Transcatheter aortic valve implantation (TAVI) has evolved into a transformative alternative to surgical aortic valve replacement (SAVR), supported by robust evidence from numerous randomised clinical trials across the spectrum of surgical risk, and features two landmark transcatheter heart valve (THV) devices: the balloon-expandable SAPIEN (Edwards Lifesciences) and the self-expanding CoreValve/Evolut (Medtronic) series. Against this solid evidence base, novel THV technologies are increasingly tested in head-to-head comparisons against these established balloon-expandable and self-expanding valve standards of care (Figure 1)12345. In the recently published LANDMARK trial, a novel balloon-expandable prosthesis, the Myval THV (Meril Life Sciences), was directly compared with contemporary THVs (SAPIEN and Evolut series)4. Of note, the Myval THV introduces a sizing strategy with 1.5 mm diameter increments between nominal device sizes, allowing for a more nuanced sizing strategy to match the aortic annulus compared to conventional devices with fixed 3.0 mm diameter increments. Out of 5,109 screened patients, 768 patients deemed eligible for all three devices were randomly assigned 1:1 to Myval (N=384) or to contemporary THVs (N=384) with subsequent stratification and equal allocation (1:1) of patients to the SAPIEN (N=192 [SAPIEN 3: 55.4% and SAPIEN 3 Ultra: 44.6%]) or Evolut (N=192 [Evolut R: 37.0%, Evolut PRO: 55.2%, Evolut PRO Plus: 5.2%, and Evolut FX: 2.6%]) devices using a covariate-adaptive randomisation process4. In the primary analysis, Myval was shown to be non-inferior to contemporary THVs with respect to the primary composite safety and effectiveness endpoint at 30 days (a composite of all-cause mortality, all stroke, major bleeding, acute kidney injury, major vascular complications, moderate or severe prosthetic valve regurgitation, and conduction disturbances requiring a permanent pacemaker implantation) (25% vs 27%, risk difference: –2.3%, one-sided upper 95% confidence interval [CI]: 3.8%; pnon-inferiority<0.0001). However, the data reported to date were limited to the comparison of the combined control cohort and did not provide detailed insights into the head-to-head comparisons of all three devices.
In this issue of EuroIntervention, van Royen et al present a prespecified analysis of the LANDMARK trial directly comparing Myval with SAPIEN and Evolut, respectively6. For the individual head-to-head comparisons, assuming an event rate of 26.1% for the primary endpoint and a non-inferiority margin of 10.4%, the sample size provided 80% power with a one-sided alpha of 0.05 to establish non-inferiority. At 30 days, Myval was found to be non-inferior for the composite primary endpoint as compared to each comparator device (24.7% vs 24.1% for SAPIEN; risk difference: 0.6%, one-sided upper 95% CI: 8.0%; pnon-inferiority=0.0033; and 24.7% vs 30.0% for Evolut; risk difference: –5.3%, one-sided upper 95% CI: 2.5%; pnon-inferiority<0.0001). In terms of echocardiographic outcomes as assessed at an independent core laboratory, Myval was associated with lower mean transprosthetic gradients, a larger effective orifice area (EOA) and a similar rate of moderate or greater prosthetic valve regurgitation compared to SAPIEN. As compared with Evolut, Myval was associated with higher mean transprosthetic gradients, a smaller EOA and a lower rate of moderate or greater prosthetic valve regurgitation. The key results of the prespecified analysis were consistent in an exploratory analysis of 245 patients with small aortic annuli, defined as an aortic annulus area ≤430 mm2 on preprocedural computed tomography (Myval 32.6%, SAPIEN 33.3%, and Evolut 29.2%) although Myval had a higher rate of mild prosthetic valve regurgitation compared to SAPIEN.
While this analysis represents the first head-to-head comparison of Myval with SAPIEN and Evolut THVs, several considerations deserve attention. First, the Myval arm predominantly used a single device generation (91.3%), whereas the respective control arms included a range of device iterations without inclusion of the newest THV generations (SAPIEN 3 Ultra RESILIA and Evolut FX Plus). Recent data suggest improved echocardiographic outcomes with the latest THV comparator generation compared to their predecessor78. Along these lines, a similar rate of moderate or greater prosthetic valve regurgitation was observed for Myval and Evolut THVs after excluding the Evolut R prostheses, and there are internal inconsistencies such as the similar effective orifice area for Evolut 26, 29 and 34 mm THVs4. Second, although the randomised allocation of study devices was robust, the selection of THV size was left to the discretion of local Heart Teams, which may have introduced performance bias. For example, despite a similar prevalence of patients with small aortic annuli, no patient in the Evolut arm received a 23 mm valve, in contrast to the use of small balloon-expandable valves (20 mm) in the Myval and SAPIEN groups. Procedural details were similarly operator dependent resulting in several inconsistencies (general anaesthesia, pre- and post-balloon dilation, vascular closure device). These differences may potentially contribute to the higher observed rates of major bleeding and acute kidney injury in the Myval group and the higher rate of permanent pacemaker implantation with SAPIEN THVs compared to other studies19. Moreover, the imbalance in crossovers (15 cases in Myval vs 2 cases in SAPIEN and 3 cases in Evolut) may have affected the results, although per-protocol and as-treated analyses for the primary endpoint are not currently reported. Finally, this study is limited to a highly selected study population (15% global inclusion rate) with a short-term follow-up (30 days) leaving uncertainties regarding the generalisability and long-term clinical outcomes and device performance.
The Compare-TAVI trial (ClinicalTrials.gov: NCT04443023) is currently underway to directly compare TAVI with Myval versus SAPIEN THVs for the primary endpoint of composite of death, stroke, moderate/severe aortic regurgitation, or moderate/severe THV deterioration at 1 year. It will provide important data complementing those of the LANDMARK trial10. Following the strategy trials comparing TAVI and SAVR across the spectrum of surgical risk in tricuspid aortic stenosis, the era of head-to-head THV trials provides a unique opportunity to standardise clinical and echocardiographic endpoints for the assessment of both early safety and long-term efficacy in order to inform clinical practice for optimal device selection and assessment of durability.
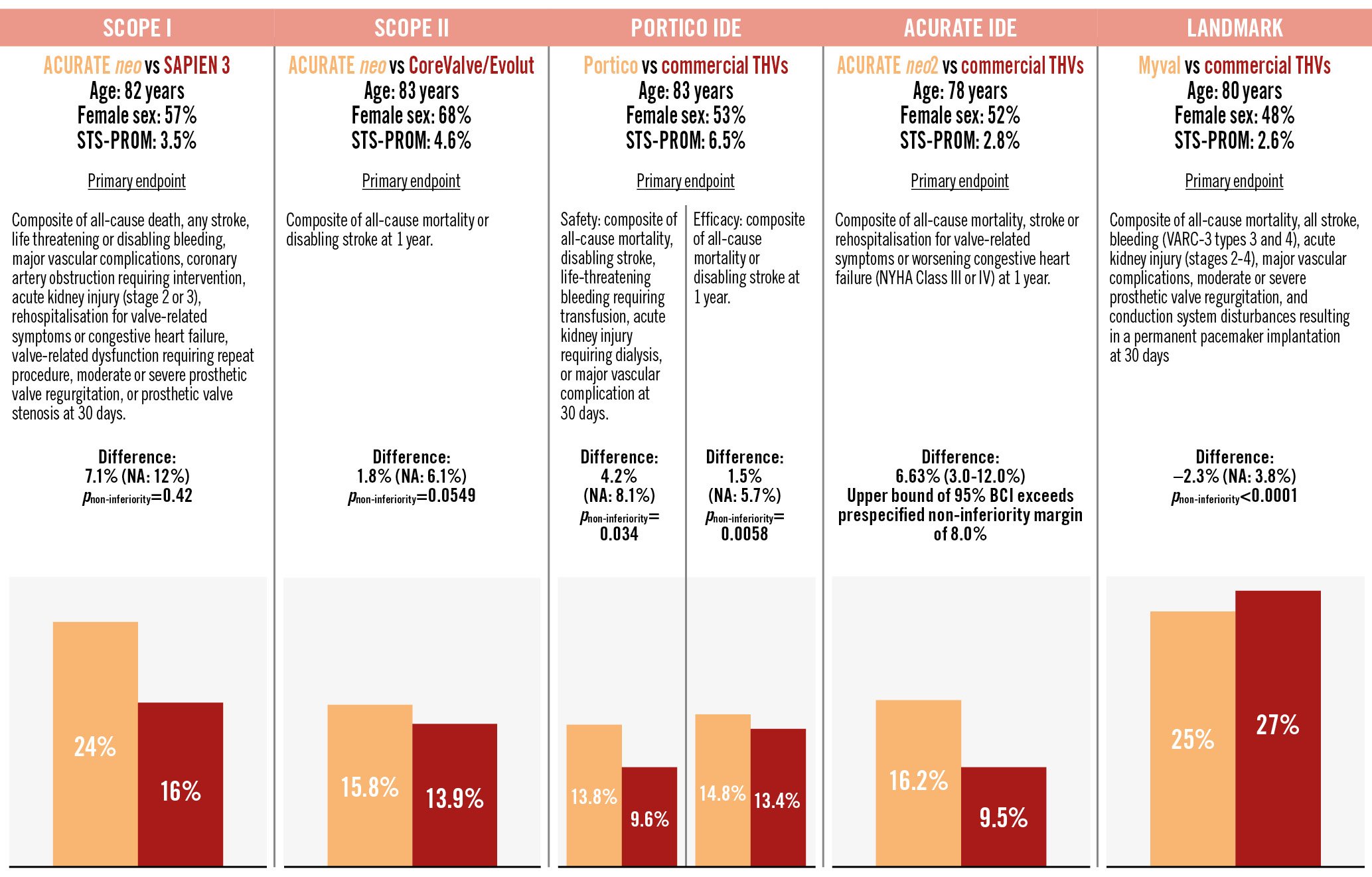
Figure 1. Primary endpoints comparing the new device versus SAPIEN/Evolut series in randomised controlled trials. The orange bar graphs indicate the experimental device and the red bar graphs indicate the established device. Commercial THVs include both SAPIEN (Edwards Lifesciences) and CoreValve/Evolut THVs (Medtronic). ACURATE neo and neo2 by Boston Scientific and Portico by Abbott. BCI: Bayesian credible interval; NA: not available; NYHA: New York Heart Association; STS-PROM: Society of Thoracic Surgeons Predicted Risk of Mortality; THV: transcatheter heart valve; VARC: Valve Academic Research Consortium
Conflict of interest statement
S. Windecker reports research, travel or educational grants to the institution without personal remuneration from Abbott, Abiomed, Amgen, AstraZeneca, Bayer, B. Braun, Biotronik, Boehringer Ingelheim, Boston Scientific, Bristol-Myers Squibb, Cardinal Health, CardioValve, Cordis Medical, CorFlow Therapeutics, CSL Behring, Cleerly, Inc., Daiichi Sankyo, Edwards Lifesciences, Farapulse Inc., Fumedica, Guerbet, Idorsia, Inari Medical, Infraredx, Janssen-Cilag, Johnson & Johnson, Medalliance, Medicure, Medtronic, Merck Sharp & Dohme, Miracor Medical, Monarch Medical, Novartis, Novo Nordisk, Organon, OrPha Swiss, Pharming Group, Pfizer, Polares, Regeneron, Sanofi-Aventis, Servier, Sinomed, Terumo, Vifor, and V-Wave; and served as advisory board member and/or member of the steering/executive group of trials funded by Abbott, Abiomed, Amgen, AstraZeneca, Bayer, Boston Scientific, Biotronik, Bristol-Myers Squibb, Edwards Lifesciences, Medalliance, Medtronic, Novartis, Polares, Recardio, Sinomed, Terumo, and V-Wave with payments to the institution but no personal payments; he is also member of the steering/executive committee group of several investigator-initiated trials that receive funding by industry without impact on his personal remuneration. D.Tomii has no conflicts of interest relevant to the contents of this article to declare.

