Resorbable scaffolds were developed to provide temporary vessel support, maintaining patency during the healing phase and subsequently dissolving to eliminate long-term complications associated with permanent metallic drug-eluting stents (DES). However, early-generation polymeric scaffolds failed to meet clinical expectations, exhibiting higher event rates than DES. In contrast, magnesium-based scaffolds have emerged as a promising alternative, given that magnesium offers mechanical properties more akin to metallic DES. While earlier iterations demonstrated an excellent safety profile, they fell short in achieving competitive angiographic performance metrics such as low late lumen loss (LLL). The third-generation sirolimus-eluting resorbable magnesium scaffold (DREAMS 3G, commercial name Freesolve [Biotronik]) was developed to address these limitations. Leveraging an enhanced magnesium alloy, DREAMS 3G features thinner struts, increased radial strength, more uniform degradation, and a prolonged scaffolding duration compared to its predecessor. One-year results from the BIOMAG-I study confirmed that the design objectives were met, with a median LLL of 0.19 mm (interquartile range [IQR]: 0.06-0.36)123. Herein, we present the 3-year clinical outcomes, two years after complete scaffold resorption.
BIOMAG-I is a prospective, multicentre, single-arm, first-in-human trial conducted in Europe (ClinicalTrials.gov: NCT04157153). Study design and primary results have been published previously3. Eligible patients presented with symptomatic coronary artery disease involving up to two de novo lesions and clinical presentations ranging from stable angina to non-ST-segment elevation myocardial infarction. The study device, DREAMS 3G, is a balloon-expandable bioresorbable scaffold made of a proprietary magnesium alloy, with radiopaque markers at both ends. It is coated with poly-L-lactic acid, incorporating sirolimus as the antiproliferative agent. Strut thicknesses range from 99 μm to 147 μm, depending on device diameter. Scheduled follow-up extends to five years, and all clinical events are adjudicated by an independent clinical events committee.
A total of 116 patients were enrolled. Three-year data are available for 112 patients. There was no cardiac death, no target vessel myocardial infarction, and no definite, probable or possible scaffold thrombosis reported. The 3-year Kaplan-Meier estimate for target lesion failure (TLF) was 3.5% (95% confidence interval: 1.3-9.0) (Figure 1), consisting of four clinically driven target lesion revascularisations (CD-TLR; on days 166, 204, 270, and 522 post-procedure). A review of the CD-TLR beyond one year identified a previously untreated plaque proximal to the scaffolded segment. By day 522 post-procedure, the previously moderate stenosis in this area progressed to 70% and therefore was treated with a permanent DES.
The low TLF rate at three years – particularly with only one event occurring beyond the scaffold resorption period – is highly encouraging and compares favourably to TLF rates of 6.7% to 13.6% reported in exemplary trials such as BIO-RESORT and BIOFLOW-V45. However, it should be noted that these trials employed broader inclusion criteria, encompassing high-risk lesion and patient characteristics.
Freedom from cardiac death, target vessel myocardial infarction, and any device thrombosis up to three years attests to excellent device performance. While the precursor devices of DREAMS 3G had already shown an excellent safety profile and lower thrombogenicity compared to other bioresorbable scaffolds13, preclinical studies suggest that DREAMS 3G further reduces thrombogenic potential2. Future randomised controlled trials are planned to validate these promising results in comparison to contemporary DES.
Study limitations include (i) the single-arm design, which precludes direct comparison with other stent technologies, particularly given the differences in patient characteristics and potential use of differing definitions; (ii) the first-in-human population, defined by its narrow inclusion and exclusion criteria, may not reflect broader real-world practice; (iii) the relatively small sample size (n=116), calculated for the primary endpoint, LLL at 6 months, results in wider confidence intervals for clinical event rates; and (iv) no imaging assessments were scheduled beyond 12 months.
In conclusion, the favourable 3-year outcomes of DREAMS 3G support renewed interest in bioresorbable scaffolds as a viable therapeutic option that combines temporary mechanical support with excellent long-term safety and efficacy. The randomised BIOMAG-II trial will determine whether DREAMS 3G can emerge as a competitive alternative to contemporary DES.
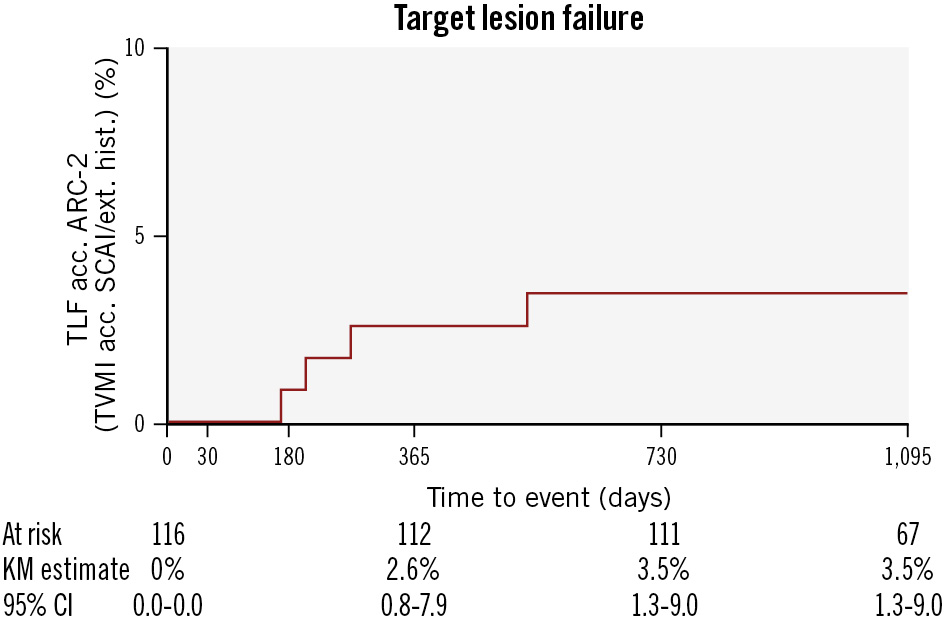
Figure 1. Kaplan-Meier estimate of target lesion failure up to three years. All events were adjudicated by an independent clinical events committee. All target lesion failures were clinically driven target lesion revascularisations. There were no cardiac deaths, nor target vessel myocardial infarctions, and no definite, probable, or possible device thrombosis was observed. acc.: according to; ARC-2: Academic Research Consortium 2; CI: confidence interval; ext. hist.: extended historical definition; KM: Kaplan-Meier; SCAI: Society for Cardiovascular Angiography & Interventions; TLF: target lesion failure; TVMI: target vessel myocardial infarction
Acknowledgements
We thank Beatrix Doerr, medical writer, for her help in preparing the manuscript. Artificial intelligence (AI) was used for language editing such as improving grammar. No AI tools were involved in the generation or interpretation of scientific content, data analysis, or the formulation of conclusions. The authors maintained full control over all aspects of the scientific content and take full responsibility for the integrity and accuracy of the work.
Conflict of interest statement
R. Waksman was a core laboratory member; the remaining authors were investigators of the trial. M. Haude reports grants/contracts from Biotronik, Cardiac Dimensions, and Philips; consulting fees from Biotronik, Cardiac Dimensions, and Shockwave Medical; honoraria/speaker fees from Biotronik, Cardiac Dimensions, Shockwave Medical, and Philips; support to attend meetings/travel support from Biotronik; is a steering committee member of the BIOSOLVE and BIOMAG trials; and is a past President of EAPCI. J. Torzewski reports grants and contracts from Abbott paid to his institution; speaker honoraria and support for attending meetings from Biotronik; and is an Associated Editor of Cardiovascular Biologics and Regenerative Medicine and Frontiers in Cardiovascular Medicine. J. Escaned reports personal fees/speaker honoraria from Abbott, Boston Scientific, Philips, and Shockwave Medical; patents from Shared; and participation in advisory boards of Abbott and Philips. J.F. Iglesias reports unrestricted research grants to his institution or contracts from Terumo, Biosensors, Concept Medical, Biotronik, and SMT; consulting fees from Biotronik, Medtronic, Cordis, and Recor Medical; speaker fees/honoraria from Biotronik, Biosensors, Bristol-Myers Squibb/Pfizer, Cordis, Concept Medical, Medtronic, Penumbra, and Recor Medical; support to attend meetings from Biotronik and Medtronic; and is a Data Safety Monitoring Board member of the Co-STAR trial. The institution of J. Bennett receives grants or contracts from Shockwave IVLS; he receives consulting fees from Biotronik and Boston Scientific; speaker fees/honoraria from Biotronik, Boston Scientific, Elixir, and Abbott; participates in the advisory board of Elixir; and has a leadership or fiduciary role for Biotronik. G. Toth reports consulting fees from Biotronik, Medtronic, Abbott, and Terumo; and honoraria from Biotronik, Medtronic, Abbott, and Terumo. M. Joner reports grant support from Boston Scientific, Cardiac Dimensions, Edwards Lifesciences, and Infraredx; consulting fees from AlchiMedics SAS, Biotronik, TRiCares, Veryan, and Shockwave Medical; speaker fees/honoraria from Abbott, Biotronik, Boston Scientific, Edwards Lifesciences, Cardiac Dimensions, AstraZeneca, Recor Medical, and Shockwave Medical; travel support from SIS Medical, Edwards Lifesciences, Boston Scientific and Cardiac Dimensions; and participation in steering committees of Biotronik and Edwards Lifesciences. R. Toelg reports lecture fees from Biotronik. M. Wiemer reports speaker honoraria and conference attendance support from Biotronik. G. Olivecrona reports lecturer honoraria from Abbott, Biotronik, and Cordis; is a DSMB member of the SCIENCE trial; and a CEC member of the BIOFREEDOM STEMI trial. R. Waksman has grants or contracts from Amgen, Biotronik, Boston Scientific, Medtronic, and Philips IGT; received consulting fees from Abbott, Biotronik, Boston Scientific, Cordis, Medtronic, Philips IGT, Pi-Cardia, Swiss Interventional Systems/SIS Medical AG, Transmural Systems, and Venus Medtech; received honoraria from AstraZeneca; participates in DSMB/advisory boards of Abbott, Boston Scientific, Medtronic, Philips IGT, and Pi-Cardia; and is an investor from MedAlliance and Transmural Systems. The other authors have no conflicts of interest to declare.

