.8%] vs 55 [8.4%], psuperiority=0.007). By landmark analysis, the primary endpoint between one and five years occurred in 17 patients (2.8%) receiving IVUS guidance and in 31 patients (5.2%) receiving angiography guidance (psuperiority=0.031). These results reiterated the beneficial effect of IVUS guidance and showed that the effect was not only sustained up to five years but also amplified between one and five years.
TECHNICAL APPROACHES
Some experienced femoral artery access interventionists still prefer this route for CAG or PCI. However, radial access has been shown to reduce mortality and bleeding events, especially in patients with ACS64. The SURF trial65 used a 2×2 factorial design to compare radial versus femoral and standard versus ultrasound-guided puncture. A total of 1,388 patients were enrolled. The primary outcome was Acute Catheterization and Urgent Intervention Triage strategY (ACUITY) major bleeding, MACE (death, stroke, MI or urgent TLR) and vascular complications at 30 days. Results showed that the transradial access reduced the primary outcome (RR 0.37, 95% CI: 0.17-0.81; psuperiority=0.013), mostly driven by ACUITY major bleeding (RR 0.34, 95% CI: 0.123-0.959; psuperiority=0.041) when compared with the transfemoral approach. There was no difference in the primary outcome between the standard and ultrasound guidance for femoral artery access (psuperiority=0.76). Ultrasound guidance, however, reduced mean access time (93 sec vs 111 sec; p=0.009), the number of attempts (1.47 vs 1.9; psuperiority<0.0001), and venepuncture occurrence (4.1% vs 9.2%; psuperiority<0.0001) and improved the success rate of difficult access (4.5% vs 9.2%; p=0.0007) and first-pass success (73% vs 59.7%; p<0.0001). The take-home message of the SURF trial is clear – with or without the ultrasound guidance, transradial access should still be the preferred vascular access route.
Intravascular lithotripsy (IVL) is a novel technology, based on an established treatment strategy for renal calculi, in which multiple lithotripsy emitters mounted on a traditional catheter platform deliver localised pulsatile sonic pressure waves to modify vascular calcium circumferentially. The recently published Disrupt CAD II study66 proved the safety and effectiveness of IVL for vessel preparation of severe coronary artery calcification (CAC) in stenotic de novo coronary lesions. One hundred and twenty patients with severe CAC with a clinical indication for revascularisation were enrolled in the trial. The post-IVL angiographic acute luminal gain was 0.83±0.47 mm, and residual stenosis was 32.7±10.4%, which further decreased to 7.8±7.1% after stent implantation. The primary endpoint (cardiac death, target MI and revascularisation) occurred in 5.8% of patients, consisting of seven non-Q-wave myocardial infarctions. There was no procedural abrupt closure, slow flow or no-reflow, or perforations. The ongoing Disrupt CAD III study is expected to provide further evidence about the safety and efficacy of IVL in the treatment of calcified lesions (NCT03595176).
Conclusion and perspectives
In 2019, robust evidence highlighted the short- and long-term efficacy of second-generation DES. The evidence that reinforces the concept of “short DAPT” has grown. The long-awaited ISCHEMIA trial confirmed that an invasive strategy cannot reduce hard endpoints but provides durable improvement in angina control and quality of life. Astonishing advances have taken place, reshaped our daily practice, and are expected to improve the quality of life and the long-term survival of patients with CAD.
Guest Editor
This paper was guest edited by Alec Vahanian, MD, PhD; Department of Cardiology, Hôpital Bichat-Claude Bernard, and University Paris VII, Paris, France.
Conflict of interest statement
R. Modolo has received research grants from SMT and Biosensors outside the present work. R-J. van Geuns has received research grants from Amgen, Abbott Vascular, Boston Scientific, and AstraZeneca, speaker’s fee from Abbott Vascular and honoraria from AstraZeneca and Amgen. P.W. Serruys reports personal fees from Abbott Laboratories, AstraZeneca, Biotronik, Cardialysis, GLG Research, Medtronic, Sino Medical Sciences Technology, Société Europa Digital & Publishing, Stentys France, Svelte Medical Systems, Philips/Volcano, St. Jude Medical, Qualimed, and Xeltis, outside the submitted work. The other authors have no conflicts of interest to declare. The Guest Editor is a consultant for Edwards Lifesciences.
Supplementary data
To read the full content of this article, please download the PDF.

