
Abstract
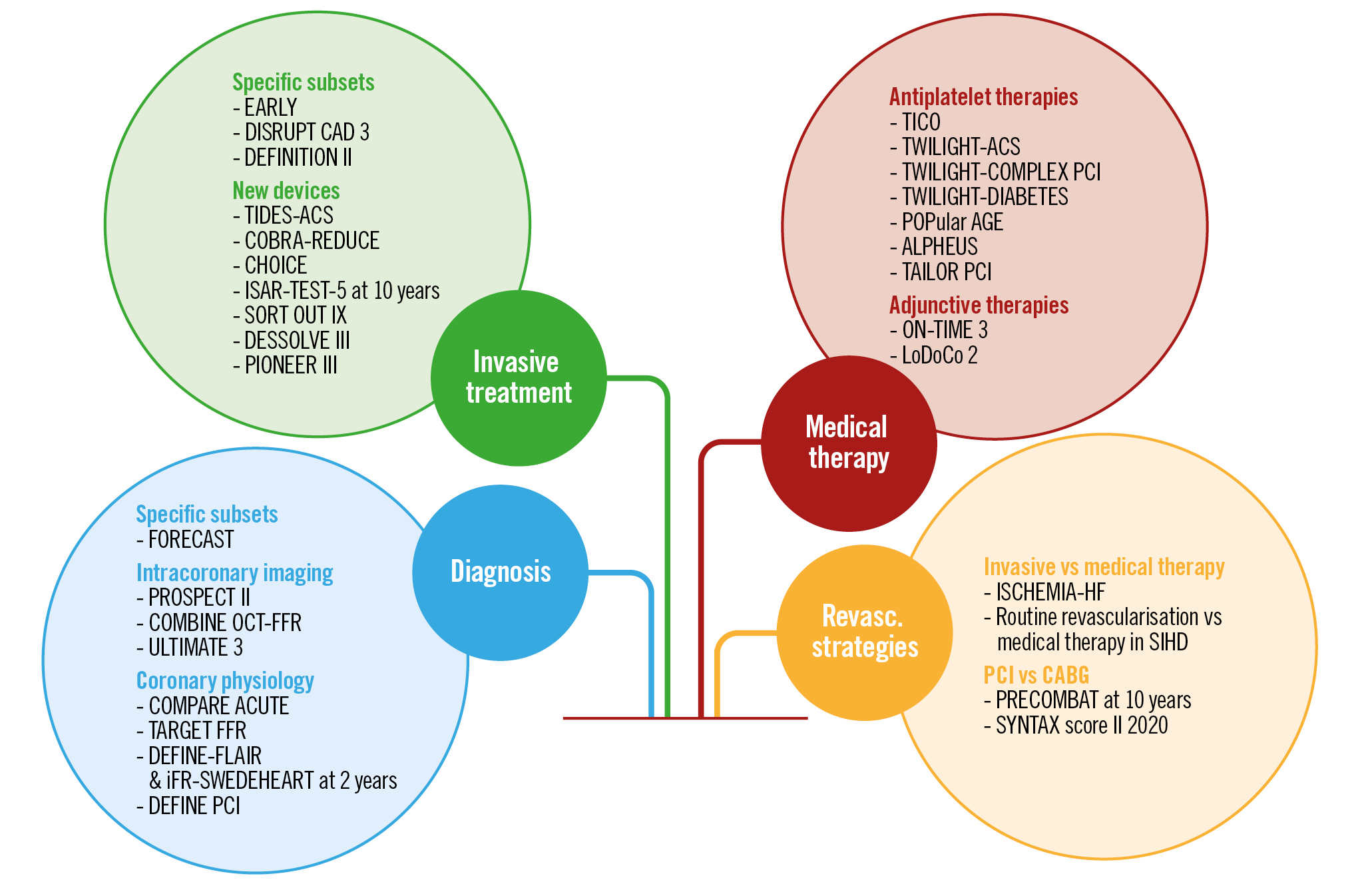
Visual summary. Coronary interventional trials in 2020.
Introduction
The coronavirus disease 2019 (COVID-19) pandemic represented – and is still representing – a challenge for clinical research which has never been seen before1. In a context where every healthcare professional is putting every effort into overcoming this catastrophe, the commitment to advance scientific progress has not disappeared among the interventional cardiology community. Although transformed into digital formats, meetings did preserve the opportunity to present the latest trial results. This review highlights the most relevant randomised trials and original research studies related to coronary interventional practice over the last year. Presentations from the PCR e-Course, the European Society of Cardiology (ESC), Transcatheter Cardiovascular Therapeutics, American Heart Association (AHA), and American College of Cardiology (ACC) meetings, as well as publications in the highest impact journals, including the New England Journal of Medicine, The Lancet, Journal of the American College of Cardiology, Journal of American Medical Association Cardiology, Circulation, European Heart Journal and EuroIntervention, were considered for the present analysis (Supplementary Table 1).
Diagnosis enlightened by intracoronary imaging and functional assessment contributes to refining treatment strategies. There is growing interest in post-percutaneous coronary intervention (PCI) functional assessment for the documentation of procedural success and prediction of outcomes. Research on new stent iterations is as creative as ever. New adjunctive pharmacotherapy studies tested different permutations between P2Y12 inhibitors, including aspirin-free monotherapy. The debate on the relative merits of medical therapy, PCI or coronary artery bypass grafing (CABG) continues but usefully focuses on the identification of the best treatment option for each individual patient. This review is an attempt to summarise all of these data, as they impact on today’s interventional practice.
Advances in the diagnosis of coronary artery disease (CAD)
NON-INVASIVE DIAGNOSIS
Although fractional flow reserve-computed tomography (FFR-CT) is still in the early stages of clinical adoption, it is already transforming clinical practice as a non-invasive alternative to coronary angiography for the diagnostic work-up of patients with chest pain. The technology provides both anatomical and functional assessment of the coronary circulation in one go, a task no other method has accomplished to date.
Until now, CAD was diagnosed by different methods, invasive or non-invasive, presenting a wide range of sensitivity, specificity and accuracy metrics. Regardless of the initial method used for gatekeeping, the majority of patients eventually ended up undergoing an invasive angiogram in the catheterisation laboratory. Of note, up to 60% of these patients had normal angiograms or non-significant stenosis not requiring revascularisation2.
This background led to the design of studies addressing the role of FFR-CT to rule out significant CAD safely in patients with new-onset chest pain and low to intermediate disease likelihood3.
The latter represented the goal of the FORECAST trial, which randomised 1,400 patients with stable angina (1:1 ratio) to undergo usual care or FFR-CT as first line. The use of FFR-CT decreased the need for invasive angiography by 22% but did not reduce costs (£1,491.46 with usual care vs £1,605.50 with FFR-CT; p=0.96). Rates of major adverse cardiac events (MACE) were similar between groups at 10.6% versus 10.1% (p>0.05)4. Although the trial failed on the economic front, the avoidance of invasive angiography for 1 out of 5 patients deserves to be highlighted.
The benefits deriving from a non-invasive diagnostic approach are demonstrated in the recent validation of a new tool, developed to estimate the likelihood of obstructive CAD. Winther et al combined the existing pre-test probability model (Diamond-Forrester approach) with clinical risk factors and coronary artery calcium score, in a cohort of 41,177 symptomatic patients5. The resulting new model was found to predict more accurately the risk of obstructive CAD than the previous one (area under the receiver operating characteristic curve 0.85 and 0.72, respectively). As a result, 54% of the patients, versus 11% with the old model, were classified to have a very low likelihood (less than 5%) of CAD requiring intervention5. When available, routine implementation of coronary CT for CAD diagnosis and management has the potential to prevent unnecessary invasive coronary angiography by more accurate risk stratification.
INVASIVE IMAGING AND INTRACORONARY PLAQUE COMPOSITION ASSESSMENT
If ever proven possible, the identification of “vulnerable” plaques before disruption leading to atherothrombosis and acute coronary syndrome (ACS) will allow preventive pharmacological or interventional strategies to stabilise them and slow their progression. In the PROSPECT I study (Providing Regional Observations to Study Predictors of Events in the Coronary Tree), the mean diameter stenosis (DS) of non-culprit plaques causing subsequent adverse coronary events at three years was 32%; almost all were initially defined as angiographically non-critical (DS <70%)6. Lipid-rich plaques detected by near-infrared spectroscopy (NIRS) were shown to be associated with adverse events7,8. Recently, the long-term outcomes of the PROSPECT II registry were presented9. The study investigated the relationship between high-risk characteristics of untreated non-culprit lesions and the occurrence of MACE (cardiac death, myocardial infarction [MI], unstable angina or progressive angina requiring revascularisation or with rapid progression) among patients presenting with MI. A total of 902 patients (3,629 non-culprit lesions) were assessed with intravascular ultrasound (IVUS)-NIRS. One or more lesions presented a high lipid content (>32.5%), high plaque burden (>70%) or a minimum lumen area (MLA) <4.0 mm2 in 58.8%, 59% and 75.6% of patients, respectively. At a median follow-up of 3.7 years, most MACE were related to the non-culprit lesions (78 out of 114). Among these, the mean DS by quantitative coronary angiography (QCA) was 46.9% at baseline and 68.4% at the time of the event. The baseline NIRS-IVUS showed a mean plaque burden of 56.2% and a median lipid content of 47.4%. The trial led to the conclusion that NIRS identified the lipid-rich plaques responsible for the majority of future coronary events. In order to prevent later plaque events, aggressive secondary prevention remains the default therapy. Whether interventional approaches, i.e., PCI with a bioresorbable vascular scaffold (BVS)10 or other devices, will be effective depends on high procedural safety and low complications such as restenosis and stent thrombosis.
The COMBINE trial (Optical Coherence Tomography Morphologic and Fractional Flow Reserve Assessment in Diabetes Mellitus Patients) evaluated 500 diabetic patients undergoing PCI for a chronic coronary syndrome (CCS) or ACS, to investigate the relationship between high-risk plaque characteristics and adverse events11. FFR was measured in the presence of ≥1 non-culprit lesion with DS 40-80%. In cases where the FFR was preserved (>0.80), medical treatment was optimised and patients divided in two groups according to the absence (group A, n=292) or presence (group B, n=98) of a thin-cap fibroatheroma (Figure 1). Patients with abnormal FFR were revascularised (group C, n=93). The primary endpoint was the composite of cardiac death, target lesion MI, clinically driven target lesion revascularisation (TLR) or hospitalisation due to ACS at 1.5 years. Optical coherence tomography (OCT) found high-risk plaque characteristics in up to 25% of lesions with preserved FFR and, most importantly, the presence of a thin-cap fibroatheroma was an independent predictor of future adverse events, despite a lack of ischaemia (primary endpoint was 13.3% in group B and 3.1% in group A, hazard ratio [HR] 4.7, 95% confidence interval [CI]: 2.0-10.9, p=0.0004). Group B presented higher rates of target lesion MI and clinically driven TLR. On top of these insights on CAD progression in diabetic patients, this study corroborates a thought-provoking, previously proposed hypothesis: ischaemia and future plaque events may proceed from different underlying backgrounds and mechanisms3,12.
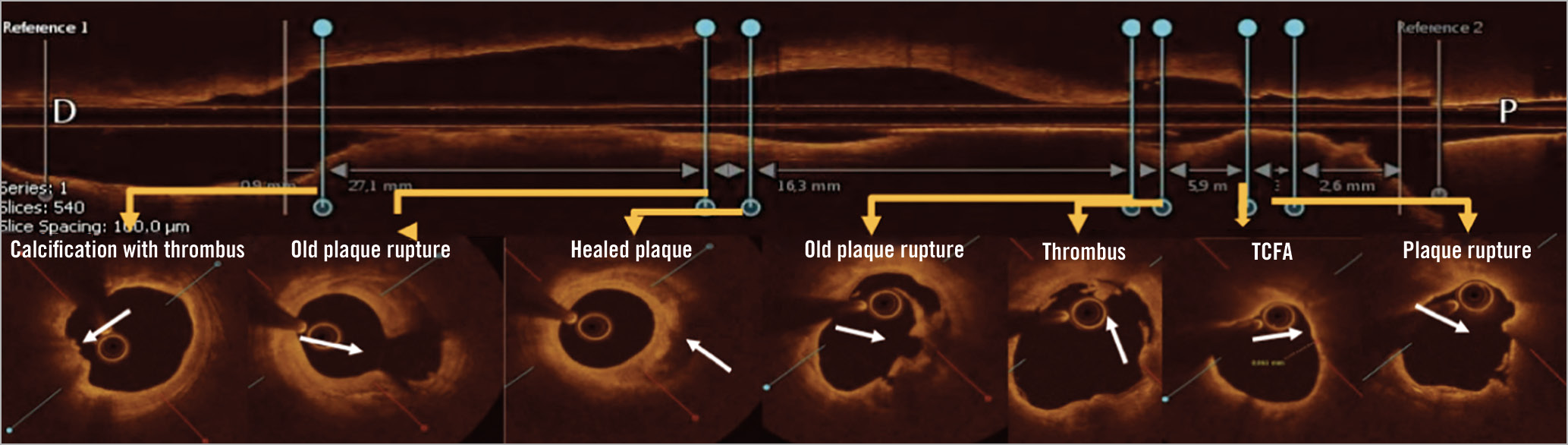
Figure 1. Coexistence of complicated plaques in the same lesion in a diabetic patient from the COMBINE OCT-FFR trial. OCT longitudinal assessment demonstrates coexistence of multiple plaque morphologies: superficial calcification with thrombus, healed plaque, plaque rupture, lipidic plaque with TCFA. Reproduced from a presentation by Kedhi, at TCT 2020 conference11. OCT: optical coherence tomography; TFCA: thin-cap fibroatheroma
The multicentre randomised ULTIMATE trial (Intravascular Ultrasound Guided Drug Eluting Stents Implantation in “All-Comers” Coronary Lesions) examined in 1,448 patients whether IVUS-guided PCI was superior to angiography-guided PCI at preventing target vessel failure (TVF), defined as the composite of cardiac death, MI, or target vessel revascularisation (TVR)13. At one year, the trial showed lower TVF after IVUS-guided drug-eluting stent (DES) implantation compared with angiographic guidance (2.9% vs 5.4%, p=0.019)14. This result improved over time, with three-year TVF rates of 6.6% in patients randomised to IVUS guidance, versus 10.7% with angiographic guidance (HR 0.60, 95% CI: 0.42-0.87; p=0.01). The benefit was mainly driven by lower rates of TVR. Optimal criteria for IVUS guidance, resulting in even better outcomes, were minimal cross-sectional area >5.0 mm² (or 90% of distal reference lumen cross-sectional area), plaque burden at proximal and distal stent edges <50%, and no edge dissection involving media with length >3 mm.
The ULTIMATE trial confirms that the benefit of IVUS guidance extends beyond one year after PCI, as already shown by the IVUS-XPL study up to five years15. The level of evidence supporting the use of intracoronary imaging guidance for optimised PCI outcomes is mounting and should be contrasted with the persistent low clinical adoption. Indeed, a recent Medicare analysis16 showed that IVUS was used in only 5.6% of all PCIs performed between 2009 and 2017.
The strengths of intracoronary imaging and its positive impact on PCI results have led to the development of catheter systems combining IVUS and OCT technologies. The Novasight Hybrid™ System (Conavi Medical Inc., Toronto, Canada) recently received Health Canada approval based on a positive feasibility and efficacy study in 20 patients with either CCS or ACS (NCT03484975). Similarly, the Terumo company (Tokyo, Japan) has developed a Dual Sensor hybrid IVUS-OCT catheter system, which was tested in post-mortem coronary arteries, with comparable outcomes to the standard stand-alone IVUS and OCT systems17. It may be anticipated that the availability of both intracoronary imaging technologies on a single catheter will increase clinical adoption of imaging guidance, especially for complex PCI.
CORONARY PHYSIOLOGY
Recent ESC guidelines for treatment of ACS support complete revascularisation in ST-segment elevation myocardial infarction (STEMI) patients with multivessel disease18. One of the pivotal supporting studies was Compare-Acute, published in 2017, showing superiority of FFR-guided complete revascularisation versus culprit-only intervention19. Findings were confirmed by three-year primary outcomes of death, MI, revascularisation or stroke rates at 15.6% (46/295 patients) with FFR-guided complete revascularisation versus 30.2% (178/590) with culprit-only PCI, a significant 54% event reduction (HR 0.46, 95% CI: 0.33-0.64; p<0.001). Similar to the one-year results, a significant reduction in the need for revascularisation (12.5% vs 25.2%; HR 0.45, 95% CI: 0.31-0.64; p<0.001) was driving the composite endpoint20. Of note, health economics are also in favour of FFR guidance with a 21% reduction in costs per patient at one year, maintained at 22% at three years (8,653 € vs 11,100 €). Now, all studies investigating the revascularisation strategy in MI patients with multivessel CAD are pointing in the same direction, in support of complete revascularisation preferably informed by physiological guidance in order to identify which bystander lesions require PCI. The best timing to revascularise the non-culprit lesions (either during the initial or as a staged procedure) needs to be further clarified and, for the time being, the decision should balance risk versus convenience.
Given all the positive data to hand, the adoption of physiological guidance is gradually increasing, also through the availability of non-hyperaemic indices, especially the instantaneous wave-free ratio (iFR). Pooled analysis of the two-year results of DEFINE FLAIR and iFR-SWEDEHEART comparing FFR with iFR in 4,486 patients has confirmed non-inferiority, with virtually identical MACE rates at two years (7.40% vs 7.43%, respectively). Overall, FFR guidance identified lesion significance and determined PCI in 5% more cases than iFR, an effect more marked among patients younger than 60, where FFR led to 12% more revascularisation procedures than iFR (54% deferral with iFR vs 42% with FFR; p<0.01). Age influenced two-year MACE only in patients deferred on an FFR basis (FFR-deferred HR 1.95, 95% CI: 1.03-3.70; FFR-treated HR 0.96, 95% CI: 0.67-1.37; p for interaction=0.06). This interaction may be explained by a varying age-related hyperaemic response to adenosine21. Results of both trials at five years are eagerly awaited in order to confirm the long-term safety of PCI deferral with either technique.
During recent years, attention has shifted to the usefulness of post-PCI physiological assessment in order to evaluate the immediate functional procedural success, in addition to the anatomic success, as estimated by angiography or intracoronary imaging. It is known that many vessels with an angiographically successful appearance may not reach post-PCI FFR values above 0.88; this finding has been associated with worse outcomes in many studies22.
In order to test whether low post-PCI FFR values can be acutely improved, 260 patients were randomised in the TARGET FFR trial to a physiology-guided post-PCI optimisation strategy versus conventional care based on angiography23. The former group underwent FFR assessment after PCI to rule out suboptimal results and optimise the revascularisation accordingly. The PCI results of the latter group were assessed by angiography only. The primary outcome of this small study failed to show a significant improvement in the proportion of patients with an optimal result (defined as FFR ≥0.9) after FFR-guided PCI optimisation: 38.1% in the physiology-guided group compared with 28.1% in the control group (p=0.09). However, 18.6% of vessels had FFR ≤0.8 in the physiology-guided arm versus 29.8% in the control arm (p=0.04).
In the DEFINE-PCI study, iFR co-registration was used in 467 patients for procedural guidance and for assessment of residual ischaemia after angiographically successful PCI, revealing residual haemodynamically significant stenosis in 24% of patients24. At one-year follow-up, a post-PCI iFR value ≥0.95 was associated with improved event-free survival and lower rates of recurrent chest pain. Other adverse events (cardiac death, spontaneous MI, or clinically driven TVR) occurred in 1.8% of patients with final iFR ≥0.95 compared to 5.7% of patients with lower post-PCI iFR (HR 3.38, 95% CI: 0.99-11.6; p=0.04).
At present, the sum of the available data confirms the strong association between suboptimal post-PCI physiology and worse outcomes, whether the obstacle to flow is located within and/or outside the stented segments.
In addition, recent advances in computational techniques for image-based calculation of flow reserve may prove very useful for immediate post-PCI functional assessment. Further studies are ongoing to establish the role of physiological indices derived from angiography or OCT25,26 in this context.
Interventional treatment for CAD
NEW DEVICES FOR INTERVENTIONAL PROCEDURES
The race for the development of more effective coronary devices, stents in particular, seems to be never-ending. One strategy continues to focus on material modification for better biocompatibility, and possibly restenosis prevention in the absence of antiproliferative drug release. The TIDES-ACS trial (Comparison of Titanium-Nitride-Oxide-Coated Bioactive Stent to the Drug [Everolimus]-Eluting Stent in Acute Coronary Syndrome) investigated the potential superiority of a bare metal stent (BMS) coated with titanium-nitride-oxide (TiNO) (Optimax; Hexacath, Paris, France) stent over the platinum-chromium-based biodegradable polymer everolimus-eluting stent (EES) (SYNERGY™; Boston Scientific, Marlborough, MA, USA)27 in 827 patients with ACS. The 18-month rate of cardiac death, MI, or major bleeding was 3.7% in the TiNO group versus 7.8% in the EES group (HR 0.64, 95% CI: 0.51 to 0.80; p=0.001), meeting the superiority hypothesis. The benefit was derived from a significant reduction in cardiac deaths (0.6% vs 2.6%; p=0.002) and MI (2.2% vs 5.0%; p=0.007). Compared to other TiNO devices, Optimax is made out of a specific cobalt-chrome alloy that preserves radial strength in spite of ultra-thin strut thickness (75 µm). Together with reduced thrombogenicity, both mechanisms may contribute to improved outcomes in ACS patients.
The COBRA-REDUCE trial28 in 996 patients requiring anticoagulation aimed to compare the standard DES with the thromboresistant Polyzene-F-coated BMS, in order to boost vessel healing and shorten the duration of DAPT. In the experimental arm, subjects received only DAPT for the first 14 days. After 14 days, they received oral anticoagulation plus aspirin. Conversely, patients treated with DES received only dual antiplatelet therapy (DAPT) for 3-6 months. After six months, subjects received oral anticoagulation plus aspirin. The trial failed to demonstrate the non-inferiority of the Polyzene-F-coated BMS over the standard DES. The primary endpoint of death, MI, stroke, or stent thrombosis at six months occurred in 7.7% of the experimental group compared to 5.2% with DES (pnon-inferiority=0.061). The co-primary endpoint of major bleeding (Bleeding Academic Research Consortium [BARC] 2-5) at 14 days was 7.5% in the experimental arm, compared to 8.9% in the DES group (p=0.48).
In an attempt to reduce potential (very) late inflammatory reactions to durable polymers, stents with bioerodible polymer or polymer-free continue to be evaluated. The CHOICE trial (Comparing Three 2nd Generation Drug-Eluting Stents in Real-World Practice) compared the 24-month safety and efficacy of the bioerodible polymer BioMatrix™ (Biosensors, Singapore) biolimus-eluting stent (BES) to the durable polymer EES and the zotarolimus-eluting stent (ZES) in 1,935 all-comer patients29. The study demonstrated the non-inferiority of BES compared to the other two stents, with a composite of cardiac death, target vessel MI, and clinically indicated TLR of 2.2% in the BES group, 3.6% in the EES group and 3.9% in the ZES group (pnon-inferiority<0.001). Landmark analysis beyond one year after the index PCI did not show benefit of the bioerodible polymer BES over the durable polymer EES or the ZES.
Another interesting technology was tested in the PIONEER III randomised trial enrolling 1,632 patients (2:1 experimental vs control) to test the efficacy and safety of a new-generation DES against the market-leading DES (XIENCE [Abbott Vascular, Santa Clara, CA, USA] or PROMUS™ [Boston Scientific]). The investigated device was the Supreme HT™ DES (HT-DES) (Sinomed, Tianjin, China), which promotes early restoration of endothelial function. The primary endpoint was a composite of cardiac death, target vessel MI and ischaemia-driven TLR at one year, which occurred in 5.4% of patients in the HT-DES group versus 5.1% in the control group (pnon-inferiority=0.002). Longer follow-up will be required to unravel potential benefit from endothelial preservation30.
Very long-term data at 10-year follow-up from the ISAR-TEST-5 trial31 (testing the efficacy of sirolimus- and probucol-eluting vs zotarolimus-eluting stents) also showed no difference in the device-oriented and patient-oriented composite outcomes between the polymer-free sirolimus- and probucol-eluting stents versus the durable polymer zotarolimus-eluting stents.
The SORT OUT IX trial32 aimed to demonstrate the non-inferiority of the polymer-free biolimus-eluting BioFreedom™ stent (Biosensors) compared to the thin-strut bioresorbable polymer sirolimus-eluting Orsiro stent (Biotronik, Bülach, Switzerland). In earlier studies, the BioFreedrom stent showed promising results in terms of efficacy and safety with short one-month DAPT, but in comparison with BMS33. On the other hand, encouraging outcomes were reported with the Orsiro stent, but against durable polymer DES34. In SORT OUT IX, the primary endpoint of MACE at 12 months was 5% in the BioFreedom group (n=1,572 patients) versus 3.7% in the Orsiro group (n=1,579 patients), not meeting the non-inferiority criteria (HR 1.34, 95% CI: 0.96-1.89, pnon-inferiority=0.14).
Based on previous findings and the above findings, it remains unclear whether the coating characteristics (durable, bioerodible, or polymer-free) confer significant outcome benefits. Most likely, strut thickness plays a more important role than coating per se. Indeed, the three-year outcomes of the DESSOLVE III trial confirm that high biocompatibility of the best-in-class current-generation DES with durable polymer is clinically equivalent to thin-strut sirolimus-eluting DES with bioerodible polymer (MiStent®; Micell Technologies, Durham, NC, USA). When compared to XIENCE everolimus DES, the device-oriented composite endpoint at three years was 10.5% for the MiStent versus 11.5% for XIENCE (p=0.55), with similar rates of probable or definite stent thrombosis35. Similar results were observed from the three-year follow-up of the TARGET All Comers study with comparable safety and efficacy profiles of the bioerodible polymer Firehawk® (MicroPort, Shanghai, China) (TLF at 11.9%) and the durable polymer XIENCE stent (11.5%, p=0.84)36.
SPECIFIC CLINICAL AND PROCEDURAL SETTINGS
NSTEMI MANAGEMENT
The optimal management of intermediate- or high-risk NSTEMI is still a matter of debate, particularly with respect to the best timing of coronary angiography and PCI, when required37. The EARLY randomised trial (Early or Delayed Revascularization for Intermediate and High-Risk Non ST-Elevation Acute Coronary Syndromes) enrolled 709 patients randomly assigned to an early invasive treatment (coronary angiography + PCI within two hours from presentation) or a delayed invasive treatment (performed 12 to 24 hours after randomisation, but up to 72 hours during weekends)38. No antiplatelet pre-treatment was allowed in either group until the time of PCI. The primary composite of cardiovascular death and recurrent ischaemic events at one month was significantly lower in the early group (4.4% vs 21.3%; HR 0.20, 95% CI: 0.11 to 0.34; p<0.001), driven mainly by a reduction in recurrent ischaemic events (2.9% vs 19.8%; p<0.001). The landmark analysis showed that the higher rate of ischaemic events in the delayed strategy was observed during the first three days, with no difference between strategies once coronary revascularisation had been performed. However, the time range to perform the invasive procedure in the delayed group was relatively wide (60 hours) and longer than in other similar trials39, resulting in a certain heterogeneity among these patients and possible bias.
This study supports the hypothesis that delaying the invasive strategy, in the absence of antiplatelet pre-treatment, increases ischaemic event rates40,41. Of note, in both arms P2Y12 was not administered until the invasive catheterisation, so that a strategy with P2Y12 early treatment and delayed procedure was not addressed in the trial. Of note, an early invasive strategy shortens the duration of hospital stay, with obvious implications for resource utilisation.
CALCIFIED STENOSES
While coronary calcifications are increasingly common, challenging optimal stent delivery and expansion, rotational atherectomy has remained until recently the only available technology for plaque debulking and modification. New techniques include the catheter-based intravascular lithotripsy, which fractures calcium through the application of acoustic pressure waves. The 30-day safety and efficacy data from the single-arm DISRUPT-CAD study have recently been presented42. Both primary safety and efficacy endpoints compared to reference point estimates were favourable. Using intravascular imaging before and after stent deployment, a mean 102% stent expansion in the area of maximum calcification was documented.
BIFURCATION LESIONS
Publication of the DKCRUSH-V study last year, showing the superiority of a double stent technique for PCI of distal left main (LM) bifurcation stenoses, has challenged the currently recommended “by default” provisional stenting strategy43. Further clarification has been provided by the DEFINITION II study (Definitions and impact of complEx biFurcation lesIons on clinical outcomes after percutaNeous coronary IntervenTIOn using drug-eluting steNts)44. Criteria for defining complex bifurcations that may benefit from a first-intention two-stent technique were derived from the 1,550 patients included in the DEFINITION I registry45. In the recently reported 653 patients, TLF at 12 months occurred in 6.1% in the two-stent group, much less than the 11.4% observed in the provisional stent group (p=0.019), a difference driven mainly by increased rates of target vessel-related MI and TLR in the provisional arm44. Of note, a standardised double kissing crush technique was the dominant approach in the two-stent group. It is therefore conceivable that the provisional stenting strategy should be applied to simple bifurcation stenoses, not meeting the DEFINITION criteria of lesion complexity.
Advances in medical therapy
ADJUNCTIVE PHARMACOLOGY FOR PCI
Further to the positive results presented in recent years46,47,48,49, more data have been released in 2020 supporting a shortened duration of DAPT treatment as well as aspirin-free strategies following PCI.
The TICO trial (ticagrelor monotherapy after three months in the patients treated with new generation sirolimus-eluting stent for acute coronary syndrome) demonstrated that stopping aspirin after three months of DAPT and continuing ticagrelor only was superior in terms of the composite of ischaemic and bleeding events after PCI for ACS50. At 12 months, the primary outcome consisting of the net adverse clinical event rate (death, MI, stent thrombosis, stroke, TVR, or Thrombolysis In Myocardial Infarction [TIMI] major bleeding) occurred in 3.9% of the ticagrelor monotherapy group (n=1,527 patients) compared with 5.9% of the standard therapy group (n=1,529 patients) (p=0.01). The main benefit resulted from the reduction in TIMI major bleeding: 0.7% with ticagrelor monotherapy compared to 3.0% with standard therapy (p=0.02). By studying ticagrelor monotherapy, TICO is addressing an important knowledge gap since prior trials evaluating aspirin-free strategies in ACS included patients mostly receiving the less effective P2Y12 antagonist clopidogrel51,52,53.
A substudy of the TWILIGHT trial focusing on ACS patients reported comparable ischaemic outcomes between the two treatment strategies (4.4% vs 4.3%, p=0.96), while bleeding events (BARC 2, 3 and 5) were reduced up to 53% in the aspirin-free treatment arm54. As already suggested by the GLOBAL LEADERS ACS substudy55, these trials indicate that ticagrelor monotherapy, following a short DAPT period, appears to be a promising alternative to 12-month DAPT with ticagrelor and aspirin, especially for patients at increased bleeding risk (Figure 2).
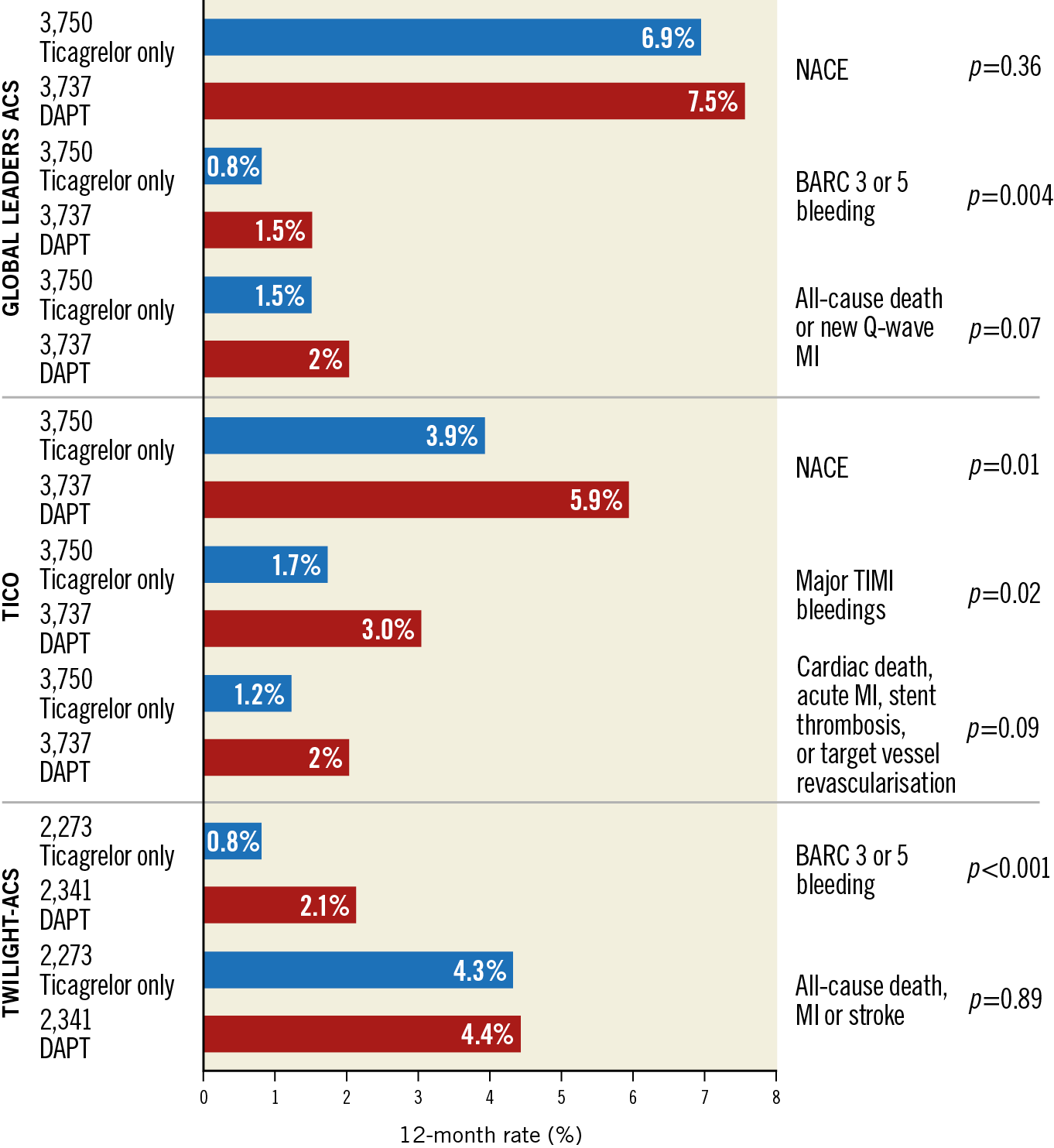
Figure 2. Ischaemic and bleeding outcomes in ACS patients undergoing ticagrelor monotherapy versus standard DAPT: results from GLOBAL-LEADERS ACS, TICO, and TWILIGHT-ACS studies. ACS: acute coronary syndrome; BARC: Bleeding Academic Research Consortium; DAPT: dual antiplatelet therapy; MI: myocardial infarction; NACE: net adverse clinical events; TIMI: Thrombolysis In Myocardial Infarction
In clinical practice, with so many available options, it becomes increasingly important to be able to evaluate ischaemic and bleeding risks in order to define a patient-tailored, most appropriate therapy. Patients undergoing complex PCI (defined as any of the following: 3 vessels treated, ≥3 lesions treated, total stent length >60 mm, bifurcation with 2 stents implanted, atherectomy device use, LM PCI, surgical bypass graft or chronic total occlusion as target lesions) seem to benefit from ticagrelor monotherapy, to the same extent as TWILIGHT patients undergoing non-complex PCI56. In patients with diabetes, data available so far show that an aspirin-free regimen reduces haemorrhagic events without increasing ischaemic events48,49,57,58.
From a pooled analysis of TWILIGHT47 and TWILIGHT-like patients in GLOBAL LEADERS59, concern was raised regarding a potential 2.2-fold increased risk of ischaemic stroke among high-risk patients treated with ticagrelor monotherapy. These data suggest that some high-risk patients will benefit from DAPT, where aspirin may still play a significant role in preventing ischaemic events by targeting a different platelet inhibition pathway than ticagrelor60.
Strategies other than aspirin-free regimens have been tested in an attempt to reduce bleeding risks. A Dutch trial randomised more than a thousand elderly patients (age >70) presenting with NSTEMI-ACS to receive either a potent P2Y12 inhibitor (ticagrelor or prasugrel) + aspirin or clopidogrel + aspirin after PCI. The primary hypothesis that clopidogrel would be superior in reducing major or minor bleeding was confirmed: a 29% lower bleeding rate in the clopidogrel arm (p=0.02) was reported. Ischaemic events did not increase, resulting in a net clinical benefit of 28%, compared to 32% in the ticagrelor/prasugrel group (p=0.03)61.
The ALPHEUS (Assessment of Loading With the P2Y12 Inhibitor Ticagrelor or Clopidogrel to Halt Ischemic Events in Patients Undergoing Elective Coronary Stenting) trial investigated the potential benefit of ticagrelor-based DAPT after elective high-risk PCI62. The trial aimed to prove the superiority of ticagrelor over clopidogrel in reducing periprocedural ischaemic complications, defined as the occurrence of PCI-related type 4 MI or major myocardial injury at 48 hours after the procedure, in 1,910 patients randomised to receive ticagrelor (n=956) or clopidogrel (n=954). The primary endpoint amounted to 334 (35%) patients in the ticagrelor group and 341 (36%) patients in the clopidogrel group (HR 0.97, 95% CI: 0.80-1.17; p=0.75). Major bleeding events (BARC type 3 or 5) were comparable between the two groups, whereas minor bleeding at 30 days was found to be more frequent in the ticagrelor group (11% vs 8%, p=0.007). Considering these results, in addition to the higher rates of dyspnoea in the ticagrelor group, and the extra cost of this molecule compared to clopidogrel, there seems to be no reason to use a more potent P2Y12 antagonist after elective PCI.
Another potential opportunity for treatment tailoring stems from genotype-guided post-procedural medical management. So far, there have been several studies describing retrospectively the association of cytochrome P450 2C19 (CYP2C19) #2 or #3 alleles (reduced ability to activate clopidogrel) with adverse clinical outcomes in clopidogrel-treated patients. Many of these have been summarised in a meta-analysis including 9,685 patients, reporting an increased risk of MACE among patients carrying one of the two allele variants (HR 1.55, 95% CI: 1.11-2.17; p=0.002)63. The weakness of these studies was that genotyping was not performed prospectively and treatment decisions were not based on genotyping results, which introduces a potential selection bias.
The TAILOR-PCI (tailored antiplatelet therapy following PCI)64 trial examined prospectively the effectiveness of preventive genetic testing to guide treatment choices (ticagrelor 90 mg twice daily for variant carriers and clopidogrel 75 mg daily for non-carriers). The study included more than five thousand patients undergoing PCI for ACS or CCS and requiring 12 months of DAPT. Among the genetic variant carriers, the primary ischaemic endpoint was 4% in the genotype-guided group versus 5.9% in the conventional group (HR 0.66, 95% CI: 0.43-1.02; p=0.056), without difference in bleeding rates. Although these data failed to demonstrate the superiority of the experimental strategy (set to a minimum threshold of 50% risk reduction), they provide a positive signal in favour of the genotype-guided therapy, with approximately one third fewer adverse events in this group. Unplanned post hoc analysis showed that 80% of the event reduction took place during the first three months of treatment. Further investigations, focusing on this time window, are required to draw definitive conclusions.
OTHER TREATMENT TARGETS
The ON-TIME 3 trial (impact of opioids on P2Y12-receptor inhibition in patients with ST-elevation myocardial infarction who are pre-treated with crushed ticagrelor: Opioids aNd crushed Ticagrelor In Myocardial infarction Evaluation) compared an opioid drug to a non-opioid analgesic drug for pain relief in STEMI patients in order to investigate P2Y12-receptor inhibition through reduced opioid-related drug absorption65. Fentanyl was compared to acetaminophen in 195 patients after crushed ticagrelor loading. The level of platelet reactivity units (PRU) measured immediately after primary PCI was not significantly different between the two groups (median PRU 104 [interquartile range: 37-215] vs 175 [interquartile range: 63-228], p=0.18). Ticagrelor blood levels were significantly higher after acetaminophen than fentanyl, maintaining the same extent of pain relief. In the absence of differences in clinical outcomes, it remains uncertain whether opioid-free strategies should be preferred for pain relief in STEMI patients.
Colchicine has recently been proven to reduce adverse cardiovascular events after MI, through its anti-inflammatory action. The LoDoCo2 trial (Low Dose Colchicine for Secondary Prevention of Cardiovascular Disease) aimed to investigate its safety and efficacy in CCS patients. A total of 5,522 patients were randomised to receive colchicine 0.5 mg/day or placebo. The primary outcome of cardiovascular death, MI, stroke, or ischaemia-driven revascularisation was reduced at 6.8% with colchicine versus 9.6% with placebo (HR 0.69, 95% CI: 0.57-0.83, p<0.001), with no difference in death or stroke66. At the same time, there was an excess in non-cardiovascular mortality with colchicine. A similar finding was reported in the Colchicine in Patients With Acute Coronary Syndrome (COPS) trial67, suggesting caution in the implementation of this treatment going forward.
Revascularisation strategies
GUIDELINE-DIRECTED MEDICAL THERAPY VERSUS REVASCULARISATION
Last year, the ISCHEMIA trial68 showed that initial invasive treatment did not lead to a reduction in MACE (cardiovascular death, MI, resuscitated cardiac arrest, or hospitalisation for unstable angina or heart failure [HF]) compared with guideline-directed medical therapy among stable patients with moderate ischaemia. An initial invasive strategy was associated with improved symptoms for those patients experiencing angina on a daily/weekly/monthly basis, while no benefit in the quality of life was observed among those without angina.
All patients underwent coronary CT angiography prior to inclusion and randomisation, with exclusion of patients with LM stenosis, severely depressed ejection fraction (EF), or NYHA III-IV HF68.
This year, the ISCHEMIA group presented the results of a pre-specified sub-analysis on 398 patients with a history of HF or depressed EF at baseline (35-45%)69. This subgroup reported a higher incidence of the four-year primary outcome (22.7% vs 13.8%), cardiovascular death or MI (19.7% vs 12.3%), and all-cause death or HF (15.0% vs 6.9%), compared to those not presenting HF or depressed EF (p<0.0001 for all). As shown in Figure 3, this subgroup of high-risk patients benefited from an invasive strategy with reduced four-year event rates (17.2% vs 29.3%, difference −12.1%; 95% CI: −22.6, −1.6%), unlike those without HF or low EF.
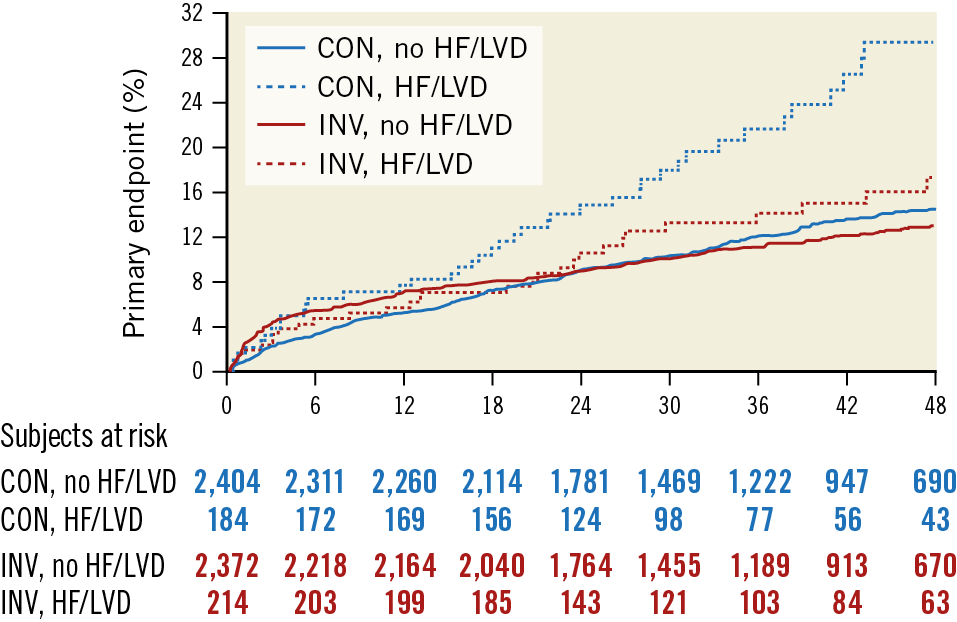
Figure 3. Cumulative incidence curves for the primary endpoint (a composite of cardiovascular death, myocardial infarction, resuscitated cardiac arrest, or hospitalisation for unstable angina or heart failure) according to randomised treatment and history of heart failure or left ventricular dysfunction, in the ISCHEMIA trial population. Reproduced from Lopes et al69, with permission. CON: conservative strategy; HF: heart failure; INV: invasive strategy; LVD: left ventricular dysfunction
On the heels of the ISCHEMIA trial, a meta-analysis including 14 randomised controlled trials that enrolled 14,877 patients with CCS compared a routine revascularisation strategy with initial guideline-directed medical therapy at 4.5 years of follow-up. Revascularisation compared with medical therapy alone was not associated with a reduced risk of death, HF or stroke (Figure 4),70. After revascularisation, the spontaneous MI rate was reduced (RR 0.76, 95% CI: 0.67-0.85) but procedure-related MI was increased (RR 2.48, 95% CI: 1.86-3.31). Episodes of unstable angina were less frequent with revascularisation. These results corroborate the ISCHEMIA trial findings and should inform decision making for the initial treatment approach in patients with CCS.
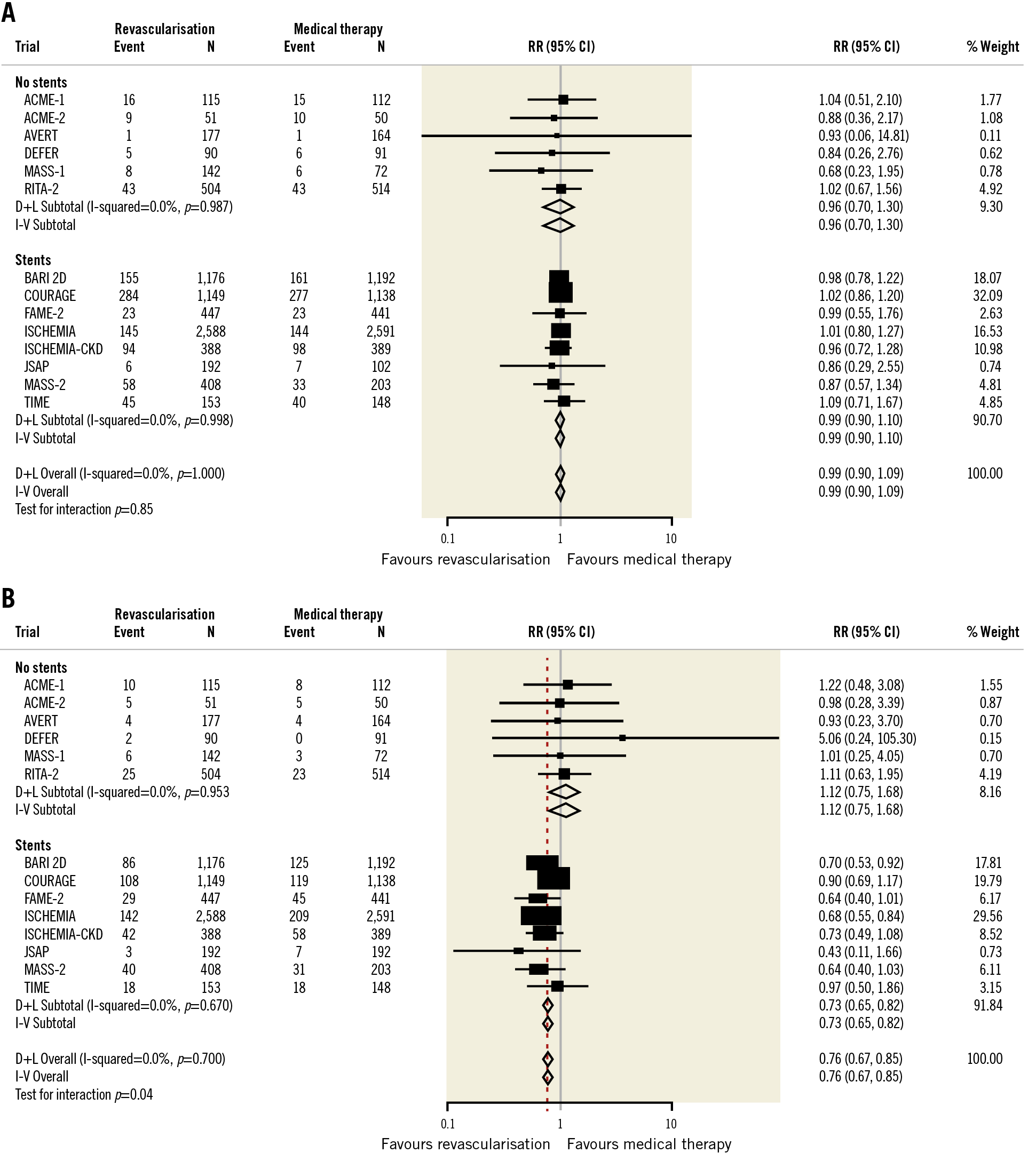
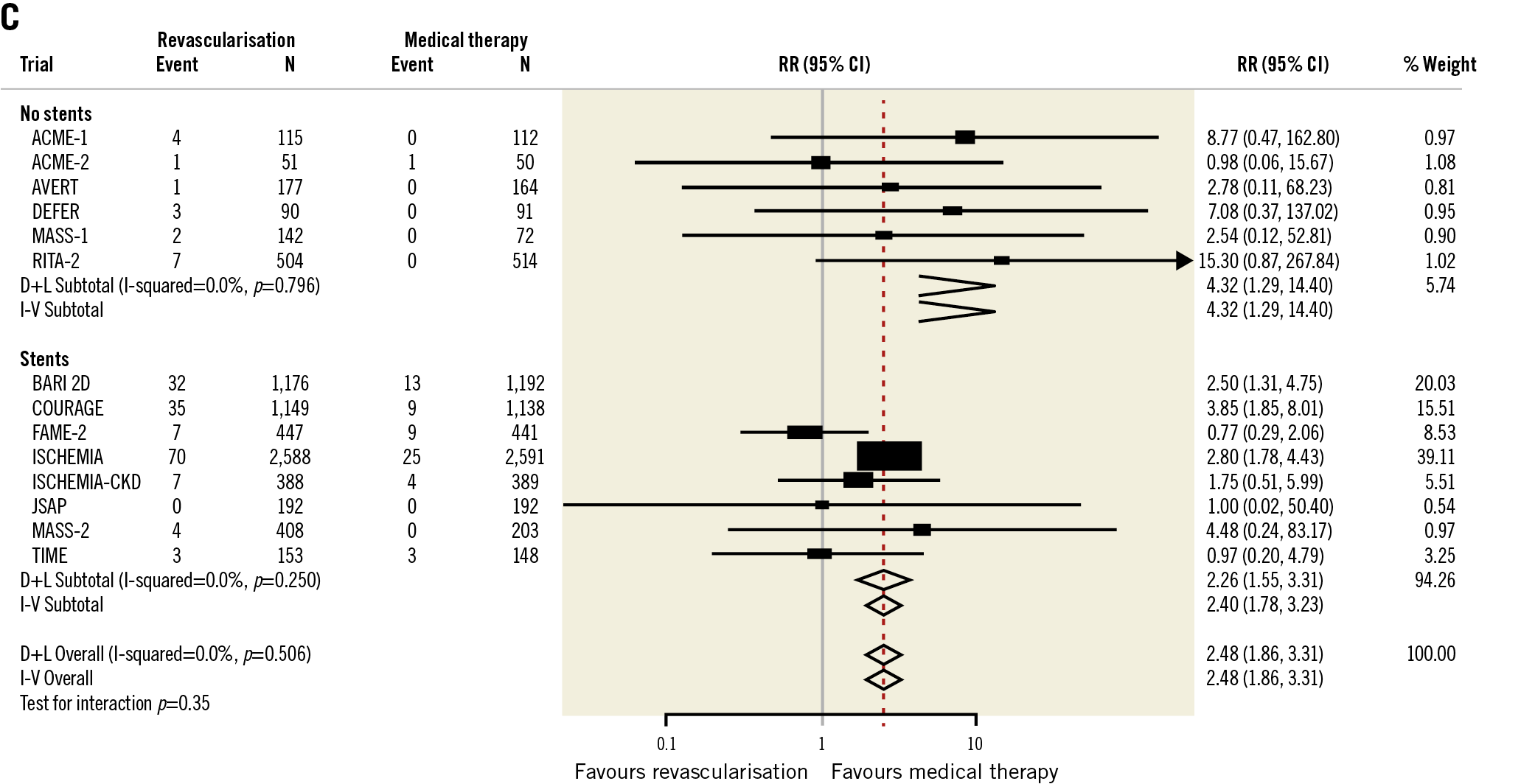
Figure 4. Meta-analysis of studies addressing revascularisation versus initial medical therapy for stable ischaemic heart disease. A) Overall death. B) Non-procedural myocardial infarction. C) Procedural myocardial infarction. Reproduced from Bangalore et al70, with permission. D+L: DerSimonian and Laird; I-V: inverse variance; RR: relative risk
PCI VERSUS CABG
Extended follow-up up to 10 years of the PRECOMBAT trial71 (Premier of Randomized Comparison of Bypass Surgery vs Angioplasty Using Sirolimus-Eluting Stent in Patients with Left Main Coronary Artery Disease) reported on the major adverse and cerebrovascular events (MACCE) rates between PCI and CABG in patients with LM disease. Repeat revascularisation rates (Figure 5) were higher with PCI at any time point and at 10 years (16.1 vs 8.0%, p<0.05), similar to other trials72,73. The composite of death, MI, stroke, or ischaemia-driven TVR was numerically higher at 29.8% after PCI versus 24.7% after CABG (HR 1.25, 95% CI: 0.93-1.69; p-value not available).
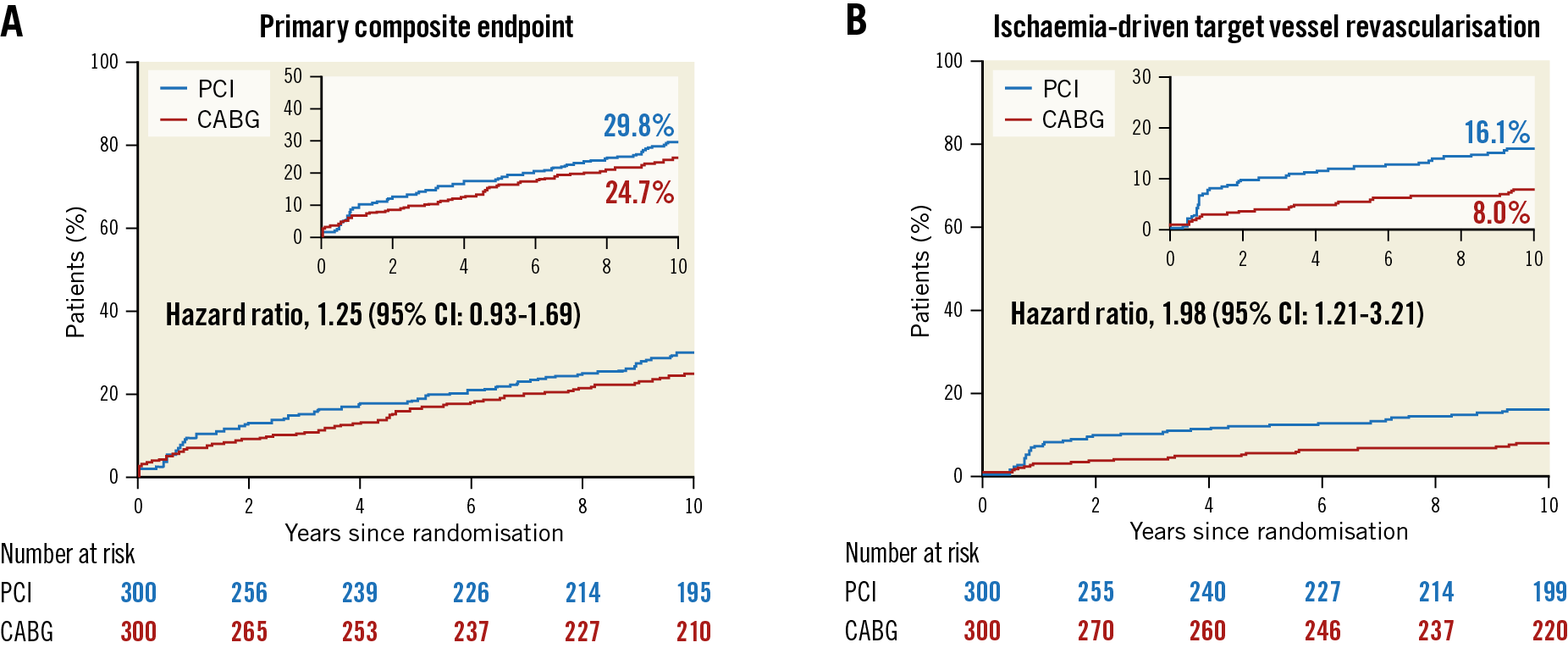
Figure 5. Cumulative incidence curves for A) the primary endpoint (composite of death from any cause, myocardial infarction, stroke, or ischaemia-driven target vessel revascularisation), and B) repeat revascularisation in the PRECOMBAT trial, at 10-year follow-up. Reproduced from Park et al71, with permission. CABG: coronary artery bypass grafting; CI: confidence interval; PCI: percutaneous coronary intervention
PCI can be proposed for treatment of LM stenosis in selected patients in the absence of extensive CAD with a high anatomic SYNTAX score. A secondary analysis of the SYNTAX database with an external validation cohort was performed in order to validate an updated version of the SYNTAX score II which compares the relative merits of PCI and CABG over a 10-year period74. The 2020 SYNTAX score II considers eight prognostic factors (age, diabetes, insulin use, creatinine clearance, left ventricular EF, chronic obstructive pulmonary disease, peripheral vascular disease, current smoker) and two effect modifiers (three-vessel disease vs LM disease only and anatomical SYNTAX score). The model has been developed to predict both the five-year risk of MACE (defined as all-cause death, non-fatal stroke, or non-fatal MI) and the 10-year mortality risk in patients receiving either PCI or CABG (Figure 6). The implementation of this score in clinical practice will play a key supportive role to Heart Teams and patients, in order to compare treatment options based on individual risk estimates.
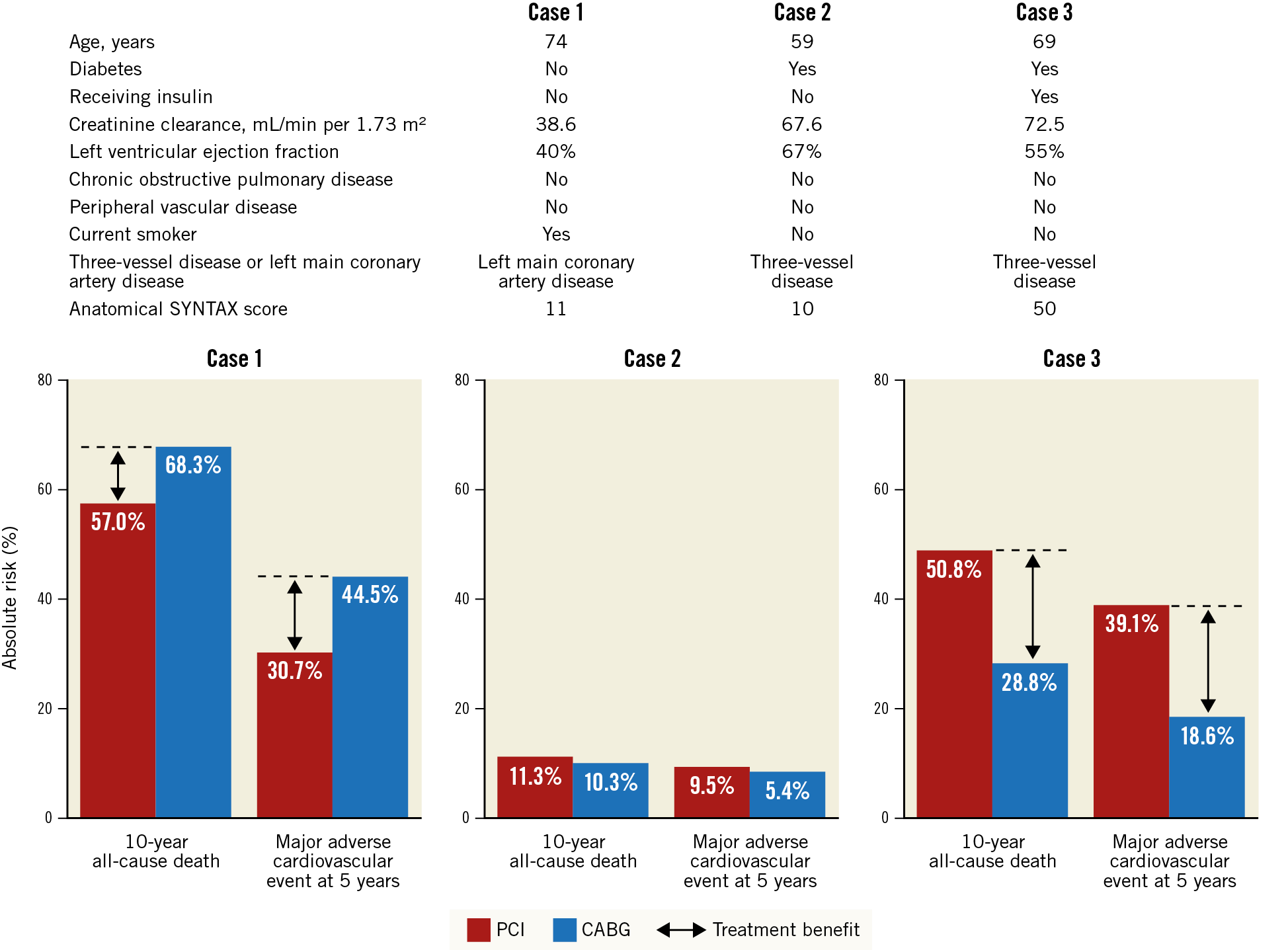
Figure 6. Example cases of the use of the 2020 SYNTAX score II for individualised decision making. The clinical characteristics of three patients considered by the new SYNTAX score to predict their risk of 10-year mortality and five-year adverse outcomes. The graph at the bottom shows the risk comparison between CABG and PCI for each patient. The 2020 SYNTAX score can be downloaded at syntaxscore2020.com. Reproduced from Takahashi et al74, with permission. CABG: coronary artery bypass grafting; PCI: percutaneous coronary intervention; SYNTAX: Synergy Between Percutaneous Coronary Intervention With Taxus And Cardiac Surgery
Interventional cardiology during the COVID-19 pandemic: “annus mirabilis”
The impact of COVID-19 on the worldwide healthcare system has drastically and suddenly disrupted consolidated processes and led to a redeployment of infrastructures and resources. Despite efforts to maintain high standards of care, the effects of the pandemic on patients’ fear and anxiety have impacted negatively on the care of cardiovascular disease. In this sense, 2020 can be described as an “annus mirabilis”, in reference to a poem written by John Dryden following the year 1666. Although the poem is entitled “Year of Wonders”, the year 1666 was one of multiple disasters and casualties, including the Plague, a number of furious wars and the Great Fire of London. It was said that Dryden used the word “mirabilis” because it was a wonder that things were not worse.
Numerous surveys and registries have reported an impressive reduction in ACS admissions all over the world, by 40% on average and peaking at 70% in specific areas75,76,77. The most consistent drop was observed among NSTEMI cases, declining by 40% in the United Kingdom and Italy, while admissions for STEMI were 20% lower than during the same period in 2018 or 2019, resulting in an inevitable PCI reduction as well75,78. There has been a prolongation in the delay between symptom onset and first medical contact, with a subsequent increased duration of the ischaemic period, whereas the “door-to-balloon” time was mostly unchanged. Different trends have been reported with respect to short-term mortality of STEMI, from no worsening in most cases to doubling in Spain79. As observed in a report from Italy, STEMI patients often presented with worse left ventricular systolic function at baseline and at discharge80, suggesting that treatment delays had an immediate impact on the extent of myocardial damage. Of note, many primary PCI centres have noticed an increase in the incidence of mechanical complications such as septal or papillary muscle rupture, or cardiogenic shock as a result of late presentation. Moreover, there is a lack of knowledge about the number of undiagnosed MI cases where patients have not contacted healthcare services in the (sub)acute phase. Governments and healthcare systems will probably be facing an excess of MI-related morbidity cases in “the people left behind”81.
In such a context, local, regional and national healthcare authorities have endeavoured to streamline diagnostic and treatment pathways for patients presenting with ACS, STEMI and other acute cardiovascular conditions, aiming to strike a proper balance between protection against COVID-19 transmission and optimal use of available resources. Scientific societies have turned best practice strategies into consensus documents or guidelines in order to disseminate and promote the implementation of lifesaving care pathways.
Conclusion
Since the first PCI was performed by Dr Grüntzig in 1977, outstanding progress has been made in multiple aspects of CAD management. From a diagnostic point of view, this year clinical research underlined the importance of the dual anatomic and functional assessment of coronary stenosis, aiming to reduce adverse events related to suboptimal PCI results or untreated high-risk plaques.
Similarly, post-PCI assessment confirms functional procedural success and may lead to further optimisation, as required. The impact of PCI complications, bleedings above all, prompted the investigation of safer antiplatelet strategies and new stent technologies. Several studies confirmed the safety of using modified antiplatelet strategies with some of the bioresorbable polymer or polymer-free DES iterations.
Long-term data from medical versus invasive management studies continue to highlight that guideline-directed medical therapy is a valuable first option approach in coronary patients with mild symptoms and low-risk anatomy. On the other hand, when revascularisation is required, the best strategy should be chosen through a tailoring process, focusing on risk mitigation in the individual patient.
Funding
This work is supported by a Science Foundation Ireland Research Professorship Award (RSF 1413) including grants to Drs M. Lunardi, C. Gao, R. Wang, and W. Wijns.
Conflict of interest statement
W. Wijns reports institutional research grants from Terumo, MiCell and MicroPort; honoraria from MicroPort; being a medical advisor of Rede Optimus Research and co-founder of Argonauts, an innovation accelerator. P.W. Serruys reports personal fees from Biosensors, MiCell Technologies, Sino Medical Sciences Technology, Philips/Volcano, Xeltis, and HeartFlow, outside the submitted work. The other authors have no conflicts of interest to declare.
References
The references can be found in the Supplementary data document.
Supplementary data
To read the full content of this article, please download the PDF.

