
Introduction
In 2017 we celebrated the 40th anniversary of one of the major breakthroughs in the history of medicine – Andreas Grüntzig’s first percutaneous coronary intervention (PCI). Since then, the field of interventional cardiology has grown exponentially, with endless possibilities, novel techniques, devices and pre-eminent research. Open heart surgery was already an absolutely astonishing advance in cardiology and, throughout the decades, the miniaturisation of these interventions has become more and more prevalent.
In 2017, expanding indications for transcatheter aortic valve replacement (TAVR) have been proved effective; a new view on the old “less is best” approach for bifurcation lesions has emerged for the left main stem (LMS); a new artery access site for coronary intervention has started to emerge; the debate on the timing of non-culprit lesions in ST-elevation myocardial infarction (STEMI) has escalated, and the hypotheses that bioresorbable scaffolds lead to vascular restoration therapy were disproven (or were they?).
During the past year, new publications and research relating to interventional cardiology have emerged. These will be highlighted in this review, which comprises the prominent interventional cardiology publications from the high-impact journals: The New England Journal of Medicine, The Lancet, Journal of the American College of Cardiology, European Heart Journal and EuroIntervention.
Coronary interventions
PERCUTANEOUS CORONARY INTERVENTION IN MULTIVESSEL DISEASE
The robustness of PCI for treating LMS disease was strengthened by results from the randomised EXCEL trial in 2016, being non-inferior to coronary artery bypass grafting (CABG) surgery in patients with low or intermediate SYNTAX scores. Now, evidence supporting the use of PCI to treat patients with multivessel disease comes from the one-year clinical outcomes of the SYNTAX II trial (Synergy between PCI with TAXUS and Cardiac Surgery II). In this study, the authors compared outcomes between state-of-the-art contemporary PCI in 454 patients with de novo multivessel disease to an equipoised sample of patients enrolled in the original SYNTAX I trial. This approach included all “the best” available current techniques: Heart Team decision making utilising the SYNTAX score II, coronary physiology-guided revascularisation, implantation of thin-strut bioresorbable polymer drug-eluting stents (DES), intravascular ultrasound-guided stent implantation, contemporary chronic total occlusion revascularisation techniques and guideline-directed medical therapy. The state-of-the-art approach led to a significant reduction in the composite of major adverse cerebrovascular and cardiovascular events (MACCE, 10.6% vs. 17.4%; HR 0.58, 95% CI: 0.39-0.85, p=0.006) compared to the equipoised patients in SYNTAX I. This was driven by significant reductions in myocardial infarction (MI) (HR 0.27, 95% CI: 0.11-0.70, p=0.007) and repeat revascularisation (HR 0.57, 95% CI: 0.37-0.9, p=0.015), but death and stroke did not differ (HR 0.69, 95% CI: 0.27-1.73, p=0.43, and HR 0.69, 95% CI: 0.10-4.89, p=0.71, respectively). Notably, the rate of definite stent thrombosis (ST) was lower using contemporary techniques (HR 0.26, 95% CI: 0.07-0.97, p=0.045)1 (Figure 1). In keeping with these results, different strategies for planning upcoming trials comparing PCI with CABG are needed, bearing in mind the improving armamentarium that interventional cardiologists and cardiac surgeons have at their disposal.
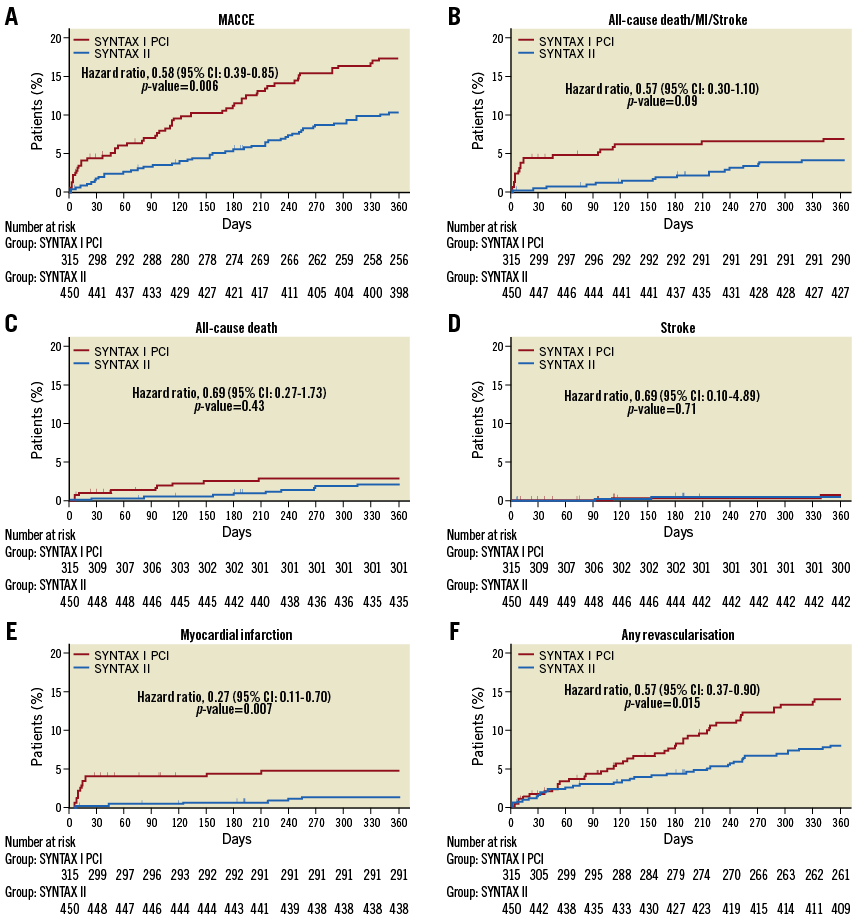
Figure 1. Time-to-event analysis of the patients from SYNTAX I and SYNTAX II. Kaplan-Meier curves of the one-year clinical outcomes among patients in the equipoise-derived SYNTAX I PCI cohort (red) and in the SYNTAX II “state-of-the-art” PCI group (blue) for the composite endpoint of MACCE (A), all-cause death, stroke and MI (B), and, separately, all-cause death, stroke, MI and any revascularisation (C, D, E, F, respectively). Reproduced from Escaned et al1.
BIFURCATION OF THE LEFT MAIN STEM
Clinically, there is a consensus among operators that for bifurcation lesions “less is best,” i.e., the provisional stent technique is preferred over the two-stent technique, whenever possible2. However, the DKCRUSH-V3 study has re-ignited this debate. This multicentre trial enrolled 482 patients with true distal LMS bifurcation lesions (Medina 1,1,1 or 0,1,1) to test the two-stent technique of double kissing crush against provisional stenting. At one-year follow-up, rates of target lesion failure (TLF) (5.0% vs. 10.7%, HR 0.42, 95% CI: 0.21-0.85; p=0.02), target vessel MI (2.9% vs. 0.4%, p=0.03) and definite/probable ST (3.3% vs. 0.4%; p=0.02) were lower in the two-stent group. Similarly, the final five-year follow-up of the DKCRUSH-II trial showed a significant reduction in target lesion revascularisation amongst the 370 patients randomised to the DK crush technique compared to the provisional approach (16.2% vs. 8.6%; p=0.027). Whilst rates of ST were similar, MACCE tended to be lower in the two-stenting group (23.8% vs. 15.7%, p=0.051)4. Of course, the results should be analysed cautiously since the sample size was small and the selection of bifurcation Medina 1,1,1 could have affected the result towards a two-stent technique.
PCI FOR STABLE ANGINA
One of the greatest highlights of the year in coronary intervention, or at least the one that gained the greatest attention from everyone, including the lay media, was the presentation and publication of the ORBITA trial5, which was designed to test whether PCI for stable angina actually relieved symptoms. It is noteworthy that PCI is commonly performed for this indication, despite the fact that no blinded randomised controlled clinical trial has ever tested its utility in this setting.
In this study, investigators randomised 200 patients with stable angina and a single coronary artery stenosis of at least 70% diameter stenosis to undergo PCI, or a sham procedure, in a blinded fashion after a six-week period of intensive medical therapy. The primary endpoint, which was the between-group difference in relief of symptoms, quantified by the change in exercise test times from before and six weeks after the PCI/sham procedure, was comparable between groups (PCI minus placebo of 16.6 sec, 95% CI: 8.9-42.0, p=0.20). No deaths were reported, whilst the only serious intraprocedural events (four dissections that were treated with PCI) all occurred in the placebo group and were related to the use of the pressure wire. Some limitations must be addressed such as the sample size, the short duration of follow-up, the training effect and the inability to detect microvascular disease – which could be a confounder for improvements with PCI. The conclusion of the trial is that PCI with stents for stable angina does not improve exercise time. Unsurprisingly, the study had extensive media coverage, with subsequent misinterpretation and overextrapolation of the results. Crucially however, this study brings us valuable information, and corroborates the results from the COURAGE trial6 which demonstrated how important optimal medical therapy was for the treatment of stable angina and that the amount of ischaemia matters. Further studies will no doubt follow, with larger sample sizes and different endpoints, with, however, the uncertainty that the results could be different. Importantly, the take-home message remains unchanged – medical therapy should never be taken for granted. A large trial comprising patients with moderate ischaemia (International Study of Comparative Health Effectiveness With Medical and Invasive Approaches – ISCHEMIA) is awaited to add valuable information to this matter. The trial (NCT01471522) is in its final phase of recruitment and will be able to address the long-term myocardial infarction and mortality endpoints – which was not possible with ORBITA.
NON-CULPRIT TREATMENT IN THE ACUTE PHASE OF ST-ELEVATION MYOCARDIAL INFARCTION
The debate regarding complete versus infarct-only revascularisation in patients with STEMI has been hotly contested ever since the publication of the PRAMI and CULPRIT trials. This has now been reinvigorated following the results of the COMPARE-ACUTE and the CULPRIT-SHOCK trials.
The COMPARE-ACUTE trial compared outcomes between 885 STEMI patients randomised to receiving immediate, fractional flow reserve (FFR)-guided, complete percutaneous revascularisation versus treatment of the culprit artery only. The primary endpoint – a composite of death, MI, repeat revascularisation and cerebrovascular events at 12-month follow-up – occurred less frequently in the complete revascularisation group (8% vs. 21%; HR 0.35, 95% CI: 0.22-0.55, p<0.001). This study showed not only the potential benefit from immediate complete revascularisation, but also the importance of revascularisation of functionally significant lesions – in this case assessed by FFR7.
At variance with this, and established practice, are the results of the CULPRIT-SHOCK (Culprit lesion only PCI versus multivessel PCI in Cardiogenic Shock) trial, which randomised 706 patients with STEMI and cardiogenic shock to receive primary PCI with either treatment of the culprit vessel only, or complete immediate revascularisation. The composite endpoint of death and renal replacement therapy after 30 days occurred in 46% of patients in the culprit lesion-only PCI group and in 55% of patients in the complete revascularisation group (RR 0.83; 95% CI: 0.71-0.96; p=0.01)8. Separately, the relative risk of death was 0.84 (95% CI: 0.72-0.98; p=0.03), and that of renal replacement therapy 0.71 (95% CI: 0.49-1.03; p=0.07), respectively, in the culprit only vs. multivessel PCI group. This extremely high-risk group of patients continues to have a high mortality rate and, interestingly, the mortality rate in CULPRIT-SHOCK (47% of all-cause mortality at 30 days) was similar to that seen in the initial SHOCK (Should We Emergently Revascularize Occluded Coronaries for Cardiogenic Shock)9 trial from almost 20 years ago – 51% of all-cause mortality at 30 days in the whole cohort. Disappointingly, despite all the advances in interventional cardiology over the past two decades, not much has changed for this increased risk set of patients.
The most recent guidelines on STEMI were from the European Society of Cardiology10. They upscaled the recommendation for complete revascularisation in STEMI from grade III (not recommended) to IIa, extending this recommendation to cardiogenic shock patients (Figure 2). However, with regard to STEMI patients with cardiogenic shock, these guidelines did not consider the results of CULPRIT-SHOCK and therefore, for this stratum, this recommendation should be evaluated cautiously in the cath lab.

Figure 2. The most important changes for the 2017 ESC Guidelines on STEMI. BMS: bare metal stent; DES: drug-eluting stent; IRA: infarct-related artery; i.V.: intravenous; LDL: low-density lipoprotein; PCI: percutaneous coronary intervention; SaO2: arterial oxygen saturation; STEMI: ST-elevation myocardial infarction; TNK-tPA: tenecteplase tissue plasminogen activator. Reproduced from Ibanez et al10. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC), European Heart Journal (2017) 00, 1-66 doi:10.1093/eurheartj/ehx393. Reproduced by permission of Oxford University Press on behalf of the European Society of Cardiology. Please visit: www.escardio.org/Guidelines/Clinical-Practice-Guidelines Acute-Myocardial-Infarction-in-patients-presenting-with-ST-segment-elevation-Ma
STENTS (DRUG-ELUTING AND BARE METAL)
Numerous DES versus DES trials have been conducted without demonstrating the superiority of one DES over another. However, this year we had the publication of the BIOFLOW V study, which randomised 1,334 patients with either elective or urgent PCI to treatment with the novel thin-strut bioresorbable polymer Orsiro stent (Biotronik, Berlin, Germany) or the durable polymer XIENCE® (Abbott Vascular, Santa Clara, CA, USA) stent. The primary endpoint of TLF occurred in 6% of the Orsiro group and 10% of the XIENCE group (p=0.039)11. This difference was mainly driven by increased target vessel myocardial infarction (8% vs. 5%, p=0.015), especially periprocedural MI. An ultra-thin strut resorbable polymer stent might be the next best thing for DES; however, larger studies with harder endpoints are needed for such conclusions. The DESSOLVE III trial – designed in a non-inferiority fashion – tested the sirolimus-eluting bioresorbable polymer-coated MiStent® (Micell Technologies, Durham, NC, USA) against XIENCE in over 2,000 lesions. At 12 months, the device-oriented primary endpoint, a composite of cardiac death, target vessel MI, or clinically indicated target lesion revascularisation occurred in 5.8% in the MiStent group and in 6.5% in the XIENCE group (difference –0.8% [95% CI: –3.3 to 1.8], pnon-inferiority=0.0001)12 (Figure 3). There was no difference in the rate of ST.
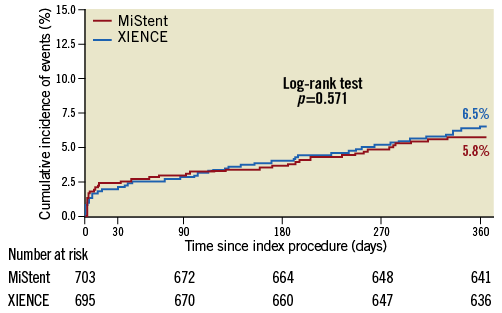
Figure 3. Time-to-event analysis of patients receiving XIENCE or MiStent. Cumulative incidence of the device-oriented composite primary endpoint – cardiac death, target vessel myocardial infarction, or clinically indicated target lesion revascularisation between patients in the XIENCE group (blue line) and those in the MiStent group (red line). Reproduced from de Winter et al12.
The LEADERS FREE trial had already shown at one year that polymer-free drug-coated stents (DCS) were safer and even more effective than bare metal stents (BMS) in over 2,000 patients at higher risk of bleeding who had received only one month of dual antiplatelet therapy (DAPT)13. At two-year follow-up, a lower incidence of the composite endpoint of cardiac death, MI or ST occurred in the DCS group compared to the BMS group (HR 0.80, 95% CI: 0.64-0.99; p=0.039), with similar rates of major bleeding14. Therefore, for high bleeding risk patients who need short durations of DAPT, the BioFreedom™ DES (Biosensors, Singapore) offers longer-term benefits over BMS, making these devices an attractive alternative to BMS.
Another high-risk group of patients is the elderly, where BMS have been used to minimise the duration of DAPT in order to avoid adverse outcomes such as bleeding. However, this exposes these patients to an increased risk of repeat revascularisation and its associated risks. The randomised single-blinded SENIOR trial was designed specifically for the purpose of addressing this issue by testing a shorter DAPT regime in patients over 75 years old who were randomised to a BMS or DES. In this trial, patients with stable coronary disease received one month of DAPT and unstable cases received six months. In this context, after randomising 1,200 patients, the investigators found that the patients who received DES had lower rates of the composite of all-cause mortality, MI, stroke, or ischaemia-driven target lesion revascularisation (12% vs. 16%; RR 0.71 [95% CI: 0.52-0.94]; p=0.02), with similar rates of bleeding complications15. This showed that a short duration of DAPT therapy can be used safely in elderly patients receiving DES. Further results with longer follow-up are needed.
INTRAVASCULAR DEVICES – ABSORB – NIGHTMARE FOR BIORESORBABLE SCAFFOLDS?
For bioresorbable vascular scaffolds (BVS), 2017 could be the year to forget, or the year of rebirth. The first-in-man analysis raised expectation on the possible healing process with the restoration of the vessel’s metabolism – vasomotion and vascular mechanotransduction. However, following the most recent data, the manufacturer interrupted the production and distribution of these scaffolds. The most important results came from the three-year clinical outcomes of the ABSORB III trial16 and from the preliminary report of the prematurely interrupted AIDA trial17. The ABSORB III trial tested the Absorb™ scaffold (Abbott Vascular) against cobalt-chromium everolimus-eluting stents (EES) for the primary endpoint of TLF in non-complex lesions. Although the primary endpoint did not differ after three years, it had a clear tendency of being higher in the Absorb group (13.4% in Absorb vs. 10.4% in EES; p=0.06). In this study, MI related to the target vessel and especially device thrombosis was higher in the Absorb group compared to EES (8.6% vs. 5.9%, p=0.03, and 2.3% vs. 0.7%, p=0.01, respectively). Hence, the AIDA trial – which randomised patients to the Absorb (BVS) or EES – had a median follow-up of 707 days, before being prematurely stopped by the Data and Safety Monitoring Board for safety concerns. The published preliminary data showed that the halt to the study was driven by a great increase in device thrombosis compared to the metallic stent (two-year cumulative event rates, 3.5% vs. 0.9%; HR 3.87, 95% CI: 1.78-8.42; p<0.001).
Despite these results, there are still some new bioresorbable vascular scaffold technologies being implemented and developed. A recent publication has shown that, in patients from the ABSORB trials (with more than 3,000 vessels treated with the BVS), technical issues relating to scaffold implantation such as proper vessel sizing, optimal predilation and adequate post-dilation were crucial for determining the outcomes in terms of TLF and scaffold thrombosis18.
FUNCTIONAL SEVERITY ASSESSMENT
FFR is already established as an important invasive tool for assessing ischaemia. A new technology for measuring the functional importance of a coronary stenosis – the instantaneous wave-free ratio (iFR) – was already proven to be feasible and to result in similar outcomes to the original FFR assessment, without the need for adenosine. The possibility of performing this evaluation without the discomfort provoked by adenosine with iFR was tested against FFR in two major trials with clinical endpoints to prove that it was non-inferior to FFR. The simultaneously published trials were the iFR-SWEDEHEART19 and the DEFINE-FLAIR20 trials. Both trials had quite similar designs, testing whether iFR-guided was non-inferior to FFR-guided revascularisation in patients with coronary disease with an indication to undergo functional assessment. Both studies randomised over 2,000 patients who were followed up for 12 months to detect the primary endpoint of death from any cause, MI or unplanned revascularisation. At follow-up, the primary endpoint was detected in 6.8% in the iFR group and in 7.0% in the FFR group of the DEFINE-FLAIR trial (difference in risk, -0.2%; 95% CI: –2.3 to 1.8; p<0.001 for non-inferiority; HR 0.95, 95% CI: 0.68-1.33; p=0.78), and in 6.7% in the iFR group and 6.1% in the FFR group (difference in event rates, 0.7%; 95% CI: –1.5 to 2.8; p=0.007 for non-inferiority; HR 1.12, 95% CI: 0.79-1.58; p=0.53) of the iFR-SWEDEHEART study – showing the non-inferiority of iFR compared to FFR. In both studies, the proportion of patients experiencing symptoms during the procedure was significantly lower in the iFR group. Thus, the new technology provides less discomfort, is cost-effective and non-inferior to FFR in terms of MACCE at one year.
Looking into the near future, the implementation of angiographically derived or tomographically derived FFR might emerge in the upcoming year. In the last year, angiography-based quantitative flow ratio (QFR) (Figure 4) has shown its feasibility and its diagnostic accuracy compared to FFR in the FAVOR II China Study21. Hence, the SYNTAX III Revolution trial is recruiting patients with multivessel disease and randomising Heart Teams to assess the results of either functional assessment with multislice computed tomography angiography or conventional angiography22.
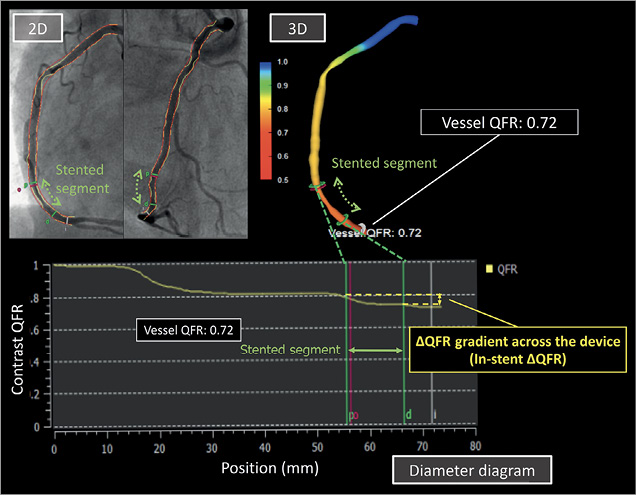
Figure 4. The quantitative flow ratio (QFR) with QAngio XA 3D. Example of a QFR calculation based on the 3D-QCA reconstructed from two angiographic projections (2D) and volumetric flow rate calculated by using contrast bolus frame count. QFR analysis was performed at the distal point of the target vessel (Vessel QFR). The graphic of contrast QFR can show the drop in the QFR as distal as you are in the vessel, even showing the delta in the stented segment. Reproduced from Asano et al49.
IMAGING GUIDANCE
The recently developed optical frequency domain imaging (OFDI) was tested against the widely used intravascular ultrasound (IVUS) with regard to clinical outcome in the OPINION trial. In this prospective, multicentre, non-inferiority trial, the investigators randomised patients who would undergo PCI to be guided by either IVUS or OFDI, having as its primary endpoint target vessel failure until 12 months after the procedure. Target vessel revascularisation (TVR) occurred in 5.2% in the OFDI-guided PCI group compared with 4.9% in the IVUS-guided PCI group (pnon-inferiority=0.042), concluding that this technology is non-inferior to IVUS in guiding PCI23. Further studies with longer follow-ups are expected.
PHARMACOLOGICAL THERAPY
The introduction and widespread use of new P2Y12 blockers, ticagrelor and prasugrel, led to improved ischaemic benefits and coronary outcomes, with the downside being an increased risk of bleeding. The assessment of bleeding risk was of paramount importance in deciding whether to use a new antiplatelet regimen, or not. A group of researchers published the TOPIC trial this year, hypothesising that one-month therapy with a new P2Y12 inhibitor subsequently replaced by DAPT with aspirin plus clopidogrel could improve bleeding without impacting on coronary-related events. With that goal in mind, the researchers randomised patients who had PCI for acute coronary syndrome and had completed one month of DAPT therapy with aspirin and the new P2Y12 inhibitors event free to switch to aspirin plus clopidogrel or to maintain the prescribed therapy. After 12 months, the group that changed therapy to clopidogrel plus aspirin had fewer events (cardiovascular death, urgent revascularisation, stroke and bleeding – BARC 2 or higher) compared to those who maintained therapy with the new P2Y12 inhibitors (13.4% vs. 26.3%; HR 0.48, 95% CI: 0.34-0.68, p<0.01)24. Surprisingly, after the unquestionable benefits of the new P2Y12 blockers over clopidogrel, the authors realised that, after the first month, the weight of bleeding on outcomes is considerable, and therapy with the “veteran” clopidogrel might be a tempting alternative – even just for the cost. However, one should bear in mind a few limitations of the study, such as the relatively small sample size, and the monocentric open-label nature of the design.
Finally, for this “seesaw” between bleeding and ischaemic events with DAPT after PCI, the PRECISE-DAPT score was proposed. This score has a simple five-item risk check that can predict the out-of-hospital bleeding risk during DAPT. The score takes into account age, creatinine clearance, haemoglobin, white blood cell count and previous bleeding, and can be of great assistance in clinical decision making regarding the duration of DAPT25.
As to anticoagulation during the PCI procedure, a large observational study comprising over 20,000 patients evaluated all-cause death at 30 days and one year in patients receiving bivalirudin or unfractionated heparin (UFH) only undergoing primary PCI. The investigators found a higher rate of death in the group that received only UFH26. However, in the context of PCI for MI, ever since the introduction of the new P2Y12 inhibitors and in the era of preferred radial access, no further information regarding bivalirudin has been obtained. In the VALIDATE-SWEDEHEART trial, Erlinge et al randomised over 6,000 patients with STEMI or non-STEMI who were undergoing PCI to receive bivalirudin or UFH. After six months, the researchers found no difference among the patients who received different anticoagulants regarding rates of MI, major bleeding, definite ST or death27. With that, the authors concluded that, in the era of potent antiplatelet agents and predominance of radial access for PCI, there was no reduction in events with the use of bivalirudin; therefore, UFH remains a good option.
CHRONIC TOTAL OCCLUSION
PCI for chronic total occlusion (CTO) is gaining ground, with an increasing number of training centres and dedicated materials; however, its benefits remain controversial. Addressing this matter, this year we had the presentation of the results of the DECISION-CTO trial (NCT01078051) at the American College of Cardiology (ACC) Congress in Washington. The presented results, as yet not published, showed that the composite endpoint of death, MI, stroke or repeat revascularisation at three years was non-inferior with medical treatment compared with PCI and that health-related quality of life was comparable between groups. The REVASC trial, presented at TCT Congress 2017, showed no benefit on left ventricular function evaluated by wall thickening in magnetic resonance imaging, compared with optimal medical treatment, but suggested, though it was underpowered, a symptom relief of the CTO procedure. The publication of these trials as well as the EuroCTO study (NCT01760083), presented at the Congress of the European Association of Percutaneous Cardiovascular Interventions (EuroPCR), are eagerly anticipated. With the data published, one can better evaluate the presented high prevalence of non-CTO lesions after enrolment, mild baseline symptoms and high rates of crossover from medical treatment to PCI in the DECISION-CTO trial. It is clear that the indications for CTO procedures are very strict and that new studies are needed to address this matter in its completeness28.
ARTERIAL ACCESS – THE SNUFFBOX TECHNIQUE
A new technique for accessing the radial artery for cardiac catheterisation has been described. It is used as an alternative for left radial access29 – thus diminishing the discomfort and malpositioning of the interventionist over the patient – or as a simple technique with possibly more comfort for the patient30. This approach rests on the fact that the puncture takes place in the anatomical snuffbox, inserting the wire in the most distal part of the radial artery, and has gained more visibility as a result of the influence of social media. Failures of this puncture site, crossover to ordinary radial access or even femoral access and patient outcomes need to be further investigated, but it seems a feasible and safe technique to be implemented (Figure 5).
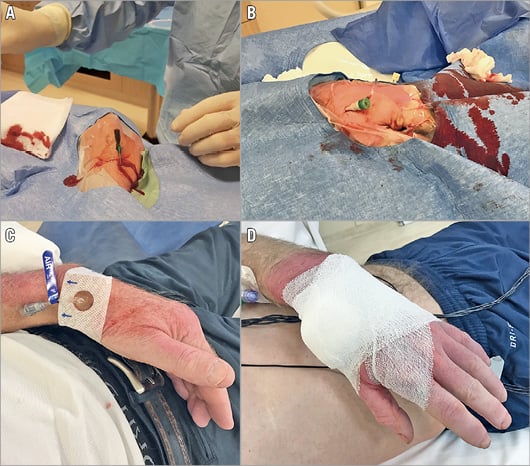
Figure 5. Demonstration of puncture and bandage in the distal left radial artery (at the anatomical snuffbox). A) The needle is directed to the point of strongest pulse, proximal in the anatomical snuffbox. B) A 6 Fr sheath is placed. C) A SafeGuard® (Merit Medical, Galway, Ireland) is left in situ for two to three hours. D) Manual compression bandage is left in situ for three hours. Reproduced from Kiemeneij et al29.
Structural heart interventions
TRANSCATHETER AORTIC VALVE REPLACEMENT
It is no surprise that the indications for TAVR are constantly expanding. In 2016 it was already considered the treatment of choice for aortic stenosis (AS) in patients considered inoperable and the preferred treatment for patients at high surgical risk. This field is growing exponentially in research and in device development, thus making the procedure steadily more beneficial for AS patients.
In 2016 we already had the publication of the PARTNER 2 trial, which showed comparable outcomes for the primary endpoint of all-cause mortality and stroke between intermediate-risk patients treated with TAVR vs. surgical aortic valve replacement (SAVR)31. This year saw the publication of the SURTAVI trial which also enrolled intermediate-risk patients randomised to receive TAVR or SAVR; however, in contrast to the PARTNER 2 trial where the valves were 100% balloon-expandable, in SURTAVI32 they were all self-expanding. In SURTAVI, TAVR was non-inferior to SAVR regarding the primary composite endpoint of all-cause death and disabling stroke (12.6% vs. 14.0%, respectively) (Figure 6). The recent ESC Guidelines on valvular heart disease33 took into account the findings of SURTAVI: for patients with STS ≥4%, the decision between surgery and TAVR should be made by the Heart Team according to the individual characteristics.

Figure 6. Non-inferiority analysis and time-to-event curves for the primary outcome. In this Bayesian analysis, the posterior probability distribution for the difference in the primary endpoint between patients who underwent transcatheter aortic valve replacement (TAVR) and those who underwent surgery confirmed that the non-inferiority margin for TAVR was met – on the left of the Figure. Also shown on the right are the time-to-event curves for the primary outcome of patients who underwent TAVR (blue dashed line) or SAVR (red line). Reproduced from Reardon et al32.
Also reported this year were outcomes for patients undergoing TAVR valve-in-valve following bioprosthetic aortic valve failure from the Valve-in-Valve (VIV) registry, which was nested in the PARTNER 2 trial. Mortality at 30 days and one year was 2.7% and 12.4%, respectively; however, of note was the significant reduction in mortality between patients included during the initial versus the latter part of the registry (30 days: 8.2% vs. 0.7%, respectively; p=0.0001, and one year: 19.7% vs. 9.8%, respectively; p=0.006). In addition, there was a decrease in ventricular mass, and improvements in Kansas City Cardiomyopathy Questionnaire score (mean: 43.1 to 77.0; p<0.0001) and the distance on the 6-minute walk test (163.6 to 252.3 m; p<0.0001)34. These results demonstrate that the VIV procedure is a technique with low mortality and a viable option for those high-risk patients with degenerative bioprosthetic aortic valves.
In terms of new devices for TAVR, the RESPOND trial showed the safety and feasibility of the LOTUS™ valve (Boston Scientific, Marlborough, MA, USA) in 996 patients. This valve can be repositioned and retrieved, and was successful in 99% and 98%, respectively, of the cases where it was attempted. Although no significant or only a trace of paravalvular leak was seen in 92% of patients, this came at the cost of an increased requirement for permanent pacemaker implantation (34.6%)35. Similarly, the FORWARD trial which tested the repositionable CoreValve® Evolut™ R (Medtronic, Minneapolis, MN, USA) in 1,058 patients demonstrated good safety, and an incidence of moderate or severe aortic regurgitation and permanent pacemaker implantation at discharge of 1.9% and 19%, respectively36.
PATENT FORAMEN OVALE CLOSURE
Until this year, data supporting closure of a patent foramen ovale (PFO) for preventing stroke were inconsistent and conflicting. Concomitantly, we had publication of three major trials for PFO closure in the second semester of last year – CLOSE, RESPECT and Gore REDUCE trials. All three randomised patients with a prior cryptogenic stroke that could be attributed to a PFO to undergo its closure compared to medical therapy for the prevention of recurrent stroke, with a few differences in each study.
In the CLOSE trial, patients with echocardiographic features symbolic of an increased risk for further stroke (atrial septal aneurysm or large interatrial shunt) were randomised to transcatheter PFO closure plus antiplatelet therapy (APT) versus APT versus anticoagulation in a total of 663 subjects. The findings included significantly less occurrence of stroke in the PFO closure groups compared to APT (14 vs. 0 patients, HR 0.03, 95% CI: 0-0.26; p<0.001), but with a higher incidence of atrial fibrillation (4.6% vs. 0.9%, p=0.02)37.
In the RESPECT trial, 980 patients with PFO and previous cryptogenic stroke were enrolled to undergo PFO closure or medical therapy (with either APT or anticoagulation) and were followed for a median of 5.9 years. In an intention-to-treat analysis, patients in whom PFO closure was performed had fewer recurrent strokes (18 vs. 28 events, HR 0.55, 95% CI: 0.31-0.999; p=0.046 by the log-rank test), with more common thromboembolic events in the PFO group38. The Gore REDUCE trial had similar enrolment of patients (PFO plus APT vs. APT); however, the investigators included as a co-primary endpoint the results from brain imaging at 24 months. The findings followed the same trend of the two reported trials, with less recurrent stroke in 3.2 years of follow-up (1.4% vs. 5.4%; HR 0.23, 95% CI: 0.09-0.62; p=0.002), but with no difference in silent stroke (from the imaging) between the groups. Atrial fibrillation occurred in 6.6% of patients after PFO closure39.
TRANSCATHETER LEFT ATRIAL APPENDAGE CLOSURE
In 2017 we also had publication of the five-year follow-up of the PREVAIL and the PROTECT-AF trials on left atrial appendage (LAA) closure. This patient-level meta-analysis comprised two studies that randomised patients with non-valvular atrial fibrillation to undergo LAA closure with the WATCHMAN® device (Boston Scientific) versus anticoagulation. The composite endpoint of stroke, systemic embolism or death was similar among the groups, but the LAA closure groups had significantly less haemorrhagic stroke, disabling/fatal stroke, cardiovascular/unexplained death, all-cause death, and post-procedure bleeding (HR 0.20, p=0.0022; HR 0.45, p=0.03; HR 0.59, p=0.027; HR 0.73, p=0.035; HR 0.48, p=0.0003, respectively)40. Further studies evaluating LAA closure are needed as the data thus far do not confirm a benefit from the procedure over standard therapy. In terms of PFO closure, 2017 possibly represents a turning point for the procedure.
MITRAL VALVE PROCEDURES
Percutaneous procedures for mitral valve disease, especially for functional mitral regurgitation, are probably the most recently developed procedures in interventional cardiology, and comprise a heterogeneous group of different devices, with diverse techniques for clipping, correcting, implanting, and so on. In this area, 2017 was marked by the introduction of novel devices, with the majority still undergoing first-in-man studies assessing their feasibility and safety with very little follow-up. There are techniques for chordal repair, annuloplasty, clipping and valve replacement. To avoid an extensive report of the literature, we restricted our report to only two new devices.
Worthy of mention is this year’s publication of the PASCAL feasibility study41 – the clips of Abbott have a rival from Edwards. In this first-in-man trial, the investigators successfully implanted the PASCAL TMVr system (Edwards Lifesciences, Irvine, CA, USA) in their entire cohort of 23 patients with mitral regurgitation (MR), with a subsequent improvement in MR to grade 2+ or less in 96% of patients. Data from the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry report regarding transcatheter mitral valve repair in the United States of America were also published. After evaluating 2,952 patients, they reported a success rate of 91.8% with 30-day and one-year mortality rates of 5.2% and 25.8%, respectively. Mortality and re-hospitalisation were associated with age, low ejection fraction prior to the procedure, lung disease, dialysis, worse post-procedure MR and tricuspid regurgitation42. Adding to the perspective of novel – and probably more sophisticated – devices, this report is encouraging for structural interventional cardiology.
As for valve replacement, the publication for the Tendyne (Figure 7) device (Tendyne/Abbott Vascular, Roseville, MN, USA) for symptomatic MR was released, showing feasibility of the procedure, and 30-day improvements in MR severity grade and a decrease in left ventricle diastolic volume index43. The data for the one-year follow-up of the Tendyne Global Feasibility Trial have been presented at TCT 2017 (but not published yet), showing great improvement in NYHA class and significant improvement in the quality of life evaluated by the Kansas City Cardiomyopathy Questionnaire (KCCQ). Duncan et al recently reported a single-centre experience with five patients followed for two years with the Tendyne device44 (Figure 8). One patient who was non-compliant with anticoagulation died nine months after the procedure. The remaining four are alive with improvements in their NYHA class and increase in exercise capacity. More recently came the release of the global pilot study on the Intrepid™ system (Medtronic). This is a nitinol, self-expanding valve, with bovine pericardial leaflets, implanted using the transapical approach, tested in 14 sites in the USA, Australia and Europe. From the fifty patients enrolled, 48 had successful valve implantation, and the 30-day mortality was 14%. The NYHA Class was I or II in 79% at follow-up (p<0.0001 vs. baseline). Longer follow-up after this pilot study is awaited45.
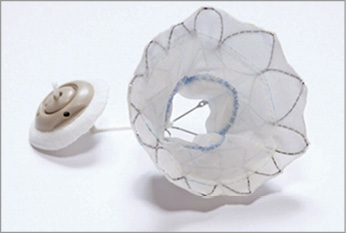
Figure 7. Tendyne device. Trileaflet porcine pericardial valve sewn within two self-expanding nitinol stents and an apical tethering/anchoring component (button-like pad and string tether). Reproduced from Duncan et al44.
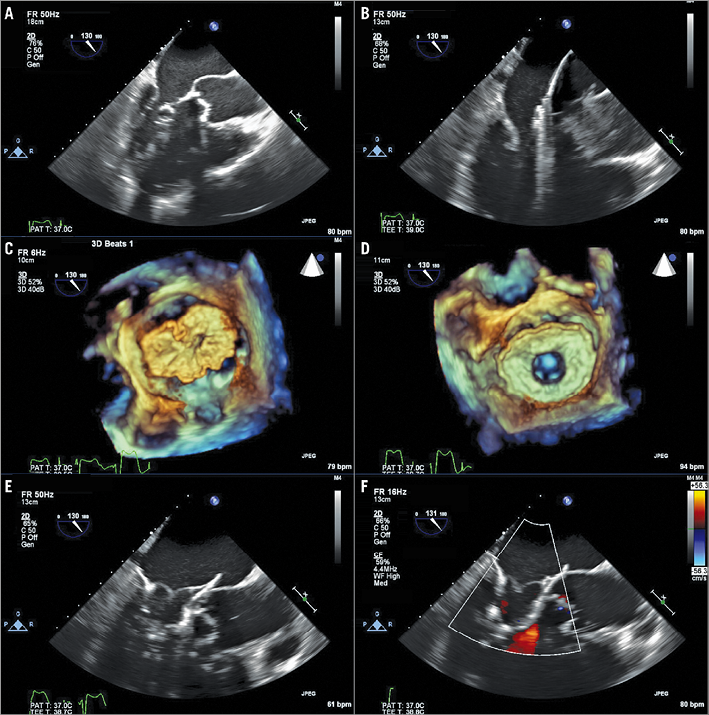
Figure 8. Transcatheter mitral valve replacement procedure. A) The balloon-tipped catheter tracked along the guidewire and advanced into the left atrium (LA). B) The balloon is removed, and a 34 Fr sheath advanced over the wire into the LA. C) The Tendyne delivery system extruded through the sheath in the mid portion of the LA and allowed expansion to 80%. D) Device rotated until correct anatomic position of the D-shaped outer stent is visualised. E) Device retracted into the mitral annulus using gentle traction and fully deployed. F) Colour Doppler assessment to confirm absence of transvalvular or paravalvular mitral regurgitation. Reproduced from Duncan et al44.
TRICUSPID VALVE PROCEDURES
Transcatheter tricuspid valve procedures, unlike mitral procedures and TAVR which can be curative, are seen as an adjunct to heart failure treatment46. Borrowing principles similar to mitral valve transcatheter repair, developments to treat tricuspid regurgitation are emerging rapidly. The most used system so far is the MitraClip® (Abbott Vascular). In a first-in-man experience published this year, researchers from Europe and Canada reported their experience with the MitraClip in 64 patients with chronic and severe tricuspid regurgitation with the edge-to-edge technique. With an implantation success in 97% and a decrease in the effective regurgitant orifice area (from 0.9±0.3 cm² to 0.4±0.2 cm²; p<0.001), in the vena contracta width (from 1.1±0.5 cm to 0.6±0.3 cm; p=0.001), and in regurgitant volume (from 57.2±12.8 mL/beat to 30.8±6.9 mL/beat; p<0.001), the data are really encouraging47. Besides MitraClip, new devices are being tested and developed such as the FORMA (Edwards Lifesciences), Cardioband (Edwards Lifesciences), Trialign™ (Mitralign, Inc., Tewksbury, MA, USA), TriCinch System™ (4Tech Cardio Ireland Ltd., Galway, Ireland), TRAIPTA concept (transatrial intrapericardial tricuspid annuloplasty), Millipede (Millipede, LLC, Ann Arbor, MI, USA) and Gate™ self-expanding tricuspid atrioventricular valved stent (NaviGate Cardiac Structures Inc., Lake Forest, CA, USA)46 (Figure 9).

Figure 9. Tricuspid device armamentarium: MitraClip (A), FORMA (B), Cardioband (C), Trialign (D), TriCinch (E), TRAIPTA (F), Millipede (G), Gate prostheses and delivery system (H). Reproduced from Taramasso et al46.
Conclusion and perspectives
The 40-year anniversary of Grüntzig’s first percutaneous angioplasty took place in a year with great advances in interventional cardiology. This year also saw the great influence of social media in trial discussion (e.g., ORBITA) and in new technique dissemination (e.g., the snuffbox technique). Greater things are expected for the upcoming year, such as the release of the GLOBAL LEADERS48 study - the largest “all-comers” trial with two years of follow-up after PCI for testing new DAPT strategies - results of the newest devices for mitral and aortic valves, and also for tricuspid valves. The achievements in this field for the next 40 years are unimaginable.
Guest Editor
This paper was guest edited by Alec Vahanian, MD, PhD; Department of Cardiology, Hôpital Bichat, and University Paris VII, Paris, France.
Conflict of interest statement
The authors have no conflicts of interest to declare. The Guest Editor declares receiving consultancy fees from Edwards Lifesciences.

