
Introduction
In the year 2018, the field of interventional cardiology continued to grow following 2017’s celebration of its 40th anniversary. Clinical trials comparing new stent technology with ultrathin biodegradable polymer drug-eluting stents and currently used stents seem to converge towards the same clinical safety and efficacy level. The introduction of new stent technology led cardiologists to challenge the need for early dual antiplatelet treatment. The new technology, with wire-free physiological non-invasive assessment, is gaining ground and computed tomography provides cardiologists with a revascularisation strategy for coronary artery disease. The transcatheter treatment of valvular heart disease opened a new era of intervention, with two trials on edge-to-edge mitral valve repair reporting conflicting results. Device treatment for hypertension returned showing its efficacy in sham-controlled trials. The aim of this review is to summarise the main results of the prominent trials in interventional cardiology in 2018: presentations from EuroPCR, ESC, AHA, ACC, PCR London Valves and TCT meetings, as well as manuscripts published in the New England Journal of Medicine, The Lancet, European Heart Journal, Journal of the American College of Cardiology and EuroIntervention. The majority of this article was presented at Gulf-PCR 2018, Dubai “Year in Review 2018”. The slides presented are available on the following website: https://www.pcronline.com/Cases-resources-images/Resources/Course-videos-slides/2018/GulfPCR-GIM-2018-Year-in-review-interventional-cardiology.
DRUG-ELUTING STENTS
The use of second-generation drug-eluting stents (DES) has shown improved clinical efficacy and safety in percutaneous coronary intervention (PCI) by reducing the risk of restenosis, stent thrombosis and myocardial infarction (MI) with the development of different stent designs, different strut thickness, different types of drug and their abluminal location when compared with first-generation DES1. Industry has been investing in new CE-mark approval trials. The clinical safety and efficacy are evaluated in relatively short periods of 30 days and 12 months after stent implantation. However, as determined by the executive summary of the European Society of Cardiology2, the follow-up must be maintained after CE-mark approval. Some stent trials for safety and long-term follow-up which reported in 2018 are available and are listed in Table 1. The second-generation thin DES have been used as benchmark and have already established their safety and efficacy; however, new-generation DES with ultrathin struts carrying a biodegradable polymer (BP) expected to show fast re-endothelialisation and less inflammation3 have been achieving their clinical efficacy and safety. The BIONYX trial4 evaluated the second-generation Resolute Onyx™ DES (Medtronic, Minneapolis, MN, USA) (n=1,243) and compared it with the ultra-thin Orsiro DES (Biotronik, Bülach, Switzerland) (n=1,245) in an all-comers population over one year; the primary endpoint of non-inferiority was met. In the ReCre8 trial5, the polymer-free amphilimus-eluting Cre8™ stent (Alvimedica, Istanbul, Turkey) (n=747) was compared with the second-generation Resolute Integrity® zotarolimus-eluting stent (ZES) (Medtronic) (n=744) and demonstrated its non-inferiority regarding target lesion failure (TLF) at 12 months. In another non-inferiority trial, TARGET All Comers6, the Firehawk® DES (MicroPort, Shanghai, China) (n=823) with a fully biodegradable sirolimus-containing polymer coating localised to recessed abluminal grooves on the stent surface was compared with the second-generation XIENCE DES (Abbott Vascular, Santa Clara, CA, USA) (n=830). Once again, the Firehawk was shown to be non-inferior to the XIENCE with regard to the primary endpoint of TLF at 12 months. Finally, the TALENT trial was presented at TCT 2018 in San Diego, USA (the slide set is available on the following website: http://www.crtonline.org/presentation-detail/randomized-trial-evaluating-ultra-thin-strut-biore). This was a prospective, randomised, single-blind, multicentre (23 centres) study in Europe randomising 1,435 patients to test non-inferiority of the Supraflex™ (APC Cardiovascular, Hartford, United Kingdom) thin-strut sirolimus-eluting stent with a biodegradable polymeric coating compared to the XIENCE stent with regard to TLF at 12 months; the non-inferiority was also met. The pre-specified two-year endpoints in the BIOFLOW V trial seem to favour the ultrathin DES Orsiro (n=884) over the XIENCE (n=450)7,8. The two-year rate of TLF was significantly better in Orsiro than XIENCE (7.5% vs. 11.9%, p=0.0015). However, the results should be carefully interpreted, because more lesions per patient, more stent overlap, and longer stent implantation were noted in the XIENCE group than in the Orsiro group. Meanwhile, a longer follow-up of five years of the same Orsiro and XIENCE comparison study (BIOSCIENCE) is now available9. In this longer follow-up, investigators observed no difference between the groups. When evaluating second-generation DES at five years, the TWENTE II trial showed low rates of adverse clinical events for both Resolute Integrity ZES (n=906) and PROMUS Element™ (Boston Scientific, Marlborough, MA, USA) (n=905)10. Ten-year results from the ISAR-TEST 4 randomised trial showed superiority of biodegradable polymer-based sirolimus-eluting stents (BP-SES; Yukon Choice PC [Translumina Therapeutics, New Delhi, India], n=1,299) and permanent polymer-based everolimus-eluting stents (PP-EES; XIENCE, n=652) over early-generation permanent polymer-based sirolimus-eluting stents (PP-SES; CYPHER® [Cordis, Cardinal Health, Milpitas, CA, USA], n=652)11. The expected treatment effect of biodegradable polymer over permanent polymer was not shown between BP-SES and PP-SES, but the need for an extended follow-up over 10 years was pronounced after implantation of first-generation PP-SES.
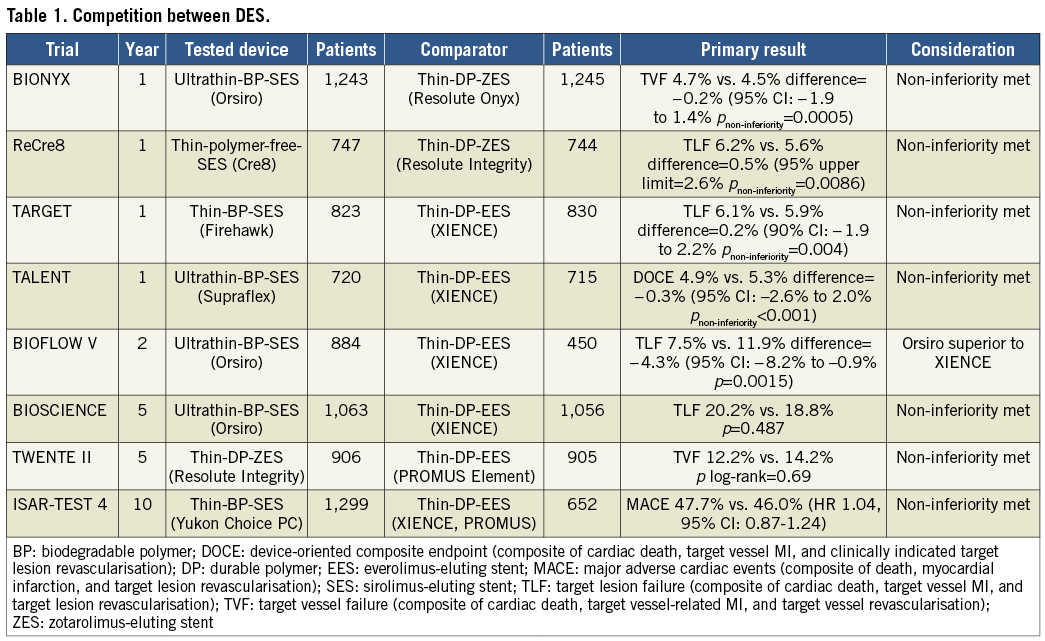
The fierce competition between drug-eluting stents seems to have come to an end with the same “me too” clinical safety and efficacy. However, in a recent meta-analysis12, the ultrathin (<70 μm) stents (Orsiro SES 60 μm, MiStent SES 64 μm [Stentys, Paris, France], and BioMime™ SES 65 μm [Meril Life Sciences Pvt. Ltd., Vapi, India]) have been shown to improve one-year clinical outcomes further compared with the second-generation DES (XIENCE EES, PROMUS EES and Resolute ZES) with a 16% reduction in TLF mainly driven by less MI attributable to the lower stent thrombosis rate, and potentially fewer cases of periprocedural MI due to less side branch coverage12. Whether strut thickness was the only factor for the clinical impact has still to be evaluated, and longer follow-up of the ultrathin stents will reveal their authentic clinical impact.
Adding stent layers in the vessel can lead to lumen narrowing by the stent itself. Even in the implantation of thin second-generation DES, angiographic late lumen loss ≥0.5 mm after two years had predictive value for subsequent revascularisation13. The drug-coated balloon (DCB) has already established its efficacy for restenosis after bare metal stent (BMS) and DES treatment but the safety and efficacy are, so far, not fully documented in comparison with DES in native coronary arteries. In the BASKET-SMALL 2 trial14, DCB was compared with the second-generation DES after successful predilatation in small vessel coronary artery disease. The proportions of major adverse cardiac events (MACE) were similar in both groups and showed non-inferiority after 12 months.
BIORESORBABLE SCAFFOLDS
Although in current guidelines the use of bioresorbable scaffolds (BRS) outside clinical studies is not recommended15, the bioresorption benefit is still expected. By using the decision analytic Markov model, the incremental benefit of biodegradable vascular scaffolds (BVS) over EES becomes apparent only after 19 years at the currently observed relative increase in device thrombosis of first-generation BRS and under the extreme hypothesis of no scaffold thrombosis and target lesion revascularisation (TLR) beyond three years16. BRS manufacturers should target scaffold thrombosis rates <1.45% at three years to make their technologies attractive for a 10-year follow-up. According to a previously published analysis17, small vessel sizing (≤2.25 mm) and operator technique (predilatation, post-dilatation with a non-compliant balloon sized up to 0.5 mm larger than the nominal scaffold diameter) were strongly associated with Absorb™ (Abbott Vascular) poly (L-lactide) everolimus-eluting BVS-related outcomes during three-year follow-up after adjustment for multivariable baseline patient and lesion characteristics. In 2018, reports about the selection of suitable vessel sizes for BVS implantation18 and implantation technique (CIAO: impaCt of Implantation strategy on Absorb long term Outcomes; presented at EuroPCR 2018, Paris, France) reached similar conclusions. The one-year results of BRS made of magnesium (Magmaris Registry: BIOSOLVE-IV) were reported at EuroPCR 2018, Paris, France. The so-called “4Ps” (patient selection, proper sizing, predilatation and post-dilatation) were required, and TLF rates at six months (2.5%) and 12 months (4.6%) showed the safety profile of the magnesium scaffold (the slide set is available on the following website: https://www.pcronline.com/Cases-resources-images/Resources/Course-videos-slides/2018/BRS-technology-clinical-outcomes-continued). The ABSORB IV randomised trial19 was designed to establish whether scaffold implantation under the optimised implantation technique and exclusion of small vessels could achieve the same late results compared to second-generation XIENCE DES. The results showed non-inferiority of the tested device at 30 days and one year for TLF and angina. TLF at 30 days occurred in 5.0% of patients assigned to BVS and 3.7% of those assigned to EES (p for non-inferiority=0.0244). TLF at one year occurred in 7.8% of patients assigned to BVS versus 6.4% assigned to EES (p for non-inferiority=0.0006). Angina, adjudicated by a central events committee at one year, occurred in 20.3% of BVS patients and 20.5% of EES patients (p for non-inferiority=0.0008). Device thrombosis within one year occurred in only 0.7% of patients assigned to BVS and 0.3% assigned to EES (p=0.1586). Still the follow-up results of BRS have not guaranteed the safety and efficacy of BRS use. Clinical trials and the new generation should primarily establish the BRS safety and efficacy.
DUAL ANTIPLATELET THERAPY
Dual antiplatelet therapy (DAPT) is currently indicated after stent implantation. The current clinically indicated DAPT duration depends on the patients’ presentation: in stable coronary artery disease, at least six months of DAPT and one month for high bleeding risk (HBR) patients; in acute coronary syndrome (ACS) patients, at least 12 months of DAPT and six months for HBR patients. The balance between ischaemic events and bleeding risk should be carefully considered especially in ACS and HBR patients. Whether patients with thin-strut stents should receive DAPT is still under exploration. The DAPT score was developed to estimate ischaemic and bleeding risks20. The DAPT score was successfully validated in a pooled cohort of three studies combining the CREDO Kyoto registry cohort-2, RESET, and NEXT conducted in Japan21 (n=22,386), whereas it failed to show its value in another nationwide registry, SCAAR, in Sweden (n=41,101)22. In 2,712 ACS patients, SMART-DATE was aimed at investigating whether a six-month duration of DAPT would be non-inferior to the conventional 12-month or longer duration of DAPT23. The primary endpoint of a composite of all-cause death, MI, or stroke at 18 months was comparable (p for non-inferiority=0.03), and the bleeding rate was 2.7% in the six-month DAPT group and 3.9% in the 12-month or longer DAPT group (p=0.09). However, MI occurred more frequently in the six-month DAPT group than in the 12-month or longer DAPT group (p=0.02). Despite encouraging results for the main analysis of non-inferiority, the increased risk of MI in shorter DAPT led the authors to conclude that prolonged DAPT in ACS patients should remain the standard of care. On the other hand, in the DAPT-STEMI trial, in which 1,100 patients were enrolled, the primary endpoint of a composite of all-cause death, MI, or stroke at 18 months was comparable without increasing MI between six months and 12 months of DAPT24. For the comparison of more potent P2Y12 inhibitors (ticagrelor versus prasugrel) for ST-elevation MI (STEMI), we have the one-year follow-up of PRAGUE-18 study results25. Although the study had a small sample size and was prematurely terminated, the authors found that economically motivated post-discharge switches from either of the potent P2Y12 inhibitors to clopidogrel were not associated with an increased risk of ischaemic events.
After the introduction of the novel and more potent P2Y12 inhibitor ticagrelor and with the intent of diminishing DAPT duration, the GLOBAL LEADERS trial26 sought to investigate whether ticagrelor in combination with aspirin for one month followed by ticagrelor monotherapy improves outcomes after PCI compared with standard antiplatelet regimens at two years. A total of 15,991 all-comer patients undergoing PCI with a biolimus A9-eluting stent for stable coronary artery disease or acute coronary syndromes were randomly assigned (1:1). At two years, 3.81% of participants in the experimental group had died or had a non-fatal centrally adjudicated new Q-wave MI, compared with 4.37% of participants in the control group (rate ratio 0.87 [95% CI: 075-1.01]; p=0.073). These results do not question the current guidelines15 which recommend DAPT following PCI. Nonetheless, with the rise and spread of the newer antiplatelet drugs27, results of the proof-of-concept ASET (Acetyl Salicylic Elimination Trial) with prasugrel, and of the TWILIGHT trial28 with ticagrelor, outcomes of trials testing monotherapy after PCI are eagerly awaited.
PHYSIOLOGY, FFR/QFR/FFRCT
In 2018, the value of invasive physiology-guided PCI in the ORBITA trial was shown in the restoration of ischaemia evaluated by stress echocardiography and the relief of patients’ symptoms29. The fractional flow reserve (FFR) index as confirmation of an ischaemic lesion in patients with coronary artery disease is well recognised. In a large cohort systematic review and individual patient data analysis from three large trials including 2,400 patients, Zimmermann et al showed that FFR-guided PCI resulted in a reduction of the composite of cardiac death or MI compared with medical therapy (hazard ratio [HR] 0.72, p=0.02), which was driven by a decreased risk of MI (HR 0.59, p<0.001) (presented at EuroPCR 2018, Paris, France)30. The results of the pre-specified five-year follow-up of stable patients enrolled in the FAME 2 trial31 (447 patients in the PCI group and 441 in the medical therapy group) showed that the rate of the primary endpoint (a composite of death, MI, or urgent revascularisation) was lower in the PCI group than in the medical therapy group at five years (13.9% vs. 27.0%; p<0.001), driven by urgent revascularisations (6.3% vs. 21.1%). There were no significant differences in the rates of death (5.1% and 5.2%) or MI (8.1% and 12.0%). Relief from angina was more pronounced after PCI than after medical therapy.
A novel non-hyperaemic index of coronary stenosis severity, resting full-cycle ratio (RFR), was evaluated. RFR, not limited by the sensitive landmarking of components of the pressure waveform or specific to the wave-free period like the instantaneous wave-free ratio (iFR), showed its potential clinical utility32. Although the verification of ischaemia by invasive testing in clinical practice is recommended in many guidelines, the actual rate of its use still remains low, due to the cost. The development of new technology with mathematical inferences allows FFR calculations derived from three-dimensional (3D) quantitative coronary angiography and computed tomography angiography (CTA). In the FAST-FFR study33, the accuracy of FFR derived from routine coronary angiography (FFRangio) had high sensitivity, specificity and accuracy compared with pressure wire-derived FFR. Quantitative flow ratio (QFR) is another diagnostic modality for functional testing. In the WIFI II (Wire-Free Functional Imaging II) study34, QFR correctly classified 83% of the lesions using FFR with cut-off at 0.80 as a reference standard. Physiological evaluation by QFR assessment showed good diagnostic accuracy.
The diagnostic performance of angiography-derived FFR for the diagnosis of haemodynamically significant coronary artery disease was determined recently by a Bayesian meta-analysis35 (Figure 1). Thirteen studies comprising 1,842 vessels were included in the final analysis. A Bayesian bivariate meta-analysis yielded a pooled sensitivity of 89%, a specificity of 90%, a positive likelihood ratio of 9.3 and a negative likelihood ratio of 0.13. The summary area under the receiver-operating curve was 0.84. The correlation between angiography-derived FFR and conventional invasive FFR is presented in Figure 2. The accuracy of angiography-derived FFR was adequate to detect haemodynamically significant lesions identified with pressure wire-measured FFR as a reference. A diagnostic strategy trial with the different technologies of angiography-derived FFR evaluating clinical endpoints is warranted.
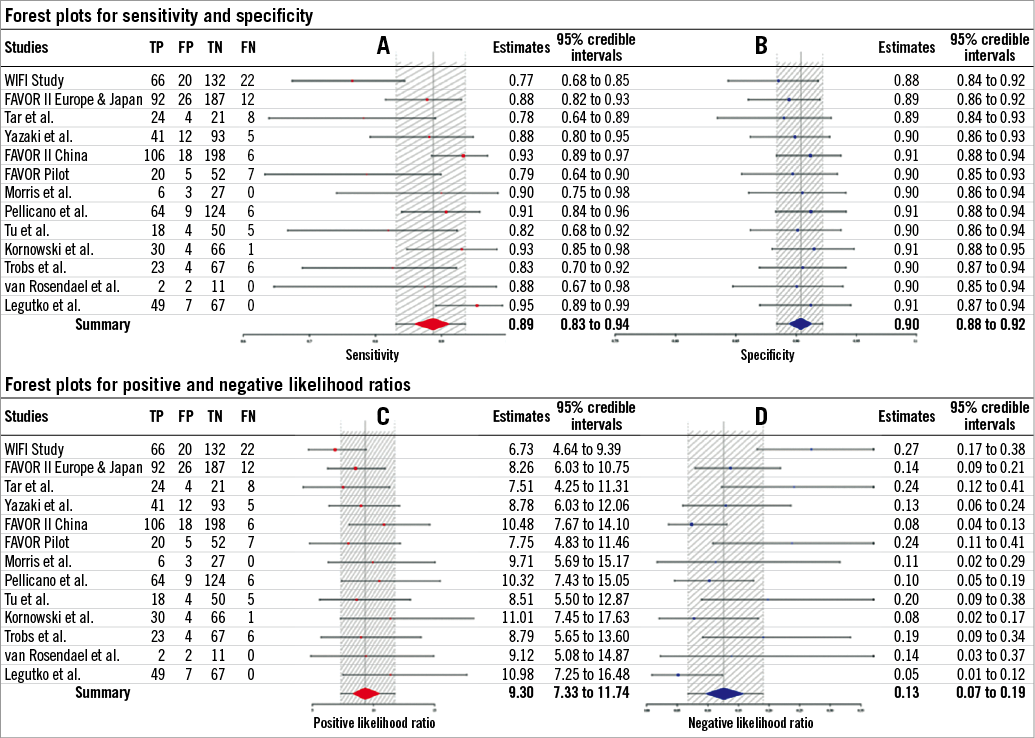
Figure 1. Individual studies of angiography-derived FFR and summary point estimates for sensitivity (A) and specificity (B), positive (C) and negative (D) likelihood ratio. Estimates within 95% credible intervals are shown. At the bottom, a summary estimate combining all studies is provided. FN: false negatives; FP: false positives; TN: true negatives; TP: true positives. (Reproduced with permission from Collet et al35).
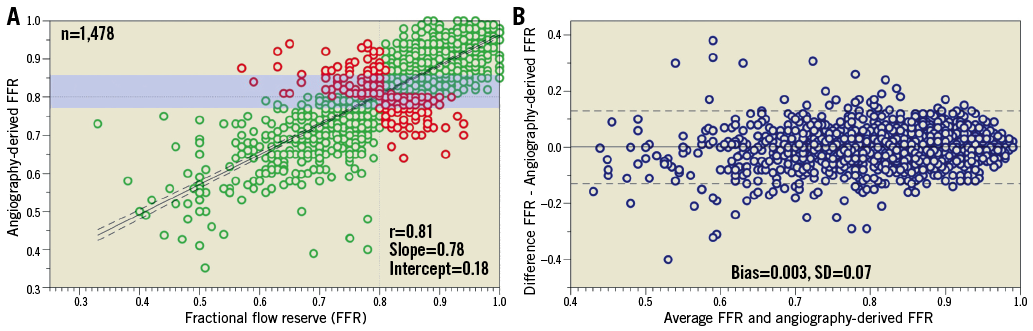
Figure 2. Lesion-level analysis of the correlation between angiography-derived FFR and conventional invasive FFR. A) Linear correlation between angiography-derived FFR and invasive FFR. The green dots represent the lesion with agreement on lesion significance whereas the red dots represent disagreement between methods. The grey dashed lines represent the cut-off values for each method. The solid black line represents the line of best fit between angiography-derived FFR and invasive FFR. The black dashed line represents the 95% confidence interval for the line of best fit; the shaded area represents the zone of uncertainty (i.e., 0.77-0.86). B) Mean difference between angiography-derived FFR and invasive FFR is shown. The solid line represents the bias and the dashed lines the limits of agreement. (Reproduced with permission from Collet et al35). FFR: fractional flow reserve
The SCOT-HEART trial36 had its five-year follow-up in 2018. Although there was an expected increase in angiograms and interventions in the CTA group during the early months of follow-up, at the end of the five-year follow-up the rates of angiograms and interventions were similar. At five years, CTA was associated with a reduction in coronary heart disease (CHD) death or MI vs. standard care (2.3% vs. 3.9%; HR 0.59, p=0.004). Benefit was largely due to a reduction in non-fatal MI. Since there was no difference in overall revascularisation rates, long-term benefit from CTA may have been due to lifestyle modification and statin therapy. SCOT-HEART shows that coronary CTA could be an attractive option for patients with stable chest pain when added to the standard clinical care.
The SYNTAX III Revolution trial was conducted to evaluate the significance of CTA for decision making in cases of complex CHD37. This trial aimed to test the agreement between two different Heart Teams (primary endpoint) on the revascularisation strategy of three-vessel disease (3VD) patients. For the investigation of the agreement between two diagnostic imaging modalities on treatment recommendation (i.e., CABG or PCI), two Heart Teams were randomised to assess the epicardial coronary artery disease with either coronary CTA or invasive coronary angiography in addition to clinical information. A case example is presented in Figure 3. The agreement on the treatment recommendation between the two imaging modalities reached an “almost perfect” (Cohen’s kappa 0.82). Moreover, the agreement between Heart Teams on which coronary vessels to be treated was 81%. As a secondary endpoint, this trial also tested the impact of the FFR derived from the CTA (FFRCT) on the decision-making process. The physiological assessment information was given to the Heart Teams only after an initial decision on the treatment had been made. This new information served to subtract points of the SYNTAX score for those lesions not considered functionally significant by FFRCT. The inclusion of FFRCT changed the treatment decision in 7% of the cases and modified treatment planning in 16% of the cases. These results support the potential role of non-invasive imaging with coronary CTA for treatment decision making and planning in patients with left main disease or 3VD.
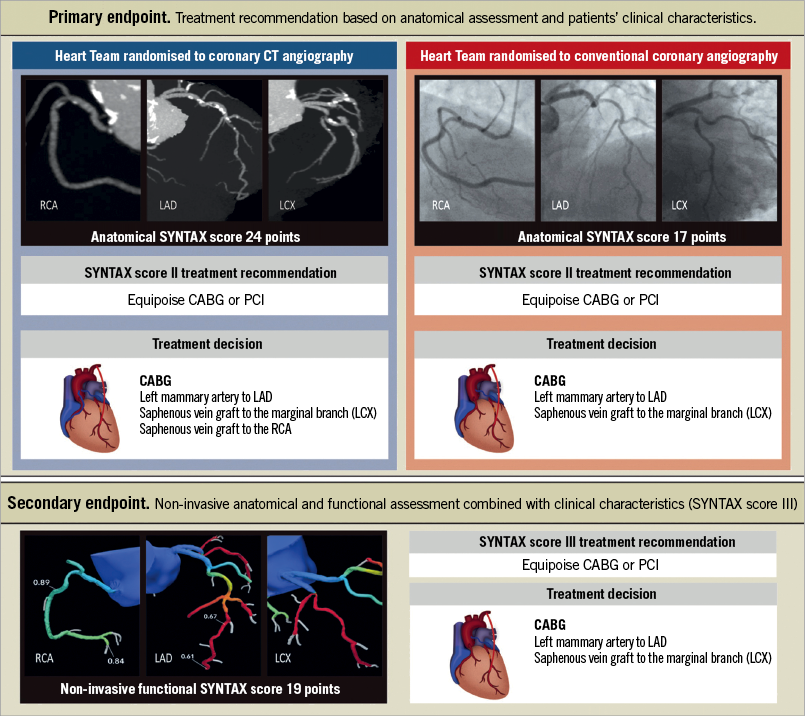
Figure 3. A case from SYNTAX III Revolution. The SYNTAX score II recommended either coronary artery bypass graft surgery or PCI based on a comparable predicted four-year mortality in this case. In the upper left column, the Heart Team recommended LAD, LCX, and RCA revascularisation by CT angiography. In the upper right column, the Heart Team recommended LAD, and LCX revascularisation depending on conventional angiography. The treatment recommendation based on coronary CTA with FFRCT remained but the treatment planning changed based on the negative FFRCT results in the right coronary artery. (Reproduced with permission from Collet et al37).
INTRAVASCULAR IMAGING
Moving from the non-invasive assessment to invasive imaging modalities, we appreciate that, in a large observational cohort study comprising 87,166 patients undergoing PCI, the investigators evaluated the role of OCT-guided intervention38. A significant difference in mortality was observed between patients who underwent OCT-guided PCI (7.7%) and those who underwent either IVUS-guided (12.2%) or angiography-guided PCI (15.7%; p<0.0001). The ULTIMATE trial aimed to determine the benefits of IVUS guidance over angiography guidance during DES implantation in all-comer patients39. A total of 1,448 all-comer patients were randomly assigned (1:1 ratio) to either IVUS guidance or angiography guidance. IVUS-guided DES implantation significantly improved clinical outcome (target vessel failure [TVF] 2.9% vs. 5.4%, HR 0.53, p=0.019). The Lipid-Rich Plaque Study, presented at TCT 2018 in San Diego, USA, aimed to detect vulnerable plaques and vulnerable patients by coronary near-infrared spectroscopy imaging (NIRS-IVUS). A total of 1,563 patients who underwent cardiac catheterisation with PCI as an index event were enrolled. In the vulnerable patient-level analysis, the risk of experiencing a non-culprit MACE event within 24 months was 18% higher with each 100-unit increase in maxLCBI4 mm. Patients with maxLCBI4 mm ≥400 had a MACE rate of 12.6% compared with 6.3% for patients with maxLCBI4 mm <400. In the vulnerable plaque-level analysis, the risk of experiencing an event in a coronary segment within 24 months was 45% higher with each 100-unit increase in maxLCBI4 mm. Plaque with maxLCBI4 mm ≥400 had a MACE rate of 3.7% compared to 0.8% for plaque with maxLCBI4 mm <400 (the slide set is available on the following website: https://www.tctmd.com/slide/detection-vulnerable-and-lipid-rich-plaque-using-nirs).
ACUTE CORONARY SYNDROME: SHOCK, STEMI, NSTEMI
The use of radial access for PCI is recommended to minimise bleeding complications; the periprocedural antithrombotic therapy is stratified in the current guidelines15. In 2018, important data from the MATRIX trial40 showed that bivalirudin significantly reduces bleeding complications not related to the access site, irrespective of the glycoprotein inhibitor (GPI) use in the heparin comparator group. Also, the one-year results of the MATRIX trial41 were reported, showing that the radial access sustained its benefits at one year compared with the femoral access. There was no superiority for the use of bivalirudin compared with heparin with or without glycoprotein IIb/IIIa inhibitors on a composite of ischaemic or ischaemic and bleeding endpoints combined, irrespective of the allocated radial or femoral access site.
The CULPRIT-SHOCK trial evaluated immediate revascularisation of the culprit lesion only or both culprit and non-culprit lesions on shock patients in a randomised fashion. In 2018, the secondary outcomes of the trial were presented with a one-year follow-up42. Death had occurred in 172 of 344 patients (50.0%) in the culprit lesion-only PCI group and in 194 of 341 patients (56.9%) in the multivessel PCI group (RR 0.88; 95% CI: 0.76-1.01). Extension of the primary endpoint follow-up to one year showed that the Kaplan-Meier curves were parallel after 30 days, maintaining the significant difference in favour of the culprit vessel-only revascularisation with regard to all-cause death and the need for renal replacement therapy (RR 0.87, 95% CI: 0.76-0.99, p=0.048).
For assessing the outcomes of complete revascularisation in non-ST-elevation MI (NSTEMI) patients, an observational multicentre cohort study was performed in the London cohort, comprising 21,857 patients with multivessel disease presenting with NSTEMI from 2005 to 201543. The investigators found that, despite higher initial (in-hospital) mortality rates, single-stage complete coronary revascularisation had fewer deaths in the long term. Kaplan-Meier analysis demonstrated significant differences in mortality rates between the two groups (22.5% complete revascularisation vs. 25.9% culprit vessel intervention; p=0.0005). The non-randomised nature of the study does not permit final conclusions. Also, the study did not assess one critical issue when evaluating complete versus culprit vessel-only treatment – the timing of complete revascularisation, i.e., whether it should be performed at once or in a staged procedure. The cohort was evaluated from 2005, therefore with the use of non-contemporary treatment (e.g., adjunctive therapy and devices); thus, contemporary results might be different. This supports the need for further randomised study to confirm these findings.
The optimal timing of invasive coronary angiography and revascularisation in patients with non-ST-segment elevation acute coronary syndrome (NSTE-ACS) is not well defined. The VERDICT study44 tested the hypothesis that a strategy of very early invasive coronary angiography (ICA) and possible revascularisation within 12 hours of diagnosis was superior to an invasive strategy performed within 48-72 hours in terms of clinical outcomes. A total of 2,147 patients with electrocardiogram (ECG) changes indicating new ischaemia and/or elevated troponin, in whom ICA was clinically indicated and deemed logistically feasible within 12 hours, were randomised 1:1 to ICA within 12 hours or standard invasive care within 48-72 hours. Within a median follow-up time of 4.3 years, a strategy of very early invasive coronary evaluation did not improve overall long-term clinical outcomes compared with an invasive strategy conducted within two to three days in patients with NSTE-ACS. However, in patients with a GRACE risk score >140, a very early invasive treatment strategy improved the primary outcome compared with the standard invasive treatment (HR 0.81, 95% CI: 0.67-1.01; p for interaction=0.023).
COMPLEX PCI: LEFT MAIN, CTO, CALCIFICATION
LEFT MAIN
The treatment for complex coronary artery disease is a growing field. PCI for left main is gaining ground in the field of myocardial revascularisation, showing comparable results with coronary artery bypass grafting (CABG). In 2018, a pooled analysis of individual patient data including 11 randomised clinical trials comparing CABG with PCI was published45. The authors estimated all-cause mortality up to five years using Kaplan-Meier analyses and compared PCI with CABG using a random-effects Cox proportional hazards model stratified by trial. The results showed no benefit for CABG over PCI in patients with left main disease, whereas CABG had a mortality benefit over PCI in patients with multivessel disease, particularly those with diabetes and higher coronary complexity. In 2018, long-term follow-up of 10 years after CABG or PCI in the SYNTAX trial (SYNTAXES) was reported at TCT 2018 in San Diego, USA. Although it was based on preliminary data, PCI had comparable survival to CABG in patients with LM disease (PCI 29.7% vs. CABG 31.9%, HR 0.89, 95% CI: 0.66-1.19; p=0.43), whereas CABG provided a survival benefit in patients with 3VD (PCI 29.2% vs. CABG 21.9%, HR 1.43, 95% CI: 1.10-1.85; p=0.007).
The Stents Versus Coronary-Artery Bypass Grafting for Left Main Coronary Artery Disease (MAIN-COMPARE) study had its ten-year follow-up released46. This registry evaluated patients with unprotected LM disease treated by PCI or CABG. At 10 years, no difference in all-cause death (21.1% vs. 23.2%; p=0.23) or in the composite of all-cause death, MI and stroke (23.8% vs. 26.3%; p=0.13) was observed between PCI and CABG, respectively. However, in the cohort comparing only DES and concurrent CABG among patients with more complex clinical and anatomic characteristics, a long-term benefit of CABG over PCI on mortality and hard clinical endpoints was detected after five years, with a significantly higher risk of death (HR 1.35, 95% CI: 1.00-1.81; p=0.05) and more severe unfavourable composite outcome (HR 1.46, 95% CI: 1.10-1.94; p=0.009) in patients with DES than in patients with concurrent CABG. One should of course bear in mind that the DES used between 2003 and 2006 in this registry were still first-generation DES; therefore, these results must not be extrapolated to the contemporary scenario.
We also saw the publication of the new consensus document on left main disease bifurcation treatment by the European Bifurcation Club47 in 2018. Although there is a multitude of strategies and approaches to bifurcation stenting within the provisional strategy as well as in all the different two-stent strategies, they maintain the recommendation to “keep it simple and safe”, limit the numbers of stents, care for the original bifurcation anatomy and try to reproduce it. However, despite the thorough guidance, the optimal bifurcation treatment is still unclear and should be elucidated in further dedicated studies and trials with the use of an imaging device (IVUS or OCT), as recommended in the guidelines also published in 201848,49.
CTO
Chronic total occlusions (CTOs) represent one of the challenging lesions associated with a lower success rate and a higher frequency of complications and the most common cause of incomplete revascularisation. The introduction of new devices and procedures significantly improved the success rate of PCI for CTO to about 90%50, but the benefit of PCI for CTO is unclear.
In 2018, the EURO-CTO trial randomised 396 CTO patients to receive treatment with PCI or optimal medical therapy. There was improved health status in the PCI group (angina frequency p=0.003, quality of life p=0.007, and physical limitation p=0.02), but the major adverse cardiac events were comparable between the two groups51. The REVASC trial was a single-centre, randomised trial which evaluated whether PCI for CTO, in addition to PCI of relevant coexisting non-CTO vessels, improves left ventricular (LV) function52. Patients with CTOs who were candidates for PCI were randomised to receive or not to receive PCI of CTOs. The primary endpoint was the change in LV wall thickness measured by cardiovascular magnetic resonance imaging (MRI) in the CTO territory between baseline and follow-up at six months. No benefit was seen for CTO PCI in terms of the primary endpoint, or other indices of LV function. However, the CTO PCI resulted in clinical benefit as evidenced by reduced MACE rates at 12 months, largely driven by the clinically indicated revascularisation compared with no CTO PCI. Since all data on CTO are still somewhat conflicting, further larger randomised controlled trials (RCTs) are eagerly awaited to generate conclusions.
CALCIFICATION
PREPARE-CALC53 was a randomised trial to evaluate high-speed rotational atherectomy prior to drug-eluting stent implantation in severely calcified lesions. A total of 200 patients with calcified lesions were assigned to modified balloon (MB, n=100) or rotational atherectomy (RA, n=100) followed by the implantation of a newer-generation, sirolimus-eluting stent with a bioabsorbable polymer. The trial had two primary endpoints – strategy success (defined as successful stent delivery and expansion with attainment of <20% in-stent residual stenosis in the presence of Thrombolysis In Myocardial Infarction (TIMI) 3 flow without crossover or stent failure; powered for superiority) and in-stent late lumen loss at nine months (powered for non-inferiority). Strategy success was significantly more common in the RA group (n=81, 81% versus n=98, 98%; relative risk of failure with an MB- versus RA-based strategy, 9.5; 95% CI: 2.3-39.7; p=0.0001). At nine months, mean in-stent late lumen loss was 0.16±0.39 mm in the MB group and 0.22±0.40 mm in the RA group (p=0.21, p=0.02 for non-inferiority). The use of rotational atherectomy prior to stent implantation was more successful and non-inferior regarding late lumen loss than MB for severely calcified lesions.
NEW GUIDELINES AND CONSENSUS
In 2018 new guidelines on myocardial revascularisation were released15. New recommendations and changes in class of recommendations are summarised in Figure 4.

Figure 4. Summary of the new recommendations and changes in myocardial revascularisation guidelines. What is new in the guidelines is summarised in the upper columns, while the changes in recommendations are shown in the lower columns. Reproduced from EuroIntervention15 with permission
Although this year there was no apparent update on patients with chronic kidney disease, the PREVENT trial54 reported that there was no favourable evidence on the use of sodium bicarbonate for the prevention of acute kidney injury after angiography. Revascularisation of a non-ischaemia-related artery in cardiogenic shock is now in Class III – not recommended. BRS use outside clinical studies is also not recommended.
The fourth universal definition of myocardial infarction was released55. In the updated concept, the difference between procedure-related myocardial injury and procedure-related myocardial infarction is emphasised. Although stand-alone post-procedural increases of cTn values are sufficient to establish a diagnosis of procedural myocardial injury, it is not for the diagnosis of type 4a MI. Type 4a MI requires an elevation of cTn values greater than five times the 99th percentile upper reference limit (URL) in patients with normal baseline values.
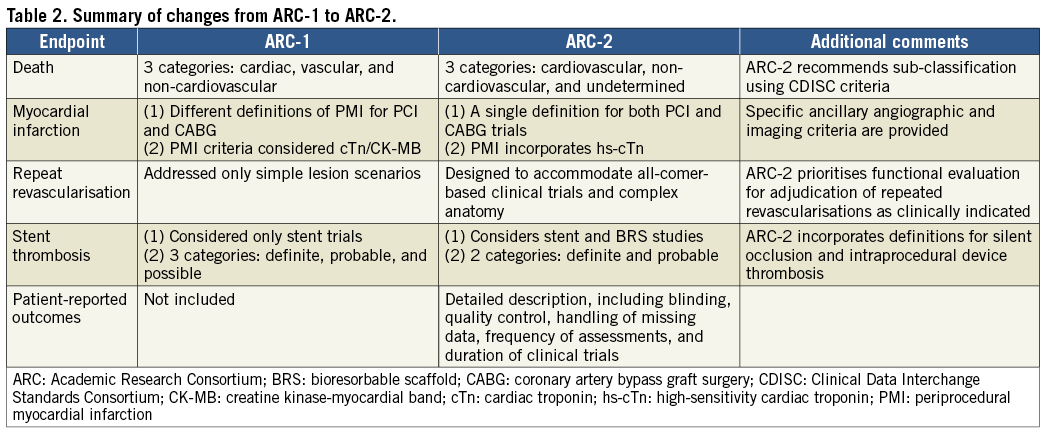
The Academic Research Consortium (ARC) initiative revised the clinical and angiographic endpoint definitions in coronary device trials proposed in 200756. The main differences between ARC-1 and ARC-2 are summarised in Table 2. The definitions of death, MI, repeat revascularisation, stent thrombosis and patient-oriented outcomes have been updated. ARC-2 definitions are suitable for use in clinical trials that include increasingly complex lesions and patient populations, and which incorporate novel devices such as bioresorbable vascular scaffolds. In addition, recommendations for the incorporation of patient-related outcomes in clinical trials are proposed.
Structural heart interventions
AORTIC INTERVENTIONS
After the publication of the SURTAVI trial in 2017 which enrolled patients with intermediate risk showing its efficacy compared to surgery, the interventional cardiology community has eagerly awaited data on lower-risk patients. This year, one publication has specifically increased expectations of the upcoming low-risk patient randomised trials on transcatheter aortic valve implantation (TAVI). This study was a sub-analysis of the SURTAVI trial, conducted by Serruys et al57. Since the inclusion criteria were adapted during the SURTAVI trial, allowing a broader inclusion with regard to the Society of Thoracic Surgeons (STS) risk score, a proportion of patients with a low STS score (<3%) could be analysed. After calibration performed with quintiles, the investigators divided the patients into three risk strata of STS PROM (<3%, ≥3 and <5%, ≥5%) to compare outcomes in these strata between TAVI and surgical aortic valve replacement (SAVR). The primary endpoint was the same as in the main study, which was a composite of all-cause death or disabling stroke. Interestingly, the lower strata of risk showed a difference in favour of TAVI for the primary endpoint (1.5% vs. 6.5%, p=0.04, respectively, for TAVI and SAVR) (Figure 5). The authors conclude that, in this lower STS risk group of patients, when compared to SAVR, TAVI could achieve a superior primary endpoint of all-cause death or disabling stroke but would require a prospective, adequately powered trial specifically using the inclusion criterion of an STS PROM score of less than 3%. Currently, there are trials in the recruitment phase that will possibly be presented in 2019 evaluating TAVI vs. SAVR in low-risk patients, namely NOTION-2 (NCT02825134), Medtronic Evolut Transcatheter Aortic Valve Replacement in Low Risk Patients (NCT02701283), and PARTNER 3 (NCT02675114).
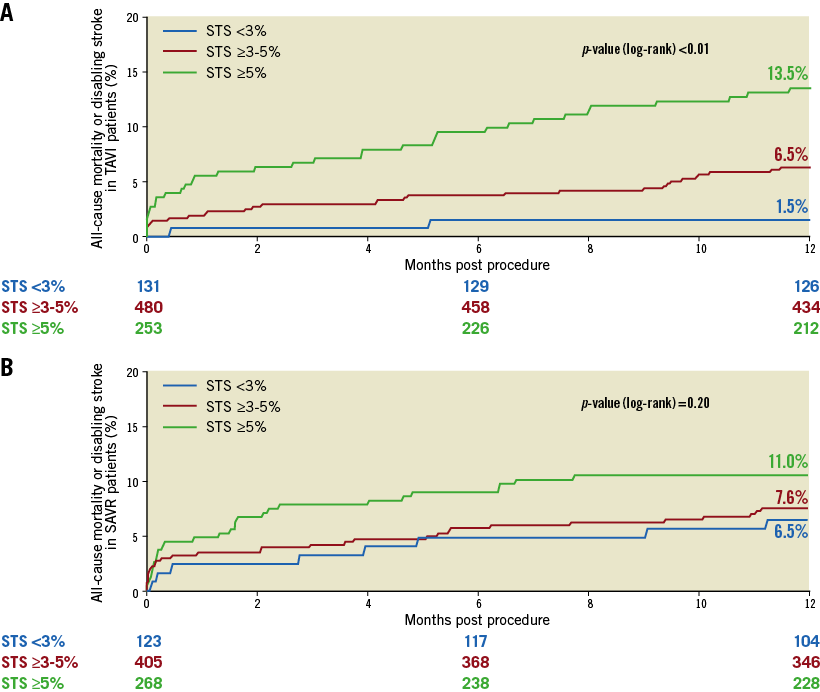
Figure 5. Primary endpoint at one year for patients randomised to TAVI or SAVR, divided among the different strata of STS PROM scores. Kaplan-Meier curves are shown for STS <3% (blue), STS ≥3% and <5% (red) and STS ≥5% (green). A) Time-to-event graph for patients undergoing TAVI. B) Time-to-event graph for patients undergoing SAVR. (Reprinted from Serruys et al57).
Setting the bar lower (low-risk patients) for the indication of TAVI has increased the concern about prosthesis durability, which was intensively discussed last year. Recently, definitions of structural valve deterioration (SVD) and bioprosthetic valve failure (BVF) in assessing long-term durability of transcatheter and surgical aortic bioprostheses have been proposed in Europe58. Holy et al have assessed the question of durability for the self-expanding CoreValve® (Medtronic) in a high-volume single centre in Germany59. With up to 8.9 years of follow-up, the investigators concluded that there was a favourable performance of the valve with regard to BVF (7.9% estimated by the Kaplan-Meier methods). Eltchaninoff et al had a larger sample size with a minimum follow-up of five years in a French institution, which included 378 patients for investigating durability of the transcatheter prostheses60. In their cohort, the incidence of SVD and BVF at eight years was 3.2% (95% CI: 1.45-6.11) and 0.58% (95% CI: 0.15-2.75), respectively. These results are encouraging and a nice teaser for the upcoming lower-risk TAVI trials.
Attempts to lower thromboembolic events after TAVI have been made. Overtchouk et al provided us with data from 12,804 patients in the FRANCE-TAVI registry, showing that, among others, anticoagulation use on discharge was associated with lower bioprosthesis valve dysfunction (odds ratio [OR] 0.54 [0.35-0.82], p=0.005)61. Since this was a registry (with its inherent limitations for proper conclusions), the results of the randomised GALILEO (Global Study Comparing a rivAroxaban-based Antithrombotic Strategy to an antipLatelet-based Strategy After Transcatheter aortIc vaLve rEplacement to Optimize Clinical Outcomes – NCT02556203) trial – that tested the superiority of rivaroxaban compared with antiplatelet-based strategy for reducing death or first thromboembolic event after TAVI – were expected. However, the data safety monitoring board recommended stopping the trial in August of 2018 after a preliminary analysis suggested higher rates of death or a first thromboembolic event, all-cause death, and primary bleeding in the rivaroxaban group versus the antiplatelet group. On this topic of anticoagulation following TAVI, cardiologists and interventionists are now eagerly awaiting the results of the ATLANTIS trial (Anti-Thrombotic Strategy After Trans-Aortic Valve Implantation for Aortic Stenosis – NCT02664649), which is testing the superiority of apixaban (Ant-Xa anticoagulant) compared with the current standard of care following TAVI in 1,510 randomised patients. Primary completion of the trial is estimated for May 2020.
MITRAL VALVE INTERVENTIONS
Mitral regurgitation (MR) is a common condition among the elderly, affecting 10% of the population over 75 years of age. It worsens heart failure and pulmonary hypertension and impacts on mortality. Dziadzko et al recently showed that, even with normal LV ejection fraction and low comorbidity, MR is associated with excess mortality and heart failure post diagnosis. Notwithstanding these poor outcomes, only a minority of affected patients undergo mitral surgery62. Treatment of MR with a less invasive approach has grown over the last few years, and it is fulfilling this unmet clinical need in untreated MR patients.
This field of interventional cardiology is extremely active and novel devices are being developed to address MR in many different ways: leaflet repair, annuloplasty, remodelling of the left ventricle and transcatheter mitral valve replacement (TMVR) (Figure 6). Recently, Bapat et al have shown the feasibility of TMVR with the Intrepid™ TMVR System (Medtronic) in their early experience with 50 patients with severe MR63. The available devices are shown in brief in Figure 7. For patients with severe mitral annular calcification and at extreme surgical risk, recent data on 116 patients with more than one year of follow-up showed that TMVR with a transcatheter balloon-expandable aortic valve is feasible, but associated with high 30-day (25%) and one-year (23.7%) mortality64. Since the purpose of the present review is to highlight the most important studies of 2018, we will focus in the next section on the most discussed edge-to-edge mitral valve repair.
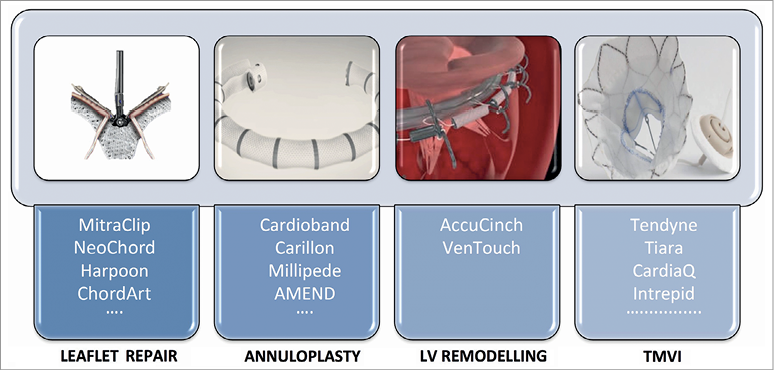
Figure 6. Different approaches for mitral valve interventions. Summary of different approaches for mitral interventions. (Reprinted from Taramasso et al70 with permission).
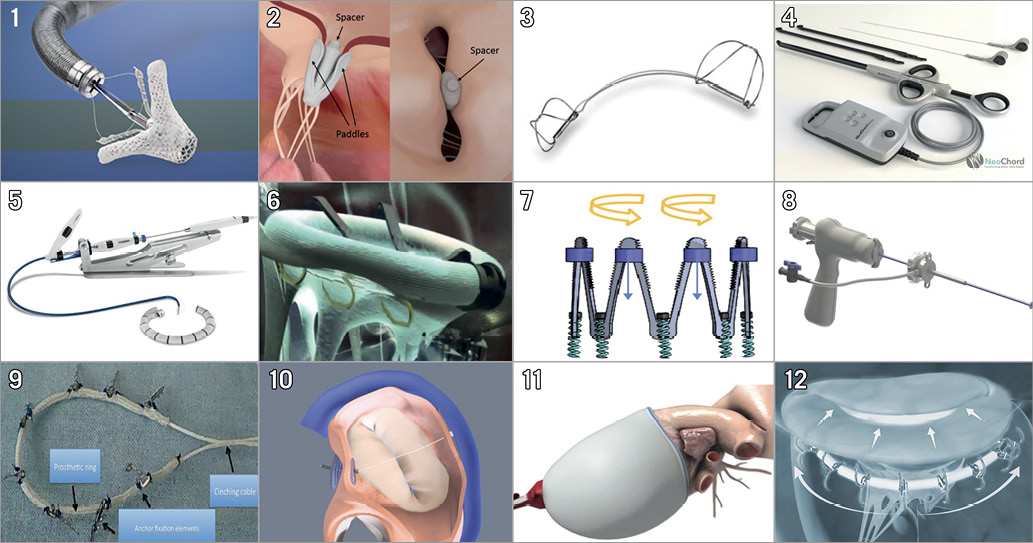
Figure 7. Transcatheter mitral valve repair devices. 1) MitraClip. 2) PASCAL (Edwards Lifesciences, Irvine, CA, USA). 3) Carillon (Cardiac Dimensions, Inc., Kirkland, WA, USA). 4) NeoChord (NeoChord, Inc., St. Louis Park, MN, USA). 5) Cardioband (Edwards Lifesciences). 6) Amend™ (Valcare Medical, Herzlyia Pituach, Israel). 7) Millipede (Millipede, Inc., Santa Rosa, CA, USA). 8) Harpoon (Edwards Lifesciences). 9) Carillon® Mitral Contour System (Cardiac Dimensions, Inc.). 10) ARTO (MVRx, Inc., San Mateo, CA, USA). 11) VenTouch™ (Mardil Medical, Inc., Champlin, MN, USA). 12) AccuCinch (Ancora Heart, Santa Clara, CA, USA). Devices of different approaches for mitral interventions. (Reprinted from Taramasso et al70 with permission).
EDGE-TO-EDGE MITRAL VALVE REPAIR: THE MITRAL PARADOX?
As in 2017, when the hot topic in interventional cardiology came from the ORBITA trial presented at TCT, the late-breaking presentation at TCT 2018 provided the key topic of this year – the edge-to-edge repair of MR with the MitraClip device (Abbott).
Before the presentation of the COAPT trial at TCT, the cardiology community became aware of the results of the MITRA-FR trial. Released in August, during the ESC Congress in Munich, the MITRA-FR trial randomised 304 patients with chronic symptomatic heart failure with reduced ejection fraction who had secondary MR to undergo transcatheter mitral repair with guideline-directed medical therapy (GDMT) or optimal medical therapy alone65. The primary endpoint was a composite of all-cause death and unplanned hospitalisation for heart failure. At 12 months, the primary endpoint was comparable between the two groups – 54.6% in the mitral repair group and 51.3% in the medical therapy alone group (p=0.53). These results came as a great disappointment to the cardiology community, making the expectations for the upcoming presentation of the COAPT trial even higher.
The primary endpoint results of the COAPT trial were received with a round of applause at TCT. This trial randomised 614 patients with symptomatic heart failure due to ischaemic or non-ischaemic cardiomyopathy with reduced ejection fraction (20 to 50%) and MR of grade 3+ or 4+66. The comparison was made between GDMT plus treatment with the MitraClip device vs. GDMT alone. In contrast to MITRA-FR, the results showed significant improvement in the primary outcome in the intervention group – represented by hospitalisations due to heart failure within 24 months of follow-up. Hospitalisations occurred in 35.8% per patient-year in the intervention group as compared with 67.9% per patient-year in the control group (HR 0.53, 95% CI: 0.40-0.70; p<0.001). As a secondary endpoint, the rate of death was also lower with the use of the MitraClip (29.1% vs. 46.1% [HR 0.62, 95% CI: 0.46-0.82]; p<0.001). A comparison of the key points of both trials is shown in Table 3. How could these somewhat paradoxical results be explained to the interventional cardiology community who are keen to implant these devices?
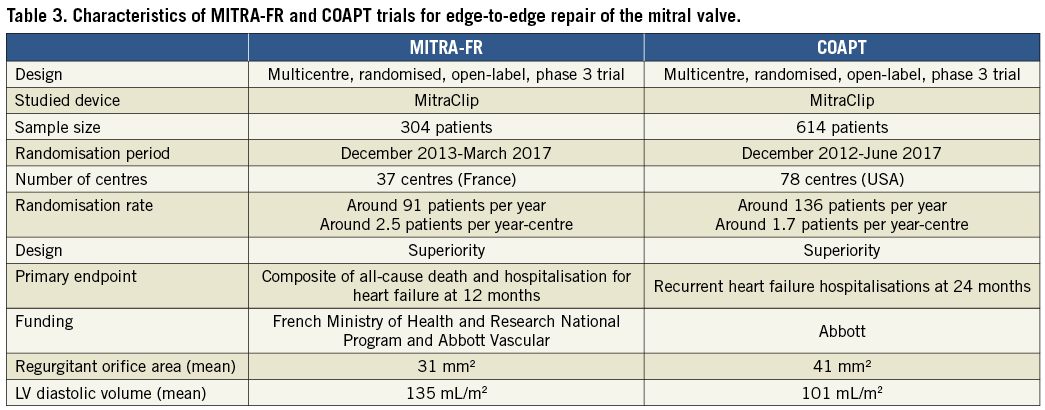
Some points must be mentioned regarding both trials. First, the population in both trials does not appear to be the same. While in COAPT the problem seemed to be in the mitral valve (greater regurgitation), in the French study the LV was more “problematic” with a much more dilated ventricle (Table 3). The randomisation rate in COAPT was much lower than in MITRA-FR (less than one device implanted per month in the highest recruiting centre), showing a possible better evaluation and selection of patients in the first. This might be considered a good message, since the liberal use of any novel device can be critical in the long-term adoption of the new technology. What at first glance could probably be called a mitral paradox can be summarised as complementary results, which, taken together, point to a precise evaluation and selection of patients for achieving very satisfactory results. A trial on this matter called RESHAPE-HF2 (A RandomizEd Study of tHe MitrACliP DEvice in Heart Failure Patients With Clinically Significant Functional Mitral Regurgitation – NCT02444338) has an estimated primary completion date of September 2019. This is being called by some the “tie-breaker” of COAPT and MITRA-FR – but most likely will be another piece of the “mitra-puzzle”. Although information on this next randomised trial is awaited, observational data published so far this year have shown results that corroborate the COAPT findings – MitraClip use in 616 patients with secondary mitral regurgitation was associated with acceptable safety, reduction of regurgitation severity, symptom improvement, and positive ventricular remodelling67.
RENAL DENERVATION
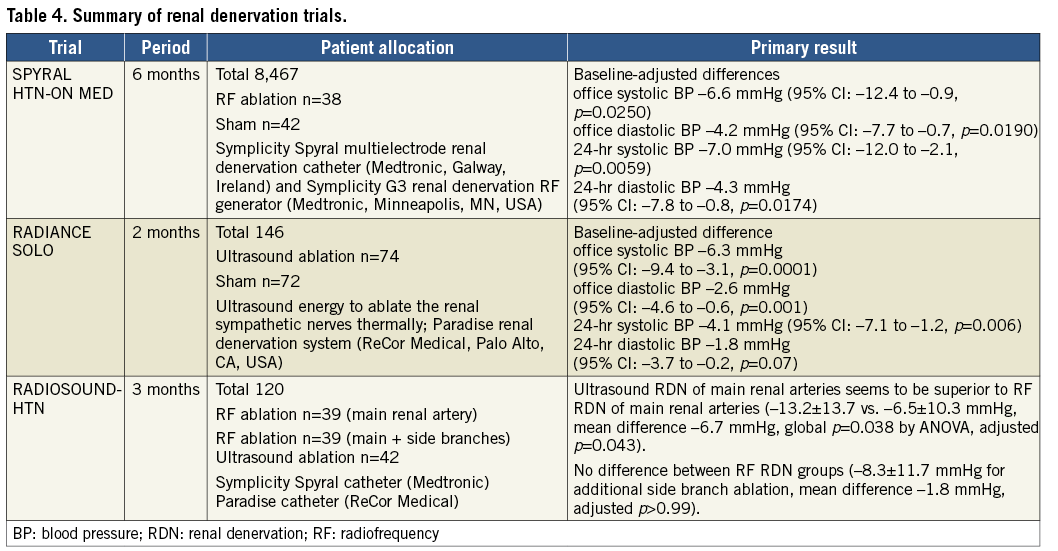
The device-based hypertension treatment returned to clinical practice. Two sham-controlled studies using radio frequency or ultrasound denervation have shown the efficacy of new device-based antihypertensive therapy. In the SPYRAL HTN-ON MED study68, in which a total of 467 eligible patients receiving one, two, or three antihypertensive drugs were randomly assigned to renal denervation and sham control, the results of the first 80 patients (denervation=38, sham=42) were reported. Renal denervation was performed with the Symplicity Spyral™ multielectrode renal denervation catheter (Medtronic, Galway, Ireland) and the Symplicity G3 renal denervation RF generator (Medtronic, Minneapolis, MN, USA) to provide circumferential radiofrequency ablation treatments in a spiral pattern in the four quadrants of the renal artery and branch vessels between three and eight mm in diameter. In the RADIANCE-HTN SOLO study69, the alternative technology of renal denervation using the endovascular ultrasound renal denervation Paradise® System (ReCor Medical, Palo Alto, CA, USA) was used. A total of 146 patients were randomised to undergo renal denervation (n=74) or a sham procedure (n=72). The main results of these studies are summarised in Table 4. When comparing these devices, in the RADIOSOUND-HTN trial, 120 patients were randomised to radiofrequency renal denervation of the main renal arteries, radiofrequency renal denervation of the main renal arteries, side branches and accessories, or an endovascular ultrasound-based renal denervation of the main renal artery. At three months, ultrasound ablation of the main renal arteries seemed to be superior to multipolar radiofrequency ablation of the main renal arteries, but there was no significant difference between the radiofrequency ablation groups. Although renal denervation returned to the clinical stage to show its efficacy, relatively small numbers of patients in these trials could lack statistical power. In addition, long-term clinical follow-up is necessary to confirm the continuity of therapy, and to evaluate whether there is a clinical benefit in lowering blood pressure to prevent cardiovascular events in this population.
Conclusion
2018 marks 41 years of PCI in interventional cardiology. The fierce competition of metallic stents seems to have come to an end; however, clarification of the safety and efficacy under the new DAPT regimen, reduction or cessation of DAPT will continue. Aortic valve intervention brought patients to a satisfactory level with safety and efficacy. The new evidence concerning mitral valve intervention has shown potential efficacy for heart failure patients. Device treatment of hypertension has returned to regain its clinical territory. Cardiologists must continue to adjust to the fast pace of interventional cardiology.
Guest Editor
This paper was guest edited by Alec Vahanian, MD, PhD; Department of Cardiology, Hôpital Bichat-Claude Bernard, and University Paris VII, Paris, France.
Conflict of interest statement
P.W. Serruys reports personal fees from Abbott Laboratories, AstraZeneca, Biotronik, Cardialysis, GLG Research, Medtronic, Sino Medical Sciences Technology, Société Europa Digital & Publishing, Stentys France, Svelte Medical Systems, Philips/Volcano, St. Jude Medical, Qualimed, and Xeltis. The other authors have no conflicts of interest to declare. The Guest Editor is a consultant for Edwards Lifesciences.

