ESC Committee for Practice Guidelines (CPG), EACTS Clinical Guidelines Committee, and National Cardiac Societies document reviewers: listed in the Appendix.
ESC entities having participated in the development of this document:
Associations: Acute Cardiovascular Care Association (ACCA), European Association of Preventive Cardiology (EAPC), European Association of Cardiovascular Imaging (EACVI), European Association of Percutaneous Cardiovascular Interventions (EAPCI), European Heart Rhythm Association (EHRA), Heart Failure Association (HFA).
Councils: Council on Cardiovascular Nursing and Allied Professions, Council for Cardiology Practice, Council on Cardiovascular Primary Care, Council on Stroke, Council on Valvular Heart Disease
Working Groups: Aorta and Peripheral Vascular Diseases, Cardiovascular Pharmacotherapy, Coronary Pathophysiology and Microcirculation, Thrombosis.
Sousa-Uva M, Neumann F-J, Ahlsson A, Alfonso F, Banning AP, Benedetto U et al. 2018 ESC/EACTS
Guidelines on myocardial revascularization. European Heart Journal 2018, doi:10.1093/eurheartj/ehy394.
Reproduced by permission of Oxford University Press on behalf of the European Society of Cardiology.
© The European Society of Cardiology 2018. All rights reserved ; no part of this publication may be reproduced, stored in a retrieval system, or transmitted in any form or by any means, electronic, mechanical, photocopying, recording, or otherwise without the prior written permission of Oxford University Press.
For permissions please email: [email protected]
Please visit: http://www.escardio.org/Guidelines/Clinical-Practice-Guidelines/ESC-EACTS-Guidelines-in-Myocardial-Revascularisation-Guidelines-for
Disclaimer. The ESC Guidelines represent the views of the ESC and were produced after careful consideration of the scientific and medical knowledge and the evidence available at the time of their dating. The ESC//EACTS are not responsible in the event of any contradiction, discrepancy and/or ambiguity between the ESC Guidelines and any other official recommendations or guidelines issued by the relevant public health authorities, in particular in relation to good use of health care or therapeutic strategies. Health professionals are encouraged to take the ESC Guidelines fully into account when exercising their clinical judgment as well as in the determination and the implementation of preventive, diagnostic or therapeutic medical strategies. However, the ESC Guidelines do not override in any way whatsoever the individual responsibility of health professionals to make appropriate and accurate decisions in consideration of each patient’s health condition and in consultation with that patient and the patient’s caregiver where appropriate and/or necessary. Nor do the ESC Guidelines exempt health professionals from taking careful and full consideration of the relevant official updated recommendations or guidelines issued by the competent public health authorities in order to manage each patient’s case in light of the scientifically accepted data pursuant to their respective ethical and professional obligations. It is also the health professional’s responsibility to verify the applicable rules and regulations relating to drugs and medical devices at the time of prescription.
Document Reviewers: William Wijns (ESC Review Co-ordinator) (Ireland), David Glineur1 (EACTS Review Co-ordinator) (Canada), Victor Aboyans (France), Stephan Achenbach (Germany), Stefan Agewall (Norway), Felicita Andreotti (Italy), Emanuele Barbato (Italy), Andreas Baumbach (UK), James Brophy (Canada), Héctor Bueno (Spain), Patrick A. Calvert (UK), Davide Capodanno (Italy), Piroze M. Davierwala1 (Germany), Victoria Delgado (The Netherlands), Dariusz Dudek (Poland), Nick Freemantle1 (UK), Christian Funck-Brentano (France), Oliver Gaemperli (Switzerland), Stephan Gielen (Germany), Martine Gilard (France), Bulent Gorenek (Turkey), Joerg Haasenritter (Germany), Michael Haude (Germany), Borja Ibanez (Spain), Bernard Iung (France), Anders Jeppsson1 (Sweden), Demosthenes Katritsis (Greece), Juhani Knuuti (Finland), Philippe Kolh1 (Belgium), Adelino Leite-Moreira1 (Portugal), Lars H. Lund (Sweden), Francesco Maisano (Switzerland), Julinda Mehilli (Germany), Bernhard Metzler (Austria), Gilles Montalescot (France), Domenico Pagano1 (UK), Anna Sonia Petronio (Italy), Massimo Francesco Piepoli (Italy), Bogdan A. Popescu (Romania), Rafael Sádaba1 (Spain), Evgeny Shlyakhto (Russia), Sigmund Silber (Germany), Iain A. Simpson (UK), David Sparv (Sweden), Giuseppe Tavilla1 (The Netherlands), Holger Thiele (Germany), Petr Tousek (Czech Republic), Eric Van Belle (France), Pascal Vranckx (Belgium), Adam Witkowski (Poland), Jose Luis Zamorano (Spain), Marco Roffi (ESC CPG Supervisor) (Switzerland)
The disclosure forms of all experts involved in the development of these Guidelines are available on the ESC website: www.escardio.org/guidelines
Table of contents
- Abbreviations and acronyms
- 1. Preamble
- 2. Introduction
- 3. Diagnostic tools to guide myocardial revascularization
- 4. Process for decision-making and patient information
- 5. Revascularization for stable coronary artery disease
- 6. Revascularization in non-ST-elevation acute coronary syndrome
- 7. Revascularization in ST-segment elevation myocardial infarction
- 8. Myocardial revascularization in patients with heart failure
- 9. Revascularization in patients with diabetes
- 10. Revascularization in patients with chronic kidney disease
- 11. Revascularization in patients requiring valve interventions
- 12. Associated peripheral artery diseases
- 13. Repeat revascularization
- 14. Arrhythmias
- 15. Procedural aspects of coronary artery bypass grafting
- 16. Procedural aspects of percutaneous coronary intervention
- 17. Antithrombotic treatments
- 18 Volume outcome relationship for revascularization procedures
- 19. Medical therapy, secondary prevention, and strategies for follow-up
- 20. Key messages
- 21. Evidence-based ‘to do’ and ‘not to do’ messages from the Guidelines
- 22. Appendix
Abbreviations and acronyms
ABC: Age, Biomarkers, Clinical History
ABSORB II: A Bioresorbable Everolimus-Eluting Scaffold Versus a Metallic Everolimus-Eluting Stent II
AIDA: Amsterdam Investigator-Initiated Absorb Strategy All-Comers
ACCOAST: Comparison of Prasugrel at the Time of Percutaneous Coronary Intervention or as Pretreatment at the Time of Diagnosis in Patients with Non-ST Elevation Myocardial Infarction
ACS: Acute coronary syndrome
ACUITY: Acute Catheterization and Urgent Intervention Triage strategy
ADAPT-DES: Assessment of Dual Antiplatelet Therapy With Drug-Eluting Stents
AF: Atrial fibrillation
ALPHEUS: Assessment of Loading With the P2Y12-Inhibitor Ticagrelor or Clopidogrel to Halt Ischemic Events in Patients Undergoing Elective Coronary Stenting
AMI: Acute myocardial infarction
AMACING: A Maastricht Contrast-Induced Nephropathy Guideline
ANTARCTIC: Platelet function monitoring to adjust antiplatelet therapy in elderly patients stented for an acute coronary syndrome
ARCTIC: Assessment by a Double Randomization of a Conventional Antiplatelet Strategy versus a Monitoring-guided Strategy for Drug-Eluting Stent Implantation and of Treatment Interruption versus Continuation One Year after Stenting
ART: Arterial Revascularization Trial
AS: Aortic stenosis
ASE: American Society of Echocardiography
ATLANTIC: Administration of Ticagrelor in the Cath Lab or in the Ambulance for New ST-Elevation Myocardial Infarction to Open the Coronary Artery
ATLAS-ACS 2-TIMI 51 Anti-Xa Therapy to Lower cardiovascular events in Addition to Standard therapy in subjects with Acute Coronary Syndrome-Thrombolysis In Myocardial Infarction 51
ATOLL: Acute STEMI Treated with primary PCI and intravenous enoxaparin Or UFH to Lower ischaemic and bleeding events at short- and Long-term follow-up
AWESOME: Angina With Extremely Serious Operative Mortality Evaluation
BARC: Bleeding Academic Research Consortium
BARI-2D: Bypass Angioplasty Revascularization Investigation 2 Diabetes
BES: Biolimus-eluting stent
BEST: Randomised Comparison of Coronary Artery Bypass Surgery and Everolimus-Eluting Stent Implantation in the Treatment of Patients with Multivessel Coronary Artery Disease
b.i.d.: Bis in die (twice daily)
BIMA: Bilateral internal mammary artery
BMS: Bare-metal stent
BRAVE: Bavarian Reperfusion Alternatives Evaluation
BRS: Bioresorbable scaffolds
BVS: Bioresorbable vascular scaffold
CABG: Coronary artery bypass grafting
CAD: Coronary artery disease
CARDia: Coronary Artery Revascularization in Diabetes
CCS: Canadian Cardiovascular Society
CEA: Carotid endarterectomy
CHA2DS2-VASc: Cardiac Congestive heart failure, Hypertension, Age ≥75 [Doubled], Diabetes mellitus, prior Stroke or transient ischaemic attack or thromboembolism [Doubled] - Vascular disease, Age 65-74 and Sex category [Female]
CHAMPION: Cangrelor versus Standard Therapy to Achieve Optimal Management of Platelet Inhibition
CI: Confidence interval
CIN: Contrast-induced nephropathy
CKD: Chronic kidney disease
CMR: Cardiac magnetic resonance
COMPASS: Rivaroxaban for the Prevention of Major Cardiovascular Events in Coronary or Peripheral Artery Disease
COURAGE: Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation
CPG: ESC Committee for Practice Guidelines
CT: Computed tomography
CT-FFR: CT-derived fractional flow reserve
CTO: Chronic total occlusion
CTSN: Cardiothoracic Surgical Trial Network
CULPRIT-SHOCK Culprit Lesion Only PCI versus Multivessel PCI in Cardiogenic Shock
CVA: Cerebrovascular accident
CvLPRIT: Complete Versus Lesion-Only Primary PCI Trial
DANAMI 3-DEFER The Third DANish Study of Optimal Acute Treatment of Patients with ST-segment Elevation Myocardial Infarction: DEFERred stent implantation in connection with primary PCI
DANAMI-3-PRIMULTI The Third DANish Study of Optimal Acute Treatment of Patients with ST-segment Elevation Myocardial Infarction: PRImary PCI in MULTIvessel Disease
DAPT: Dual antiplatelet therapy
DCB: Drug-coated balloon
DEFINE-FLAIR: Define Functional Lesion Assessment of Intermediate Stenosis to Guide Revascularization
DES: Drug-eluting stents
DUS: Duplex ultrasound
EACTS: European Association for Cardio-Thoracic Surgery
EAPCI: European Association for Percutaneous Cardiovascular Interventions
EBC TWO: European Bifurcation Coronary TWO
ECG: Electrocardiogram
ECLS: Extracorporeal life support
ECMO: Extracorporeal membrane oxygenation
EES: Everolimus-eluting stent
EF: Ejection fraction
EMS: Emergency medical service
EROA: Effective regurgitant orifice area
ENTRUST-AF-PCI Evaluation of the safety and efficacy of an edoxaban-based antithrombotic regimen in patients with atrial fibrillation following successful percutaneous coronary intervention
ESC: European Society of Cardiology
EUROCTO: Randomized Multicentre Trial to Compare Revascularization With Optimal Medical Therapy for the Treatment of Chronic Total Occlusions
EuroSCORE: European System for Cardiac Operative Risk Evaluation
EUROMAX: European Ambulance Acute Coronary Syndrome Angiography
EXCEL: Evaluation of XIENCE Versus Coronary Artery Bypass Surgery for Effectiveness of Left Main Revascularization
FAME: Fractional Flow Reserve versus Angiography for Multivessel Evaluation
FDG-PET: Fluorodeoxyglucose positron emission tomography
FFR: Fractional flow reserve
FITT-STEMI: Feedback Intervention and Treatment Times in ST-Elevation Myocardial Infarction
FMC: First medical contact
FREEDOM: Future Revascularization Evaluation in Patients with Diabetes Mellitus
GLOBAL LEADERS Long-term ticagrelor monotherapy versus standard dual antiplatelet therapy followed by aspirin monotherapy in patients undergoing biolimus-eluting stent implantation
GP IIb/IIIa: Glycoprotein IIb/IIIa
GRAVITAS: Gauging Responsiveness with A VerifyNow assay-Impact on Thrombosis And Safety
HAS-BLED: Hypertension, Abnormal renal/liver function, Stroke, Bleeding history or predisposition, Labile INR, Elderly, Drugs/ alcohol
HEAT-PPCI: How Effective are Antithrombotic Therapies in primary PCI
HF: Heart failure
HFrEF: Heart failure with reduced ejection fraction
HORIZONS: Harmonizing Outcomes with Revascularization and Stents in Acute Myocardial Infarction
HPR: High platelet reactivity
HR: Hazard ratio
i.v.: Intravenous
IABP: Intra-aortic balloon pump
IABP-SHOCK II: Intraaortic Balloon Pump in Cardiogenic Shock II Trial
ICD: Implantable cardioverter defibrillator
iwFR: Instantaneous wave-free ratio
IMA: Internal mammary artery
IMR: Ischaemic mitral regurgitation
INR: International normalized ratio
IRA: Infarct-related artery
ISAR-CABG: Is Drug-Eluting-Stenting Associated with Improved Results in Coronary Artery Bypass Grafts
ISAR-REACT: Intracoronary Stenting and Antithrombotic Regimen Rapid Early Action for Coronary Treatment
ISCHEMIA: International Study of Comparative Health Effectiveness With Medical and Invasive Approaches
IVUS: Intravascular ultrasound imaging
LAA: Left atrial appendage
LAD: Left anterior descending
LEAD: Lower extremity artery disease
LGE-CMR: Late gadolinium enhancement cardiac magnetic resonance
LIMA: Left internal mammary artery
LM/LMS: Left main/left main stem
LMWH: Low-molecular-weight heparin
LPR: Low platelet reactivity
LV: Left ventricle/left ventricular
LVAD: Left ventricular assist device,
LVEF: Left ventricular ejection fraction
MACCE: Major adverse cardiac and cerebrovascular events
MACE: Major adverse cardiac events
MADIT II: Multicenter Automatic Defibrillator Implantation Trial II
MATRIX: Minimizing Adverse Haemorrhagic Events by Transradial Access Site and Systemic Implementation of AngioX
MCS: Mechanical circulatory support
MI: Myocardial infarction
MINOCA: Myocardial infarction with non-obstructive coronary arteries
MLA: Minimal luminal area
MR: Mitral regurgitation
MSCT: Multi-slice computed tomography
MT: Medical therapy
MVD: Multivessel coronary artery disease
MVO: Microvascular obstruction
NAC: N-acetylcysteine
NNT: Number needed to treat
NOAC: Non-vitamin K antagonist oral anticoagulant
NOBLE: Nordic-Baltic-British Left Main Revascularization Study
NSTE-ACS: Non-ST-segment elevation acute coronary syndrome
NSTEMI: Non-ST-segment elevation myocardial infarction
NYHA: New York Heart Association
OAC: Oral anticoagulation
OASIS-5: Optimal Antiplatelet Strategy for Interventions-5
OCT: Optical coherence tomography
OR: Odds ratio
ORBITA: Objective Randomised Blinded Investigation with optimal medical Therapy of Angioplasty in stable angina
PARR-2: PET and Recovery following Revascularization
PCI: Percutaneous coronary intervention
Pd/Pa: Distal coronary to aortic pressure
PES: Paclitaxel-eluting stent
PET: Positron emission tomography
PF: Platelet function
PIONEER: Prevention of bleeding in patients with AF undergoing PCI
PLATFORM: Prospective LongitudinAl Trial of FFRct: Outcome and Resource Impacts,
PLATO: Study of Platelet Inhibition and Patient Outcomes
pLVAD: Percutaneous left ventricular assist device
p.o.: Per os (orally)
POSEIDON: Prevention of Contrast Renal Injury with Different Hydration Strategies
PPI: Proton pump inhibitor
PRAGUE-18: Comparison of Prasugrel and Ticagrelor in the Treatment of Acute Myocardial Infarction
PRAMI: Preventive Angioplasty in Acute Myocardial Infarction
PRECISE-DAPT: PREdicting bleeding Complications In patients undergoing Stent implantation and subsEquent Dual Anti Platelet Therapy
PRECOMBAT: Premier of Randomised Comparison of Bypass Surgery versus Angioplasty Using Sirolimus-Eluting Stent in Patients with Left Main Coronary Artery Disease
PRESERVE: Prevention of Serious Adverse Events Following Angiography
q.d.: Quaque die (once daily)
RCT: Randomized controlled trial
RE-DUAL: Randomised Evaluation of Dual Antithrombotic Therapy with Dabigatran versus Triple Therapy with Warfarin in Patients with Nonvalvular Atrial Fibrillation Undergoing Percutaneous Coronary Intervention
REMEDIAL II: Renal Insufficiency After Contrast Media Administration II
REPLACE-2: The Randomised Evaluation in PCI Linking Angiomax to Reduced Clinical Events 2
RIVAL: Radial versus femoral access for coronary angiography and intervention in patients with acute coronary syndromes
ROMA: Randomization of Single vs. Multiple Arterial Grafts
RR: Relative risk
SASSICAIA: Comparison of Loading Strategies With Antiplatelet Drugs in Patients Undergoing Elective Coronary Intervention
SAVR: Surgical aortic valve replacement
s.c.: Subcutaneous
SCAD: Stable coronary artery disease
SCD-HEFT: Sudden Cardiac Death in Heart Failure Trial
SES: Sirolimus-eluting stent
SHOCK: Should We Emergently Revascularize Occluded Coronaries for Cardiogenic Shock
SIMA: Single internal mammary artery
SMART-DATE: Smart Angioplasty Research Team-safety of 6-month duration of Dual Antiplatelet Therapy after percutaneous coronary intervention in patients with acute coronary syndromes
SPECT: Single-photon emission computed tomography
SR: Sinus rhythm
STEEPLE: Safety and Efficacy of Intravenous Enoxaparin in Elective Percutaneous Coronary Intervention Randomised Evaluation
STEMI: ST-segment elevation myocardial infarction
STICH: Surgical Treatment for Ischemic Heart Failure
STICHES: STICH Extension Study
STS: Society of Thoracic Surgeons
SVG: Saphenous vein graft
SVR: Surgical ventricular reconstruction
SWEDEHEART: Swedish Web-system for Enhancement and Development of Evidence-based care in Heart disease Evaluated According to Recommended Therapies
SYNTAX: Synergy between Percutaneous Coronary Intervention with TAXUS and Cardiac Surgery
TAP: T and protrusion
TAVI: Transcatheter aortic valve implantation
TIA: Transient ischaemic attack
TIMI: Thrombolysis in Myocardial Infarction
TLR: Target lesion revascularization
TOTAL: Trial of Routine Aspiration Thrombectomy with PCI versus PCI Alone in Patients with STEMI
TRIGGER-PCI: Testing platelet Reactivity In patients underGoing elective stent placement on clopidogrel to Guide alternative thErapy with pRasugrel
TRITON-TIMI 38 TRial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet InhibitioN with Prasugrel-Thrombolysis In Myocardial Infarction
TROPICAL-ACS: Testing responsiveness to platelet inhibition on chronic antiplatelet treatment for acute coronary syndromes
TVR: Target vessel revascularization
TWILIGHT: Ticagrelor With Aspirin or Alone in High-Risk Patients After Coronary Intervention
UFH: Unfractionated heparin
VA: Veno-arterial
VACARDS: Veterans Affairs Coronary Artery Revascularization in Diabetes Study
VALIDATE: Bivalirudin versus Heparin in ST-Segment and Non-ST-Segment Elevation Myocardial Infarction in Patients on Modern Antiplatelet Therapy
VKA: Vitamin K antagonist
1 Preamble
Clinical practice guidelines summarize and evaluate all available evidence at the time of the writing process on a particular issue with the aim of assisting physicians in selecting the best management strategies for an individual patient with a given condition, taking into account the impact on outcome as well as the risk-benefit ratio of particular diagnostic or therapeutic means. Clinical practice guidelines are no substitutes for textbooks, but complement them, and cover the European Society of Cardiology (ESC) Core Curriculum topics. As such they should help physicians to make decisions in their daily practice. However, final decisions should be individualized by responsible physicians and the patient.
A great number of clinical practice guidelines have been issued in recent years both by the ESC as well as by other societies and organizations. Because of the impact on clinical practice, quality criteria for the development of guidelines have been established in order to make all decisions transparent to the user. The recommendations for formulating and issuing ESC and joint society guidelines can be found on the ESC website (https://www.escardio.org/Guidelines/Clinical-Practice-Guidelines/Guidelines-development/Writing-ESC-Guidelines). These Guidelines represent the official position of the ESC and the European Association for Cardio-Thoracic Surgery (EACTS) on this given topic and will be regularly updated.
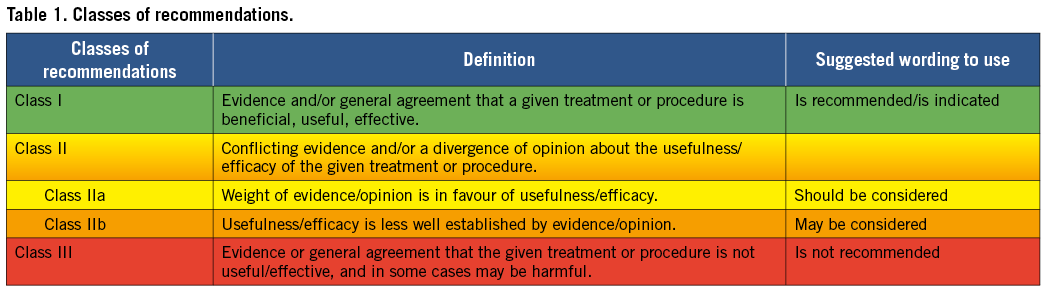
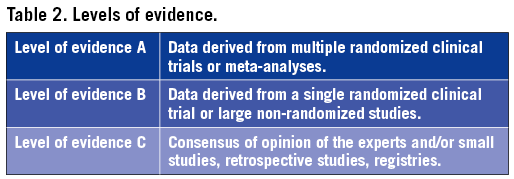
Members of this Task Force were selected by the ESC and EACTS to represent professionals involved with the medical care of patients with this pathology. Selected experts in the field undertook a comprehensive review of the published evidence for diagnosis, management (including treatment) and/or prevention of a given condition according to the ESC Committee for Practice Guidelines (CPG) and EACTS policy. A critical evaluation of diagnostic and therapeutic procedures was performed including assessment of the risk-benefit ratio. Estimates of expected health outcomes for larger populations were included, where data exist. The level of evidence and the strength of recommendation of particular treatment options were weighed and graded according to predefined scales, as outlined in Tables 1 and 2.
The experts of the writing and reviewing panels completed declarations of interest forms on what might be perceived as real or potential sources of conflicts of interest. These forms were compiled into one file and can be found on the ESC and EACTS websites http://www.escardio.org/guidelines and http://www.eacts.org). Any changes in declarations of interest that arise during the writing period must be notified to the ESC and EACTS and updated. The Task Force received its entire financial support from the ESC and EACTS without any involvement from the healthcare industry.
The CPG-ESC and EACTS supervised and coordinated the preparation of these new Guidelines produced by the joint Task Force. These entities are also responsible for the endorsement process of these Guidelines. The ESC/EACTS Guidelines underwent extensive review by a wide panel of relevant external experts. After appropriate revisions it was approved by all the experts involved in the Task Force. The finalized document was approved by the ESC CPG and EACTS for joint publication in the European Heart Journal and the European Journal of Cardio-Thoracic Surgery.
The task of developing clinical practice guidelines covers not only the integration of the most recent research, but also the creation of educational tools and implementation programmes for the recommendations. To implement the guidelines, condensed pocket guidelines, summary slides, booklets with essential messages, and an electronic version for digital applications (smartphones, etc.) are produced. These versions are abridged and, thus, if needed, one should always refer to the full text version, which is freely available on the ESC and EACTS websites. The National Societies of the ESC are encouraged to endorse, translate, and implement the ESC Guidelines. Implementation programmes are needed because it has been shown that the outcome of disease may be favourably influenced by the thorough application of clinical recommendations.
Surveys and registries are needed to verify that real-life daily practice is in keeping with what is recommended in the guidelines, thus completing the loop between clinical research, writing of guidelines, and implementing them in clinical practice.
The guidelines do not, however, override the individual responsibility of healthcare professionals to make appropriate decisions in the circumstances of the individual patients, in consultation with that patient, and where appropriate and necessary the patient’s guardian or carer. It is also the health professional’s responsibility to verify the rules and regulations applicable to drugs and devices at the time of prescription.
2 Introduction
These Guidelines represent the third time that the ESC and EACTS have brought together cardiologists and cardiac surgeons in a joint Task Force to review the ever-increasing body of evidence, with the mission of drafting balanced, patient-centred practice Guidelines on myocardial revascularization. Summaries of the key changes in comparison with the previous Guidelines are provided in Figures 1 and 2.
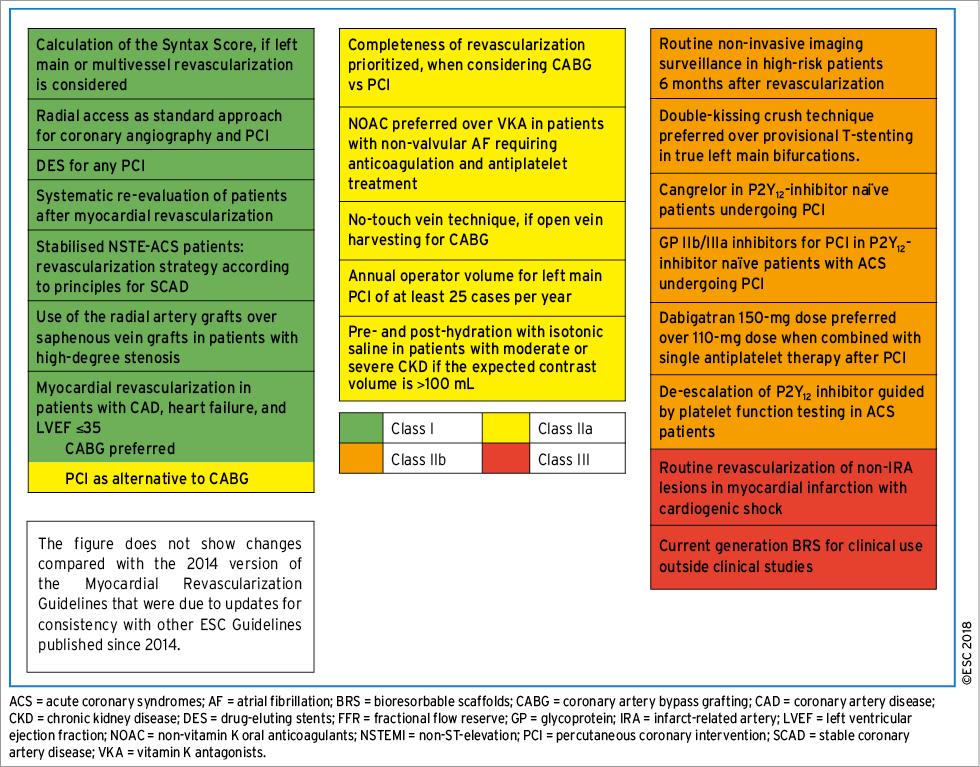
Figure 1. New recommendations.
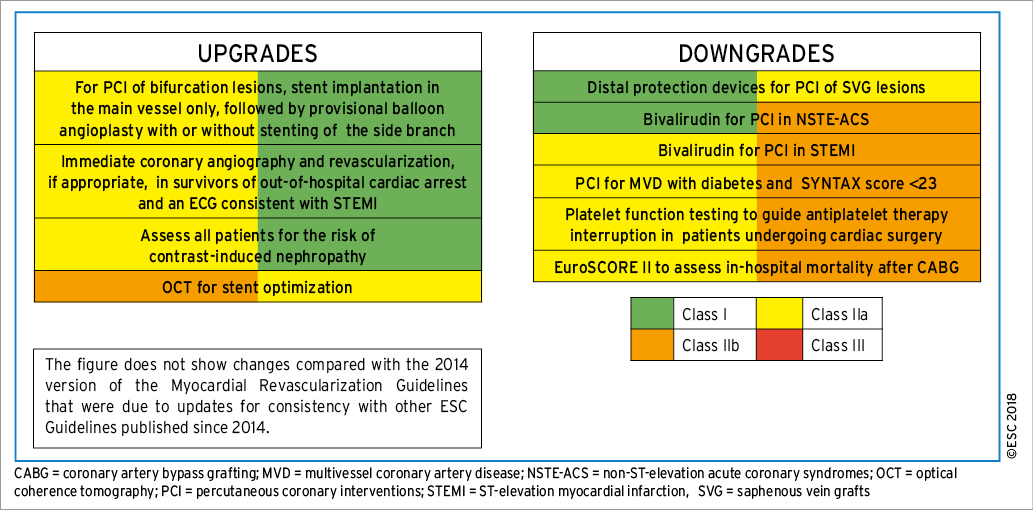
Figure 2. Changes in class of recommendation.
There is considerable overlap of the current document with other Guidelines, specifically those on stable coronary artery disease, non-ST-elevation myocardial infarction, ST-elevation myocardial infarction, heart failure, valvular heart disease and the Focused Update on Dual Antiplatelet Therapy. Unless supported by new evidence, we followed the recommendations of these Guidelines where pertinent to our Guidelines, and refer the reader to the respective sections in those documents for detailed discussion. We reserve more in-depth discussion for topics that are specific to issues pertaining to myocardial revascularization that are not covered in other Guidelines. To keep the current document concise and reader-friendly, we also moved some of the detailed descriptions of study results to the online Supplementary Data.
2.1 WHAT IS NEW IN THE 2018 GUIDELINES?
3 Diagnostic tools to guide myocardial revascularization
The use of diagnostic imaging and functional testing modalities to detect patients with coronary artery disease (CAD) is discussed in detail in the clinical practice Guidelines for patients with SCAD.1 Further diagnostic assessment of patients with obstructive CAD is critical in order to identify patients and select specific lesions that are likely to benefit from myocardial revascularization, in addition to optimal medical therapy.
3.1 NON-INVASIVE DIAGNOSTIC TOOLS
3.1.1 ASSESSMENT OF MYOCARDIAL ISCHAEMIA
Non-invasive diagnostic assessment of patients with CAD being considered for myocardial revascularization comprises the assessment of ischaemia and the evaluation of viability in patients with regional wall motion abnormalities or reduced ejection fraction (EF).
Functional testing to assess ischaemia is critical for the assessment of stable patients with CAD. Documentation of ischaemia using functional testing before elective invasive procedures for CAD is the preferred approach. It may also have a role in the assessment of some patients presenting with acute coronary syndrome (ACS). Because of the low sensitivity of exercise electrocardiogram (ECG) testing in the assessment of patients with symptoms of angina, non-invasive imaging is recommended as the first-line test.1 Detection of a large area of myocardial ischaemia by functional imaging is associated with impaired prognosis of patients and identifies patients who should undergo revascularization (see section 5).
In patients undergoing coronary computed tomography (CT), both CT-derived fractional flow reserve (CT-FFR) and CT perfusion represent possible approaches to evaluate lesion-specific ischaemia. Although the evidence for both is limited at present, there are considerably more data from clinical investigations of CT-FFR. A number of trials have shown that correlation between CT-derived FFR and invasive FFR is high.2,3 The non-randomized PLATFORM (Prospective LongitudinAl Trial of FFRct: Outcome and Resource Impacts) study showed that in patients referred for invasive angiography due to chest pain (predominantly atypical angina) and intermediate pre-test probability of CAD, assessment with CT and CT-FFR reduced the number of patients with subsequently normal invasive coronary angiograms compared with standard care.4 Currently, clinical trial data with CT-FFR are insufficient to make a recommendation for its use in clinical practice.
3.1.2 ASSESSMENT OF MYOCARDIAL VIABILITY IN PATIENTS WITH HEART FAILURE AND CORONARY ARTERY DISEASE
In patients with regional wall motion abnormalities or ventricular dysfunction, heart failure (HF) can be caused by stunned or hibernating myocardium and may be reversed by revascularization. Assessment of myocardial viability may be done in order to select patients that are more likely to benefit from myocardial revascularization and can be achieved with several imaging modalities: myocardial contrast echocardiography, single-photon emission CT (SPECT), and late gadolinium enhancement cardiac magnetic resonance (LGE-CMR) all assess cellular integrity; positron emission tomography (PET) assesses cellular metabolism; and dobutamine techniques assess contractile reserve.1,5 Assessment of ischaemia provides incremental benefit over viability in mild to moderate CAD, but with extensive CAD viability assessment may be sufficient.6 Patients with advanced HF and viable myocardium should first undergo revascularization with coronary artery bypass grafting (CABG) or percutaneous coronary intervention (PCI) before being considered for mechanical circulatory support (MCS) or heart transplantation.7,8
The PARR-2 (PET and Recovery following Revascularization) trial included patients with severe left ventricular (LV) dysfunction being considered for revascularization or HF/transplantation workups, and randomized them to management assisted by fluorodeoxyglucose PET (FDG-PET) or standard care.6 The primary outcome of cardiac death, myocardial infarction (MI), or recurrent hospital stay for cardiac cause at 1 year was not improved in the group managed by FDG-PET [relative risk (RR) 0.82, 95% confidence interval (CI) 0.59-1.14, P=0.16], though the rate of compliance with the treatment recommended by FDG-PET was variable.
The viability substudy of the STICH (Surgical Treatment for Ischemic Heart Failure) trial found viable myocardium in 487/601 patients (81%) and none in 114 (19%).9 There was a significant association between myocardial viability and outcome by univariate analysis, but not on multivariable analysis. The lack of correlation between myocardial viability and benefit from revascularization indicates that this strategy should not be the only test when selecting the optimal therapy.
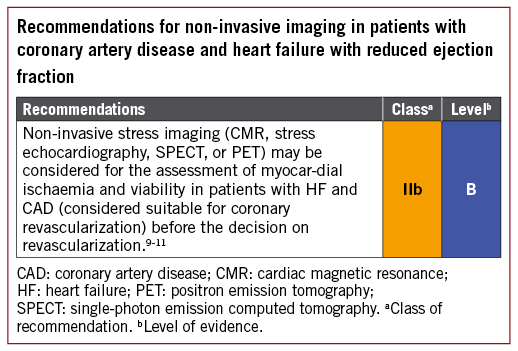
3.2 INVASIVE DIAGNOSTIC TOOLS
3.2.1 PRESSURE-DERIVED FRACTIONAL FLOW RESERVE
3.2.1.1 Use of fractional flow reserve in patients with intermediate-grade coronary stenosis including left main stenosis
Coronary pressure-derived FFR is the current standard of care for the functional assessment of lesion severity in patients with intermediate-grade stenosis (typically around 40-90% stenosis) without evidence of ischaemia in non-invasive testing, or in those with multivessel disease.
Multiple studies have shown that PCI can be safely deferred if FFR is >0.75.12-15 The DEFER trial enrolled 325 patients scheduled for PCI of an intermediate stenosis.15 If FFR was ≥0.75, patients were randomly assigned to deferral (defer group; n=91) or performance (perform group; n=90) of PCI. The composite rate of cardiac death and acute MI (AMI) in the defer and perform groups was 3.3 vs. 7.9% (P=0.21).
However, most contemporary studies use an FFR cut-off of 0.80. A recent large-scale observational study supports the use of FFR >0.80 rather than 0.75 as a cut-off.16 Indeed, the two largest studies in this field, DEFINE-FLAIR (Define Functional Lesion Assessment of Intermediate Stenosis to Guide Revascularization DES drug-eluting stent)17 and iFR-SWEDEHEART (Swedish Web-system for Enhancement and Development of Evidence-based care in Heart disease Evaluated According to Recommended Therapies),18 used the 0.80 cut-off for lesion selection by FFR, with favourable event rates at 1 year. Thus, 0.80 is the accepted FFR threshold for defining haemodynamically relevant lesions.
Haemodynamic relevance, as defined by FFR ≤ 0.80, correlates poorly with diameter stenosis by visual assessment. In the FAME (Fractional Flow Reserve versus Angiography for Multivessel Evaluation) trial, only 35% of the 50-70% stenoses were haemodynamically relevant and, of the 71-90% stenoses, 20% were not. Only an estimated diameter stenosis >90% predicted haemodynamic relevance with high accuracy (96% correct classification). A number of studies have shown that utilization of an FFR-based assessment strategy at the time of angiography results in reclassification of the revascularization strategy (PCI, bypass surgery, or medical therapy) in a high proportion of patients with intermediate-grade lesions (>40% of patients are reclassified).19-22 In addition, separate and pooled analyses of the patients included in those studies have shown that the end results of `FFR-based reclassification’ in patients investigated at the time of diagnostic angiography is neutral overall for the number of patients indicated for revascularization.23
A patient-level and study-level meta-analysis of 9173 lesions demonstrated that with lesions with FFR <0.75, revascularization reduced the 1 year risk of major adverse cardiac events (MACE), including a reduction in the composite risk of death and MI.24 Thus, the FFR threshold of 0.75 is used to define more severe ischaemia that is of prognostic relevance.
The presence of intermediate grade left main stem (LMS) disease is not infrequent and angiographic evaluation may be challenging. Assessment using pressure-derived FFR is more challenging in comparison with non-LMS stenosis due to the requirement for disengagement of the guiding catheter and an inability to administer intracoronary adenosine. Some observational data exist to support the use of FFR in order to decide if revascularization should be deferred or performed.25 In the largest study, which included 230 patients with intermediate-grade LMS stenosis, only 23% showed an FFR <0.80. Treatment was deferred in patients with an FFR ≥0.80 and bypass surgery was done in patients with an FFR <0.80.26 Clinical outcomes at 5 years were similar in both groups. However, it is important to consider the potential influence of any untreated downstream disease in the left anterior descending (LAD) or left circumflex arteries, which may be associated with an increased risk of a false negative FFR.27
The value of FFR to evaluate intermediate stenosis and guide selection of lesions for revascularization at the time of bypass surgery has been shown in an observational study.28 Of the 627 patients with intermediate stenosis that were evaluated, 429 had bypass without FFR and 198 had bypass with FFR; in the latter group, the proportion of patients with three-vessel disease was reclassified from 94 to 86%. Outcomes were similar in both groups at 3 years [hazard ratio (HR) for death/MI/target vessel revascularization (TVR)=1.03, 95% CI 0.67-1.69], though the group with FFR guidance was associated with a lower number of graft anastomoses and a lower rate of on-pump surgery compared with angiography-guided CABG surgery.
3.2.1.2 Use of fractional flow reserve to identify lesions requiring revascularization in patients with multivessel coronary artery disease undergoing percutaneous coronary intervention
FFR may also be useful for the selection of lesions requiring revascularization in patients with multivessel CAD. The FAME trial showed that in patients with multivessel disease randomized to an FFR-guided PCI strategy (using a cut-off ≤0.80 to indicate requirement for PCI), outcomes at 12 months in terms of death, non-fatal MI, and repeat revascularization were superior compared with angiography-guided PCI and utilized fewer resources.29 In addition, the 2 year composite risk of death or MI was significantly lower with the FFR-guided PCI strategy.30 Long-term follow-up at 5 years showed broadly consistent findings, although differences between groups in relation to the primary endpoint were no longer significant.31 This suggests that FFR-guided PCI should be the preferred management strategy in these patients.
3.2.1.3 Fractional flow reserve-guided management vs. medical therapy in patients with coronary artery disease
In patients with SCAD and at least one stenosis with FFR ≤0.80, the FAME 2 trial showed that PCI using drug-eluting stent (DES) implantation improved the primary endpoint of death, non-fatal MI, or urgent revascularization within 2 years compared with medical treatment alone, which was driven by a lower need for urgent revascularization.32 The advantage of FFR-guided PCI over medical therapy alone was maintained at 3 years.33
3.2.2 OTHER PRESSURE-DERIVED INDICES
FFR evaluation requires maximal and stable hyperaemia, which is usually obtained by the administration of intravenous (i.v.) adenosine. Recently, there has been renewed interest in resting indices derived from resting gradients alone [distal coronary to aortic pressure (Pd/Pa) or instantaneous wave-free ratio (iwFR)]. Two recent large-scale randomized trials showed broadly comparable results between FFR-guided and iwFR-guided revascularization strategies in patients with intermediate-grade stenosis.17,18 Revascularization was indicated in both trials if FFR was ≤0.80 or if iwFR was ≤0.89. In the DEFINE-FLAIR trial, the primary endpoint of MACE at 1 year occurred in 6.8% in patients randomized to iwFR-guided revascularization vs. 7.0% in patients randomized to FFR-guided revascularization (P <0.001 for non-inferiority; HR 0.95, 95% CI 0.68-1.33, P=0.78).17 In the iFR-SWEDEHEART trial, the primary endpoint of death from any cause, non-fatal MI, or unplanned revascularization was 6.7% in the iwFR group and 6.1% in the FFR group (P=0.007 for non-inferiority; HR 1.12, 95% CI 0.79-1.58, P=0.53).18 In this trial, 17.5% of patients had ACS at the time of presentation. There was no interaction with outcomes. Both trials are limited by having a follow-up duration of only 1 year.
The SYNTAX II study (Synergy between Percutaneous Coronary Intervention with TAXUS and Cardiac Surgery), a single-arm, prospective study in patients with multivessel disease incorporating a management strategy including combined iwFR/FFR assessment of stenosis severity in addition to intravascular ultrasound (IVUS)-guided stent implantation and guideline-directed medical therapy, showed encouraging outcomes compared with a historical cohort enrolled in the SYNTAX trial.34
Randomized trials comparing iwFR-guided revascularization with angiography-guided revascularization or medical therapy are not available. iwFR has not been extensively validated for patients with LMS stenosis.
There is no adequate randomized controlled trial (RCT) data to support the use of whole-cardiac cycle Pd/Pa for the guidance of revascularization decisions.
3.2.3 USE OF FRACTIONAL FLOW RESERVE AND PRESSURE-DERIVED INDICES IN PATIENTS WITH SEVERE AORTIC STENOSIS
In patients with intermediate coronary stenosis and concomitant severe aortic stenosis, although some observational studies exist (see section 11), there are no adequate RCT data to support the use of FFR or iwFR for the guidance of revascularization decisions.
3.2.4 USE OF INTRAVASCULAR IMAGING FOR THE DIAGNOSTIC ASSESSMENT OF STENOSIS
IVUS is an ultrasound-based modality of intravascular imaging with an axial resolution of about 150 mm. IVUS imaging allows real-time tomographic assessment of vessel size, lumen area, and plaque composition and volume. In comparison with optical coherence tomography (OCT), it has more limited spatial resolution, but better penetration depth and potential advantages in terms of vessel sizing. OCT is a light-based modality of intravascular imaging with higher axial resolution compared with IVUS (15 vs. 150 mm). The disadvantages of OCT imaging are that it requires complete blood clearance from the lumen for imaging and that it has more limited penetration, which can limit the assessment of complete plaque burden and may impair accurate vessel sizing.
Potential clinical uses of intravascular imaging for diagnostic assessment in patients being considered for myocardial revascularization are the evaluation of stenosis severity in lesions with intermediate-grade stenosis, evaluation of lesion morphology in lesions ambiguous with angiographic assessment, and the characterization of plaque composition. The majority of the existing data from clinical trials relate to the use of intravascular imaging guidance during PCI and are discussed in section 16. The use of intravascular imaging to evaluate patients with stent failure is discussed in section 13.
Regarding the assessment of intermediate-grade stenosis, a number of studies have evaluated the optimal cut-off of minimal lumen area for the identification of haemodynamically relevant lesions. One prospective registry showed overall moderate correlation of minimal lumen area with FFR values, with cut-off values for detecting haemodynamically relevant stenosis (<2.4, <2.7, and <3.6 mm2) dependent on vessel size (reference vessel diameters <3.0, 3.0-3.5, and >3.5 mm, respectively).34a Generally, haemodynamic assessment with FFR should be preferred for this indication.
The presence of intermediate-grade LMS disease is not infrequent and angiographic assessment may be challenging. Assessment using IVUS evaluation of intermediate-grade LMS disease in patients being considered for bypass surgery or PCI is supported by data from a number of observational studies.35-38 In a multicentre, prospective study, revascularization was mainly deferred if the minimal luminal area (MLA) was ≥6 mm2 and performed in cases of an MLA <6 mm2.37 After a 2 year follow-up, cardiac death-free survival was similar in both groups (98 and 95%, respectively). Another study suggested that deferral of intervention in 131 patients with an MLA ≥7.5 mm2 showed favourable clinical outcomes.36 In Asian patients with generally smaller heart sizes, studies have suggested that an IVUS MLA of 4.5-4.8 mm2 may be the most appropriate.38

3.3 GAPS IN THE EVIDENCE
Further studies investigating the role of novel, combined, non-invasive anatomical and functional imaging are needed, such as randomized clinical trials with CT-FFR in patients with suspected and known CAD, as well as further clinical investigation of perfusion CT.
Randomized trials comparing iwFR-based management of patients with intermediate-grade stenosis compared with medical therapy are missing. Further study of whole-cardiac cycle Pd/Pa for the guidance of revascularization in the setting of randomized clinical trials is also required.
Further studies including randomized trials are needed to assess the value of functional vs. anatomical guidance for CABG.
4 Process for decision-making and patient information
4.1 PATIENT INFORMATION AND INFORMED CONSENT
Informed consent requires transparency, especially if there is debate over various treatment options. Active patient participation in the decision-making process should be encouraged. Patient information needs to be unbiased, evidence-based, up-to-date, reliable, accessible, relevant, and consistent with legal requirements. Use of terminology that the patient understands is essential. Short-term procedure-related and long-term risks and benefits – such as survival, relief of angina, quality of life, the potential need for late reintervention, the need for prevention measures, and uncertainties associated with different treatment strategies – should be thoroughly discussed. Although current recommendations are mostly based on the ability of treatments to reduce adverse events including mortality, there is growing interest in patient-reported outcome measures.40,41 Patients are not only interested to know how recommended treatment impacts on prognosis but also on their quality of life in the way they perceive it. A written evidence-based patient information document should be provided, potentially with decision aids.
Patients must have the time to reflect on the trade-offs imposed by the outcome estimates. In order to seek a second opinion or to discuss the findings and consequences with referring physicians, enough time should be allowed – up to several days, as required – between diagnostic catheterization and intervention. These recommendations pertain to patients in a stable condition, for whom various treatment options exist and who can make a decision without the constraints of an urgent or emergent situation (Table 3). The patient’s right to decline the treatment option recommended by the Heart Team has to be respected. Patient refusal of a recommended treatment should be acknowledged in a written document after the patient has received the necessary information by the Heart Team members. In this case, the patient may be offered an alternative treatment option by the Heart Team.
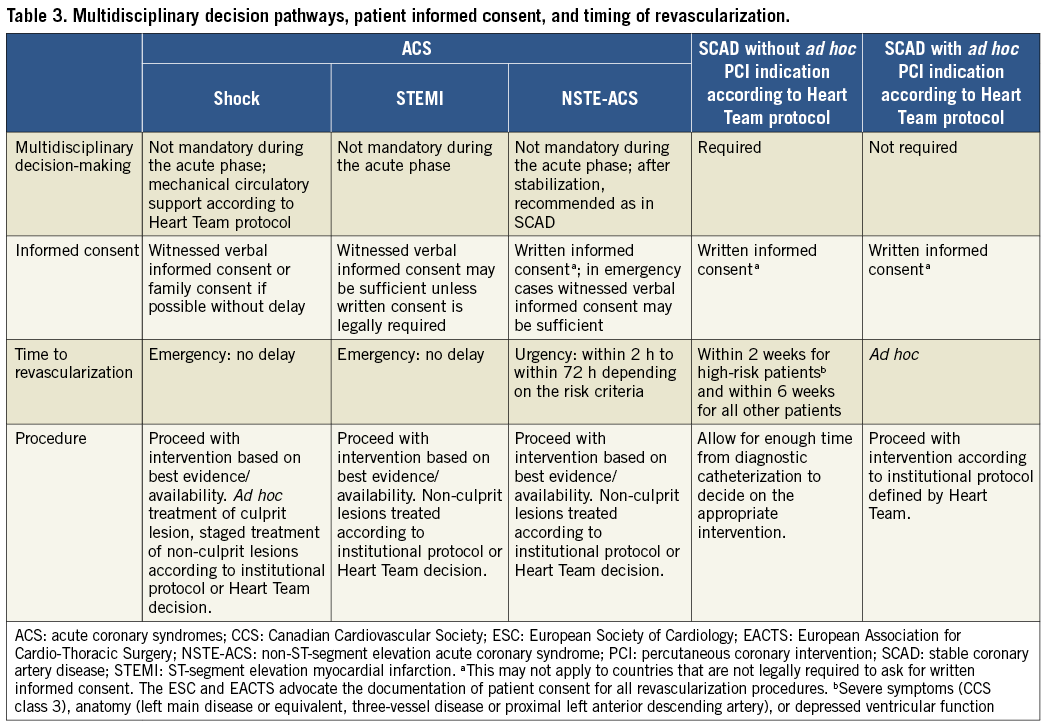
The patient has the right to obtain information on the level of expertise of the operator, the workload of the centre, whether all treatment options – including surgery – are available on-site, and local results in the performance of percutaneous and surgical myocardial revascularization procedures. Patients considered for revascularization should also be clearly informed of the continuing need for medical therapy, as well as lifestyle modification and other secondary prevention strategies (see section 19).42
4.2 MULTIDISCIPLINARY DECISION-MAKING (HEART TEAM)
The Heart Team – comprising clinical or non-invasive cardiologists, cardiac surgeons, and interventional cardiologists, as well as anaesthetists and other specialists if deemed necessary – should provide a balanced, multidisciplinary decision-making process.43 Additional input may be needed from other specialties involved in the care of the patient. The Heart Team should meet on a regular basis to analyse and interpret the available diagnostic evidence, determine the need for myocardial revascularization, and assess the relative short- and long-term safety and effectiveness of the percutaneous and surgical options. Ad hoc meetings of the Heart Team should facilitate and support efficient clinical workflows.
The need for an interdisciplinary approach is underlined by reports on (i) the underuse of revascularization procedures in 18-40% of patients with CAD44 and (ii) inappropriate use of revascularization strategies with a lack of case discussions.45 The marked variability in PCI-to-CABG ratios between European countries (ranging from 2.4-7.6 in 2013, for example) has raised concerns regarding the appropriate selection of revascularization strategies.46 Rates for the inappropriate use of PCI (10-15%)43,47,48 and CABG (1-2%) are reported. Multidisciplinary decision-making in a Heart Team can minimize specialty bias and prevent self-referral from interfering with optimal patient care.49
Several reports from different centres have established that the treatment recommendations made in multidisciplinary Heart Team discussions are reproducible and implemented in the vast majority of cases (93-95%).50,51
Interdisciplinary institutional protocols should be developed for common case scenarios to avoid the need for systematic case-by-case review of all diagnostic angiograms. However, complex cases – defined by the protocols – should be discussed individually. In these cases, revascularization should not be performed at the time of diagnostic angiography, to allow sufficient time to assess all available information and clearly explain and discuss the findings with the patient. The rationale for a decision and consensus on the optimal revascularization treatment should be documented on the patient’s chart. In institutions without an on-site cardiac surgery unit, collaboration with an external cardiac surgery unit is required to design protocols that define when Heart Team discussion is needed.
4.3 TIMING OF REVASCULARIZATION
Patients requiring myocardial revascularization may be at increased risk of adverse events during the waiting period.52 A recent meta-analysis of observational studies calculated that a waiting period of 3 months for surgical myocardial revascularization may be associated with the risk of 1 death among 80 patients.53 Table 3 shows the preferred timing of revascularization depending on the clinical presentation and the extent and localization of CAD.54 Sections 7 and 8 show additional and more specific information in this regard for patients with ACS.
Ad hoc PCI is defined as a therapeutic intervention performed within the same procedure as the diagnostic coronary angiography. Ad hoc PCI is convenient, often cost-effective and safe, and is associated with fewer access site complications and lower radiation exposure.55,56 However, in the USA, up to 30% of patients undergoing ad hoc PCI are potential candidates for CABG.56 This number may be lower in Europe.45 Although it is not advisable for ad hoc PCI to represent the default approach for complex SCAD, it may be justified if a full diagnostic work-up, including functional testing, is available and the patient is adequately informed on both percutaneous and surgical myocardial revascularization options (see section 4.1). Institutional protocols developed by the Heart Team in accordance with current Guidelines should define specific anatomical criteria and clinical subsets that may be – or should not be – treated ad hoc. Stable patients with complex CAD, as reflected by a high SYNTAX score, should in general be discussed by the Heart Team and not be treated ad hoc.

5 Revascularization for stable coronary artery disease
5.1 RATIONALE FOR REVASCULARIZATION
The indications for revascularization in patients with SCAD who receive guideline-recommended medical treatment are the persistence of symptoms despite medical treatment and/or the improvement of prognosis.1
Several studies have shown that myocardial revascularization by PCI or CABG more effectively relieves angina, reduces the use of anti-anginal drugs, and improves exercise capacity and quality of life compared with a strategy of medical therapy alone during short- and long-term follow-up (Supplementary Table 1).32,33,57-62 Recently, the ORBITA (Objective Randomised Blinded Investigation with optimal medical Therapy of Angioplasty in stable angina) trial randomly compared PCI with placebo (sham procedure) in patients with SCAD due to single-vessel CAD (diameter stenosis >70%) and preserved LV function in the presence of moderate symptoms of angina [Canadian Cardiovascular Society (CCS) class II in 59% of patients, duration 9 months] for the first time.63 After 6 weeks of medication optimization (mean number of anti-anginal drugs: 3) and baseline cardiopulmonary exercise testing, 200 patients were randomized (105 PCI and 95 placebo). Following a 6-week post-randomization period, the primary endpoint of increment in exercise time was not significantly different, but estimates were imprecise (PCI minus placebo 16.6 sec, 95% CI –8.9 to 42.0, P=0.20). The dobutamine stress echocardiography peak stress wall motion score index improved with PCI (–0.09, 95% CI –0.15 to –0.04, P=0.001). ORBITA raises the issue of whether the symptom relief of PCI in the specific setting of stable single-vessel CAD may be related at least in part to a placebo effect. Limitations of the study, as acknowledged by the investigators and outlined elsewhere, include the short observation period (6 weeks), the inclusion of patients with mild symptoms pre-randomization (CCS class 0-I in 25% of patients), the group imbalance in ostial and proximal lesions (37 vs. 57%, P=0.005), loss to follow-up after randomization, and the insufficient power to detect a true difference.64 This precludes definite conclusions at this stage. Nevertheless, the ORBITA study underlines the value of optimal medical therapy in the management of SCAD.
Three year follow-up of the FAME 2 study indicated yearly and sustained improvement of angina (10.2 vs. 28.5% at 1 month and 5.2 vs. 9.7% at 3 years) in favour of FFR-guided PCI, despite considerable crossover in the medical therapy arm.33 Among patients with multivessel disease, the assessment of frequency of angina and quality of life measures in the SYNTAX, FREEDOM (Future Revascularization Evaluation in Patients with Diabetes Mellitus), and EXCEL (Evaluation of XIENCE Versus Coronary Artery Bypass Surgery for Effectiveness of Left Main Revascularization) trials consistently showed early and sustained improvement for both PCI and CABG during long-term follow-up.65-67
5.2 EVIDENCE BASIS FOR REVASCULARIZATION
The indications for revascularization in patients with stable angina or silent ischaemia are summarized in the recommendation table.
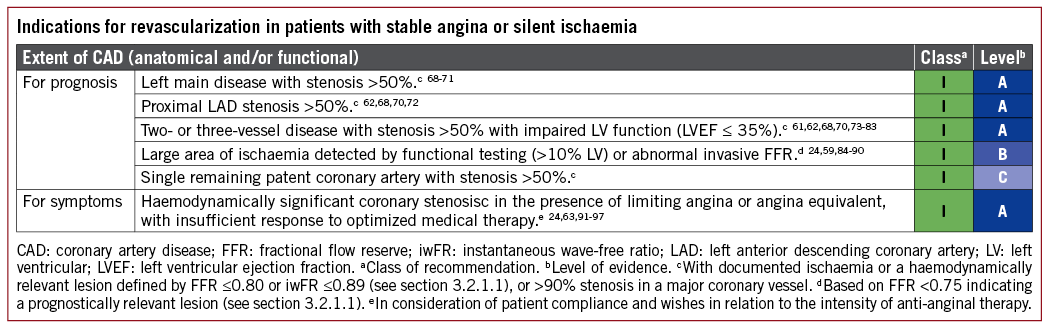
5.2.1 REVASCULARIZATION WITH THE USE OF PERCUTANEOUS CORONARY INTERVENTION
Several meta-analyses comparing a strategy of PCI with initial medical therapy among patients with SCAD found no or only modest benefits in terms of survival or MI for an invasive strategy, taking into account the fact that up to 40% of patients crossed over after to revascularization during longer-term follow-up.91,98,99 A network meta-analysis of 100 trials with 93553 patients and 262090 patient-years of follow-up comparing a strategy of initial medical therapy with revascularization reported improved survival using PCI with new-generation DES (everolimus: rate ratio 0.75, 95% CI 0.59-0.96; zotarolimus: rate ratio 0.65, 95% CI 0.42-1.00) compared with initial medical treatment.100
In the FAME 2 trial,32 patients with SCAD and at least one functionally significant stenosis (invasive FFR ≤0.80) were randomly assigned to medical therapy or medical therapy plus FFR-guided PCI using new-generation DES. The 3 year report of the FAME 2 trial reported a lower incidence of the primary composite endpoints of death, MI, and urgent revascularization (10.1 vs. 22.0%; P <0.001), driven by a lower incidence of urgent revascularization in the PCI group (4.3 vs. 17.2%; P <0.001) and without significant differences in the rates of death and MI.33 At 2 years of follow-up, the rate of death or MI was lower in the PCI than the medical therapy group (4.6 vs. 8.0%; HR 0.56, 95% CI 0.32-0.97, P=0.04) in a landmark analysis between 8 days and 2 years of follow-up, whereas event rates were higher during days 0-7 due to periprocedural MI (for overview of studies see Supplementary Table 2).97
5.2.2 REVASCULARIZATION WITH THE USE OF CORONARY ARTERY BYPASS GRAFTING
The superiority of CABG over a strategy of initial medical therapy was established in a meta-analysis of seven RCTs68 more than two decades ago, demonstrating a survival benefit of CABG in patients with SCAD and left main (LM) or three-vessel disease, particularly when the proximal LAD coronary artery was involved, and has been corroborated in more recent studies.100,101 A network meta-analysis of 100 trials with 93553 patients comparing a strategy of initial medical therapy with revascularization reported improved survival (RR 0.80, 95% CI 0.63-0.99) and a reduced risk of MI (RR 0.79, 95% CI 0.83-0.99) among patients undergoing CABG compared with initial medical treatment.100
In the STICH trial, 1212 patients with CAD and an LV ejection fraction (LVEF) ≤35% were randomized to initial medical therapy or CABG. The extended 10 year follow-up of the STICH trial reported a significant reduction in all-cause (59 vs. 66%; HR 0.84, 95% CI 0.73-0.97; P=0.02) and cardiovascular mortality (41 vs. 49%; HR 0.79, 95% CI 0.66-0.93; P=0.006).81 For an overview of studies, see Supplementary Table 2.
5.3 PERCUTANEOUS CORONARY INTERVENTION VS. CORONARY ARTERY BYPASS GRAFTING
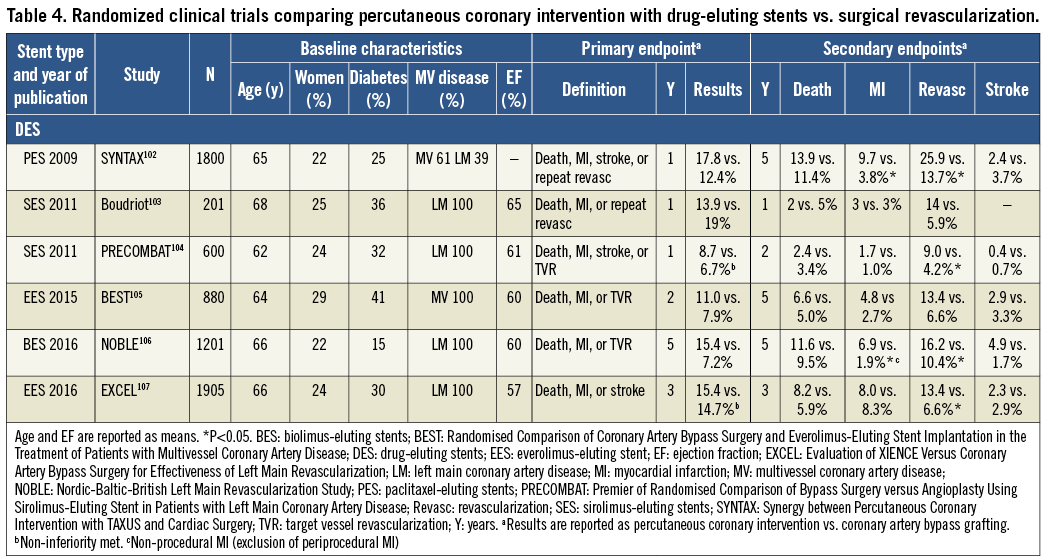
The recommendations for the type of revascularization (CABG or PCI) in patients with SCAD with suitable coronary anatomy for both procedures and low predicted surgical mortality are summarized below. The Heart Team should take into consideration the individual cardiac and extracardiac characteristics, in addition to patient preference, in the overall decision-making process (Figure 3). A summary of trials comparing the outcomes of patients treated with angioplasty vs. CABG and bare-metal stent (BMS) vs. CABG is shown in Supplementary Table 3, and of studies comparing DES and CABG in Table 4.
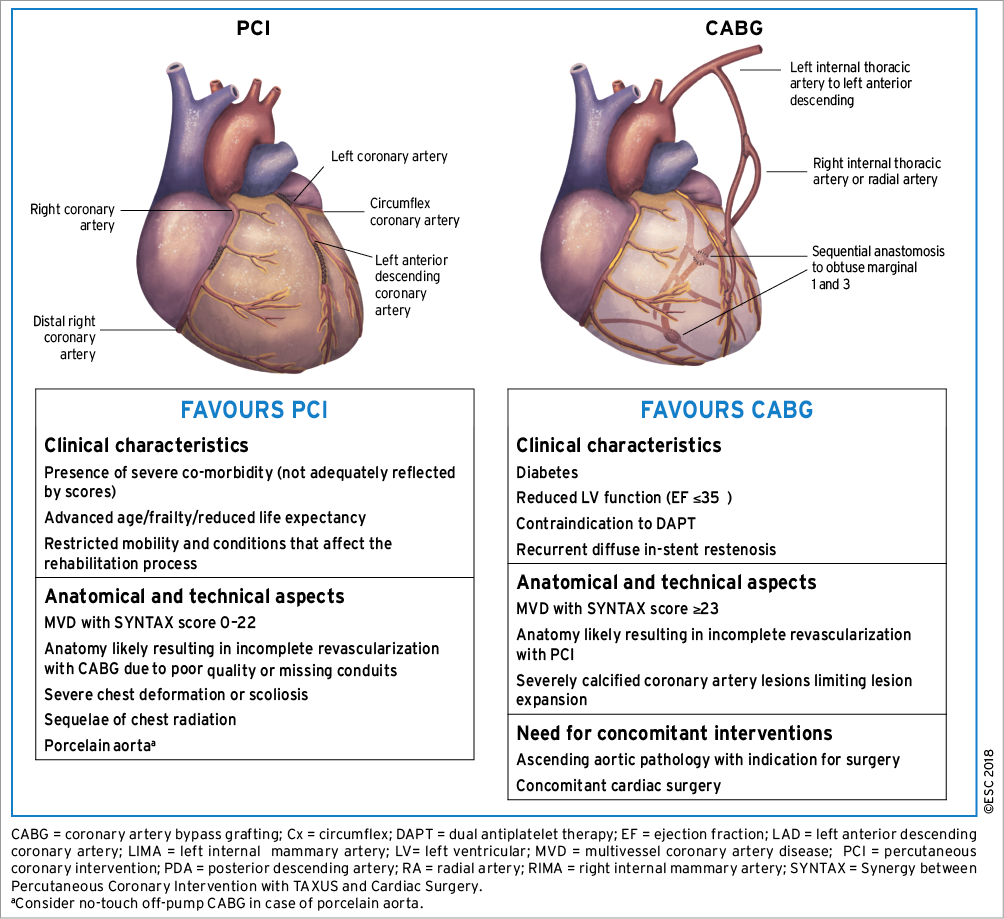
Figure 3. Aspects to be considered by the Heart Team for decision-making between percutaneous coronary intervention and coronary artery bypass grafting among patients with stable multivessel and/or left main coronary artery disease.
5.3.1 CRITERIA FOR DECISION-MAKING
Predicted surgical mortality, the anatomical complexity of CAD, and the anticipated completeness of revascularization are important criteria for decision-making with respect to the type of revascularization (CABG or PCI). Whether conservative therapy, PCI, or CABG is preferred should depend on the risk-benefit ratios of these treatment strategies, weighing up the risks of periprocedural complications (e.g. cerebrovascular events, blood transfusions, renal failure, new onset arrhythmias, or wound infections) against improvements in health-related quality of life, as well as long-term freedom from death, MI, or repeat revascularization.
5.3.1.1 Predicted surgical mortality
To assess the predicted surgical mortality, the European System for Cardiac Operative Risk Evaluation (EuroSCORE II) (www.euroscore.org/calc.html) and the Society of Thoracic Surgeons (STS) score (http://riskcalc.sts.org) were both developed based on clinical variables to estimate the operative in-hospital or 30 day mortality risk108-110 (Supplementary Table 4). Both scores have demonstrated their value in specific cohorts of patients undergoing CABG.111 Calibration of the STS score is updated on a regular basis. It has been suggested that the STS score outperforms the EuroSCORE II when compared directly in a cohort of CABG patients,112 although other studies have found comparable performance of both models.113,114
There are no established cut-offs for low predicted surgical mortality based on the EuroSCORE II or STS score. Thus, individualized treatment decisions are needed. These decisions should respect the range of predicted surgical risks in the major RCTs that inform the choice of revascularization modality (Table 5). In these studies, the predicted surgical risk was assessed by the logistic EuroSCORE. Compared with the more recent EuroSCORE II, the logistic EuroSCORE has similar discrimination but poorer calibration and, thus, overestimates surgical mortality by roughly two-fold.115
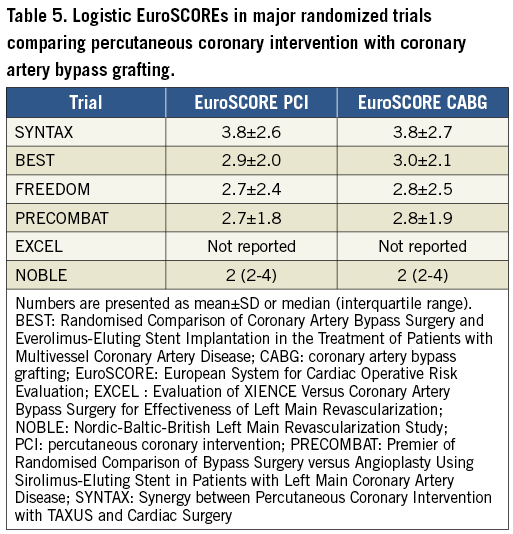
Despite the usefulness of these scores, there is not a single risk model that provides perfect risk assessment because the scores are limited by (i) the specific definitions used or the methodology applied, (ii) the absence of important variables such as frailty, (iii) the practicability of calculation, (iv) a failure to reflect all relevant mortality and morbidity endpoints, and (v) limited external validation. Decision-making should not be solely dependent on risk scores. These scores should be used as a guide within the multidisciplinary Heart Team discussion.
To combine clinical and anatomical risk estimation, the SYNTAX II score was retrospectively derived from the SYNTAX cohort127 and subsequently externally validated.120,128,129 Nevertheless, compared with the SYNTAX score, its value in assigning patients to PCI or CABG is less well investigated. The fact that the SYNTAX II score failed to predict the outcome of the EXCEL trial raises additional concern.130
5.3.1.2 Anatomical complexity of coronary artery disease
The SYNTAX score (http://www.syntaxscore.com) was prospectively developed for the SYNTAX trial to grade the anatomical complexity of coronary lesions in patients with LM or three-vessel disease (Table 6 and Supplementary Table 4).116 In the cohort of the SYNTAX trial, and subsequently in external validation cohorts, the SYNTAX score was found to be an independent predictor of long-term major adverse cardiac and cerebrovascular events (MACCE) and of death in patients treated with PCI but not CABG.117-120
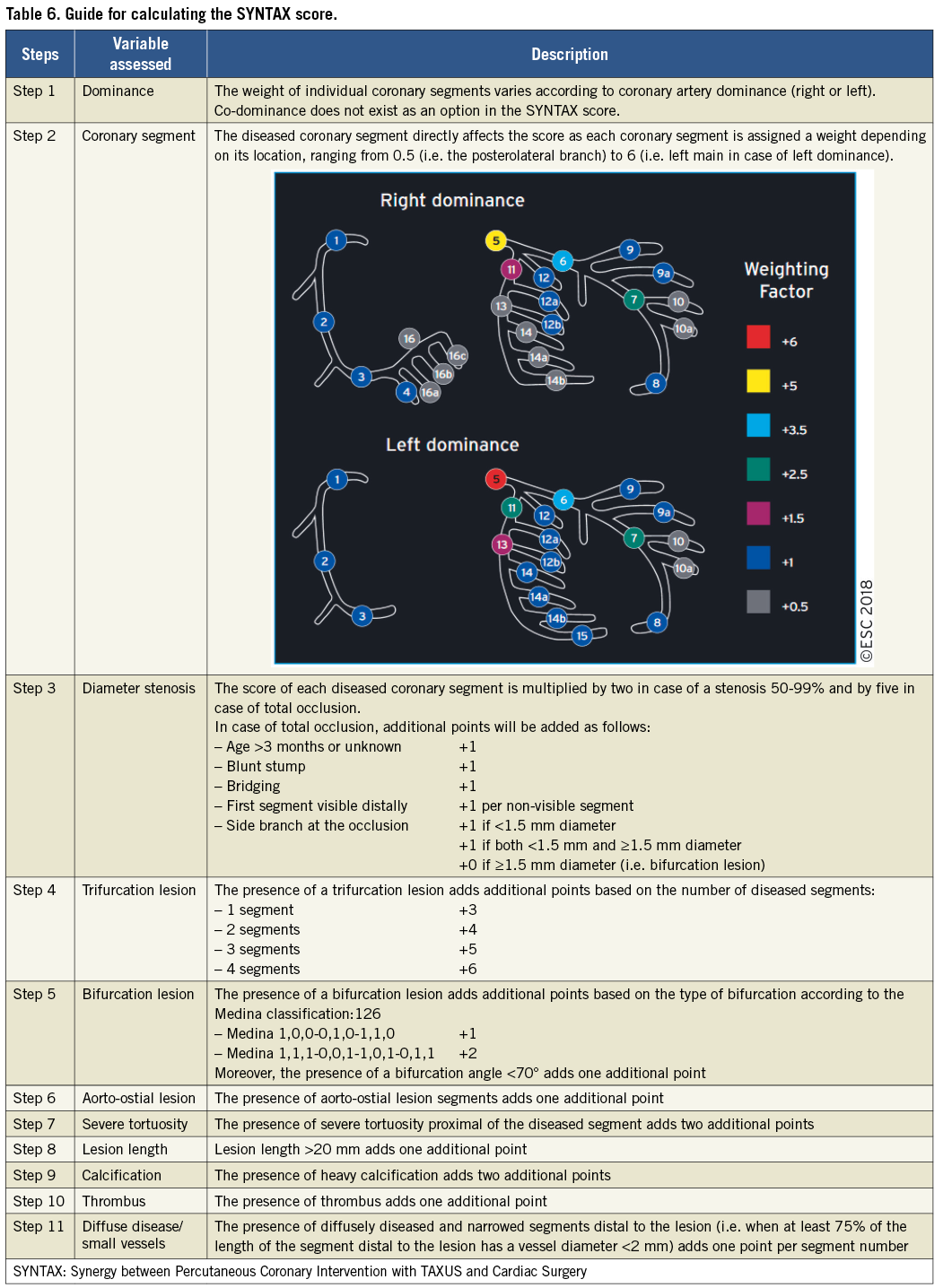
In the SYNTAX trial, tertiles of SYNTAX score with low, intermediate, and high anatomical complexity stratified patients into those who had similar outcomes with both PCI and CABG and those who derived significant benefit from CABG.121-123 In subsequent RCTs, the interaction of the strata of SYNTAX score with the effect of the randomized treatment was less pronounced and did not reach statistical significance.105-107 However, in a recent collaborative individual patient pooled analysis of randomized trials including 11518 patients,124 the test for trend across the ordered tertiles of the SYNTAX score of the SYNTAX study was positive at P=0.0011 (unpublished analysis), confirming the strata of the SYNTAX score as an effect modifier to be considered. There is concern about bias and inter-individual variability in calculating the SYNTAX score.125 This should be minimized by adequate training.
5.3.1.3 Completeness of revascularization
The aim of myocardial revascularization is to minimize residual ischaemia. In support of this concept, the nuclear substudy of the COURAGE (Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation) trial demonstrated an incremental benefit in reducing the risk of death and MI by reducing residual stress-induced ischaemia from >10% of the myocardium to ≤5%.86
In the SYNTAX trial, anatomical complete revascularization was defined as PCI or bypass of all epicardial vessels with a diameter exceeding ≥1.5 mm and a luminal reduction of ≥50% in at least one angiographic view.131 A meta-analysis of 89883 patients enrolled in RCTs and observational studies revealed a lower long-term mortality (RR 0.71, 95% CI 0.65-0.77, P <0.001), MI (RR 0.78, 95% CI 0.68-0.90; P=0.001), and repeat myocardial revascularization (RR 0.74, 95% CI 0.65-0.83; P <0.001) by complete revascularization (based on anatomical definition in 87% of the patients) as compared with incomplete revascularization.132 The benefit of complete revascularization was independent of the treatment modality. A more recent meta-analysis suggested enhanced benefit when complete revascularization is performed with state-of-the-art techniques in high-risk patients.133 Likewise, in a post hoc analysis of the SYNTAX trial, anatomical incomplete revascularization was associated with inferior long-term outcomes after both CABG and PCI.131 A residual SYNTAX score >8 after PCI was associated with significant increases in the 5-year risk of death and of the composite of death, MI, and stroke, and any residual SYNTAX score >0 was associated with the risk of repeat intervention.134 In an observational study from the New York State registry that compared CABG with PCI using new-generation DES [everolimus-eluting stent (EES)] in 9223 pairs of propensity-matched patients with multivessel CAD, the significantly higher risk of MI associated with PCI as compared with CABG was not seen among matched pairs of patients in which the PCI group had complete revascularization (Pinteraction=0.02).135 Consistent findings were obtained in a pooled analysis of 3212 patients of the SYNTAX, BEST (Randomised Comparison of Coronary Artery Bypass Surgery and Everolimus-Eluting Stent Implantation in the Treatment of Patients with Multivessel Coronary Artery Disease), and PRECOMBAT (Premier of Randomised Comparison of Bypass Surgery versus Angioplasty Using Sirolimus-Eluting Stent in Patients with Left Main Coronary Artery Disease) trials.136 A mean SYNTAX score of 27 and an LVEF of 59% were obtained. In a propensity-matched analysis, mortality and the composite risk of death, MI, and stroke were significantly lower after PCI with complete vs. incomplete revascularization. After PCI with complete revascularization, the risk of death or of the composite of death, MI, or stroke was not significantly different from that after CABG with complete revascularization (adjusted HR 1.16, 95% CI 0.83-1.63, P=0.39, and 1.14, 95% CI 0.87-1.48, P=0.35, respectively), whereas these risks were significantly elevated after PCI with incomplete revascularization.
Functional complete revascularization is achieved when all lesions causing resting or stress-induced ischaemia are bypassed or treated by PCI. Given the limitations of non-invasive imaging techniques (see section 3), these lesions are best identified by FFR or iwFR during diagnostic angiography. For PCI, the FAME study demonstrated that the more restrictive selection of target lesions by functional guidance conferred superior long-term outcomes compared with anatomically guided lesion selection (see section 3).31 In contrast, leaving functionally relevant lesions untreated resulted in a high rate of reinterventions in the FAME 2 study.33 Based on the data of the FAME and FAME 2 studies, complete revascularization based on the functional definition is the preferred strategy for PCI.
The role of functional guidance for CABG is less clear.28,137 One of the potential benefits of CABG is protection against disease progression in proximal segments, which may be diminished by restricting the bypass targets to functionally relevant lesions. This has to be weighed against the risk of bypass closure when native vessel flow is high. Thus, for ambiguous lesions, functional testing may also help guide the surgical revascularization strategy.

5.3.2 ISOLATED PROXIMAL LEFT ANTERIOR DESCENDING CORONARY ARTERY DISEASE
Comparing CABG and PCI among patients with isolated proximal LAD disease, the available evidence suggests similar outcomes in terms of death, MI, and stroke, but a higher risk of repeat revascularization with PCI.68,70,73,101,139-144
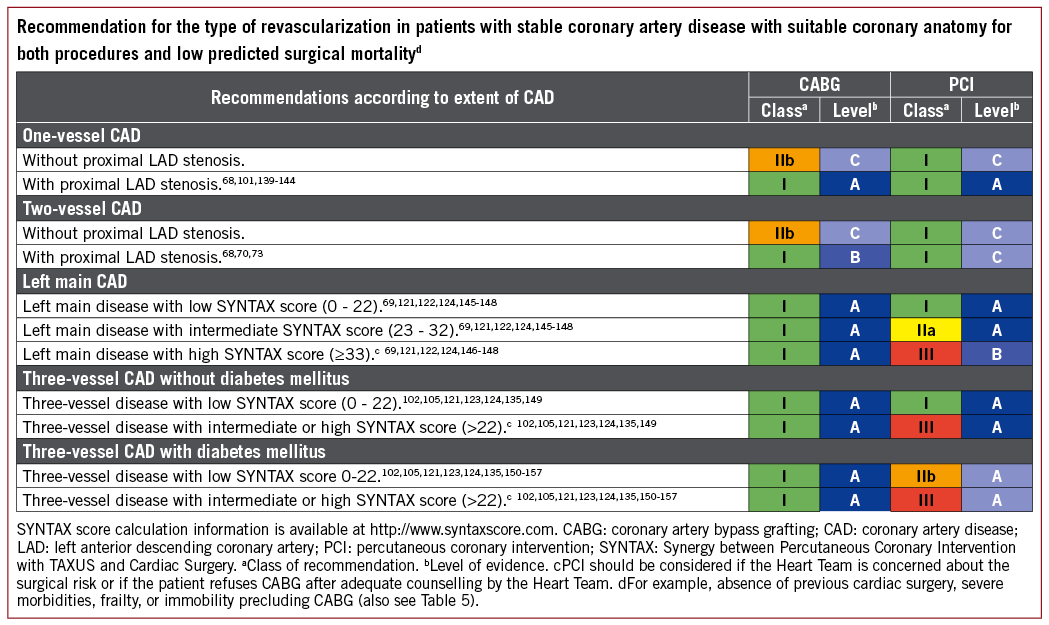
5.3.3 LEFT MAIN CORONARY ARTERY DISEASE
The available evidence from RCTs and meta-analyses comparing CABG with PCI using DES among patients with LM disease suggests equivalent results for the safety composite of death, MI, and stroke up to 5 years of follow-up.148 A significant interaction with time is notable, providing early benefit for PCI in terms of MI and peri-interventional stroke, which is subsequently offset by a higher risk of spontaneous MI during long-term follow-up. The need for repeat revascularization is higher with PCI than with CABG.
The EXCEL trial compared CABG with PCI using new-generation DES (EES) among 1905 patients with significant LM disease.107 At 3 years of follow-up, the primary endpoint of death, stroke, or MI occurred with similar frequency in the CABG and PCI group (14.7 vs. 15.4%; HR 1.00, 95% CI 0.79-1.26, P=0.98). The pre-planned landmark analysis from 30 days to 3 years showed a significant difference for the primary endpoint in favour of CABG (7.9 vs. 11.5%, P=0.02).
The NOBLE (Nordic-Baltic-British Left Main Revascularization Study) trial compared CABG with PCI using new-generation DES [biolimus-eluting stents (BES)] among 1201 patients with significant LM disease (mean SYNTAX score of 23).106 At a median follow-up of 3.1 years, the primary endpoint of death, non-procedural MI, stroke, and repeat revascularization occurred more frequently in the PCI than the CABG group (29 vs. 19%; HR 1.48, 95% CI 1.11-1.96, P=0.007).
A recent collaborative individual patient pooled analysis of randomized trials including 11 518 patients reviewed the currently available evidence from randomized trials comparing CABG with PCI for LM or multivessel disease.124 The primary outcome was all-cause mortality. In the overall cohort, CABG was associated with a significant survival benefit during a mean follow-up of 3.8±1.4 years (5 year all-cause mortality 11.2% after PCI vs. 9.2% after CABG; HR 1.20, 95% CI 1.06-1.37, P=0.0038). There was a linear trend for HRs of death increasing with increasing SYNTAX tertiles [P=0.0011 for trend (unpublished analysis)]. However, among 4478 patients with LM disease, those randomly assigned to CABG or PCI with a mean follow-up of 3.4±1.4 years reported similar risks for the primary outcome all-cause mortality (PCI 10.7 vs. CABG 10.5%; HR 1.07, 95% CI 0.87-1.33, P=0.52) at 5 years. There were no significant differences in mortality between PCI and CABG in subgroup analyses according to SYNTAX scores. Nevertheless, in patients with a high SYNTAX score, a trend towards better survival was noted with CABG. The proportion of patients with a high SYNTAX score was limited in view of the inclusion criteria of the respective studies.
Current evidence indicates that PCI is an appropriate alternative to CABG in LM disease and low-to-intermediate anatomical complexity. Among patients with LM disease and low anatomical complexity, there is evidence that the outcomes with respect to major clinical endpoints are similar for PCI and CABG, resulting in a class I recommendation. Among patients with LM disease and high anatomical complexity, the number of patients studied in RCTs is low due to exclusion criteria; the risk estimates and CIs are imprecise, but suggest a trend towards better survival with CABG. Therefore, PCI in this setting cannot be endorsed as reflected by a class III recommendation. For PCI in LM with intermediate anatomical complexity, the previous class IIa recommendation was maintained in view of the incomplete 5 year follow-up of the two largest RCTs in this setting.
5.3.4 MULTIVESSEL CORONARY ARTERY DISEASE
The observation of a survival advantage of CABG over PCI has been consistent among patients with severe three-vessel CAD (intermediate to high SYNTAX score), and has been attributed at least in part to the placement of bypass grafts to the mid coronary vessels providing prophylaxis against the development of new proximal disease.
The BEST trial, comparing CABG with PCI using new-generation DES (EES) among patients with multivessel CAD (77% three-vessel CAD and 23% two-vessel disease, mean SYNTAX score 24), prematurely stopped enrolment after the inclusion of 880 patients due to slow recruitment.105 At a median follow-up of 4.6 years, PCI was associated with a higher incidence of the primary endpoint (death, MI, and TVR) (15.3 vs. 10.6%; HR 1.47, 95% CI 1.01-2.13, P=0.04) than CABG. The risk of death, MI, and stroke was not statistically different between the two treatment groups (11.9 vs. 9.5%; HR 1.26, 95% CI 0.84-1.89, P=0.26), whereas repeat revascularization of any vessel (11.0 vs. 5.4%; HR 2.1, 95% CI 1.28-3.41, P=0.003) but not target lesion revascularization (5.7 vs. 3.8%; HR 1.51, 95% CI 0.82-2.80, P=0.19) was more frequent in the PCI group. CABG resulted in more complete revascularization (71.5 vs. 50.9%; P<0.001) and a lower incidence of revascularization for new lesions (5.5 vs. 2.3%; HR 2.47, 95% CI 1.18-5.17, P=0.01).
Consistent with findings in the overall cohort (see section 5.3.3), the collaborative individual patient pooled analysis found that in 7040 patients with multivessel disease, those assigned to CABG had significantly lower 5 year all-cause mortality than those assigned to PCI (PCI 11.5 vs. CABG 8.9%; HR 1.28, 95% CI 1.09-1.49, P=0.0019).124 Outcomes for the endpoint all-cause mortality were modified by two variables, diabetes and disease complexity, as assessed by the SYNTAX score. Compared with patients without diabetes (8.7 vs. 8.0%; HR 1.08, 95% CI 0.86-1.36, P=0.49), mortality was higher after PCI than CABG in patients with diabetes (15.5 vs. 10.0%; HR 1.48, 95% CI 1.19-1.84, P=0.0004, Pinteraction=0.045). There was a gradient of risk with a stepwise increase in mortality for PCI according to SYNTAX score tertile (SYNTAX score 0-22: 10.5 vs. 8.4%; HR 1.11, 95% CI 0.77-1.62, P=0.57; SYNTAX score 23-32: 14.0 vs. 9.5%; HR 1.50, 95% CI 1.09-2.08, P=0.0129; SYNTAX score >32: 19.2 vs. 11.2%; HR 1.70, 95% CI 1.13-2.55, P=0.0094).
An individual patient data pooled analysis of SYNTAX and BEST, comparing CABG with PCI using DES among 1275 patients with multivessel disease in the absence of diabetes (89% three-vessel CAD, mean SYNTAX score 26), reported a lower risk of death (6.0 vs. 9.3%; HR 0.65, 95% CI 0.43-0.98, P=0.04) and MI (3.3 vs. 8.3%; HR 0.40, 95% CI 0.24-0.65, P <0.001) in the CABG group at a median follow-up of 61 months.149 The risk of death was not significantly different among patients with a low (0-22) SYNTAX score (6.0 vs. 7.5%, P=0.66), whereas the benefit of CABG over PCI was greater in patients with an intermediate-to-high (>22) SYNTAX score (7.1 vs. 11.6%, P=0.02). Another individual patient data pooled analysis of SYNTAX and BEST, comparing CABG with PCI using DES among 1166 patients with multivessel disease involving the proximal LAD (88% three-vessel CAD, mean SYNTAX score 28), reported a higher risk of the composite of death, MI, and stroke (16.3 vs. 11.5%; HR 1.43, 95% CI 1.05-1.96, P=0.02), cardiac death, MI, and repeat revascularization in the PCI group at 5 years of follow-up.147 Of note, outcomes were not significantly different for CABG and PCI for any endpoint except for MI among the subgroup of patients with low SYNTAX score (0-22).
The available evidence suggests that in multivessel CAD without diabetes and low anatomical complexity, PCI and CABG achieve similar long-term outcomes with respect to survival and the composite of death, MI, and stroke, justifying a class I recommendation for PCI. In patients with multivessel CAD and intermediate-to-high anatomical complexity, the two large trials using DES, SYNTAX and BEST, found a significantly higher mortality and a higher incidence of death, MI, and stroke with PCI in the absence of diabetes. Consistent results were also obtained for patients with multivessel CAD in the recent individual patient-level meta-analysis.124 Thus, the previous class III recommendation for PCI in multivessel CAD and intermediate-to-high complexity was maintained.
5.4 GAPS IN THE EVIDENCE
It remains to be determined whether revascularization by PCI improves prognosis in patients with SCAD. The ISCHEMIA (International Study of Comparative Health Effectiveness With Medical and Invasive Approaches) study (NCT01471522) is currently recruiting 5000 patients with SCAD and evidence of moderate-to-severe ischaemia detected by non-invasive imaging, who are randomized before coronary angiography to medical therapy or an invasive strategy to detect differences in the primary endpoint of death or MI. Current techniques rely on coronary angiography and the detection of ischaemia-producing lesions. However, future adverse events are related at least in part to non-flow limiting, vulnerable plaques. Better identification of vulnerable plaques and the development of appropriate treatment strategies is needed. Along the same lines, the completeness and timing of revascularization are not well defined, and neither are the roles of residual ischaemia and lesions. Moreover, we need more research on the use of the SYNTAX and other scores for informing treatment allocation, as well as dedicated trials in specific subsets. Very long-term, extended follow-up (10 years) of trials comparing PCI and CABG, particularly in the setting of LM disease, will provide further insights into the relative merits of both revascularization techniques.
6 Revascularization in non-st-elevation acute coronary syndrome
Myocardial revascularization in patients with non-ST-segment elevation ACS (NSTE-ACS) is addressed by prior Guidelines that are endorsed by the current Task Force.158 In the present Guidelines, we discuss new evidence where previous recommendations require an update.
6.1 EARLY INVASIVE VS. CONSERVATIVE STRATEGY
An invasive strategy has become the standard of care for high-risk patients.158 This approach allows prompt diagnosis of the underlying CAD, identification of the culprit lesion, guidance for antithrombotic management, and the assessment of the suitability of coronary anatomy for PCI or CABG. Numerous factors interplay in the decision-making process, including clinical presentation, comorbidities, risk stratification (Figure 4), and high-risk features specific for a revascularization modality such as frailty, cognitive status, estimated life expectancy, and the functional and anatomical severity of CAD.
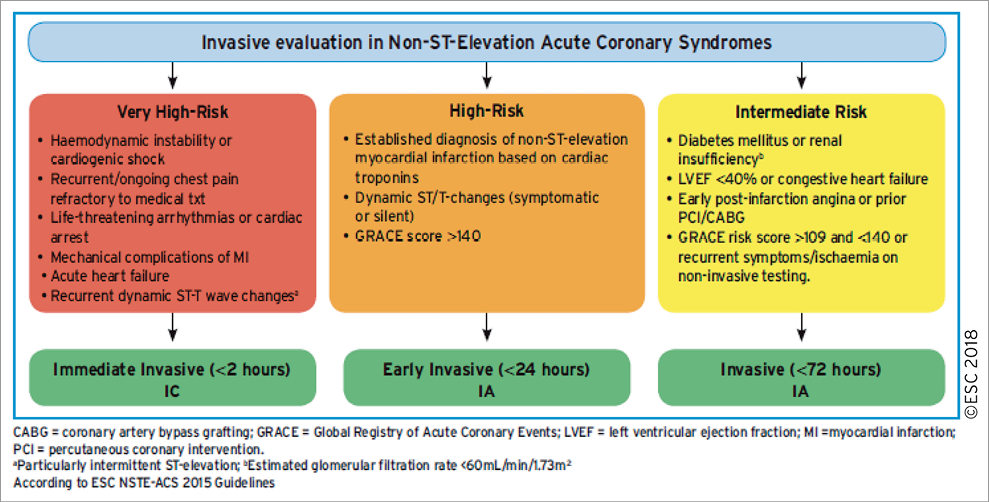
Figure 4. Selection of non-ST-elevation acute coronary syndrome treatment strategy and timing according to initial risk stratification.
Up to 40% of NSTE-ACS patients with obstructive CAD present with multiple complex plaques159-162 and 25% with an acute occluded coronary artery,163 so that identification of the culprit lesion may be challenging. Correlation with ECG or echo changes and the use of OCT in the 25% of NSTE-ACS patients with angiographically normal epicardial coronary arteries164-166 may be helpful for identifying the culprit lesion, or rule out other mechanisms such as dissection or haematomas [MI with non-obstructive coronary arteries (MINOCA)].167-169
A routine invasive strategy in NSTE-ACS has been shown to improve clinical outcomes,170 and benefit was mainly confined to biomarker-positive patients171 and patients with other high-risk features as defined in Figure 4. Of importance, the use of a radial approach, new-generation DES, and more effective P2Y12-inhibitors were not available or broadly implemented in these trials, and led to a magnified benefit in frail ACS populations.172,173
6.2 TIMING OF ANGIOGRAPHY AND INTERVENTION
The current recommendations on the timing of angiography and intervention, as defined in Figure 4, are based on evidence discussed in detail by the prior Guidelines on NSTE-ACS.158 Specifically, a reduction in recurrent or refractory ischaemia and length of hospital stay was found with early intervention.174,175 More recently, an updated collaborative meta-analysis on individual published and unpublished data (n=5324 patients with a median follow-up of 180 days) suggested that early intervention might also be associated with decreased mortality.176 This meta-analysis showed a statistical trend towards decreased mortality with an early invasive strategy compared with a delayed invasive strategy in unselected patients with NSTE-ACS (HR 0.81, 95% CI 0.64-1.03, P=0.088). The survival benefit of the early invasive strategy appeared more pronounced in high-risk subsets, including elevated cardiac biomarkers at baseline (HR 0.76, 95% CI 0.58-0.996), diabetes (HR 0.67, 95% CI 0.45-0.99), a Global Registry of Acute Coronary Events risk score >140 (HR 0.70, 95% CI 0.52-0.95), and age 75 years or older (HR 0.65, 95% CI 0.46-0.93), although tests for interaction were inconclusive.
6.3 TYPE OF REVASCULARIZATION
6.3.1 PERCUTANEOUS CORONARY INTERVENTION
6.3.1.1 Technical aspects
Implantation of new-generation DES is the standard treatment strategy even when dual antiplatelet therapy (DAPT) cannot be sustained beyond 1 month post-intervention173,177-179 (see section 17), and the radial approach has also become the standard of care.172 DAPT is recommended for 12 months irrespective of stent type, while in patients at high ischaemic risk not experiencing bleeding events, DAPT may be extended (see section 17). There is no evidence for any additional benefit of thrombectomy in patients undergoing PCI in the setting of NSTE-ACS.180 While FFR is considered the invasive gold standard for the functional assessment of lesion severity in SCAD, it has been shown to be feasible, reliable, safe, and effective in NSTE-ACS patients with multivessel disease, although its prognostic value is unclear.22,137,181
6.3.1.2 Revascularization strategies and outcomes
Complete revascularization of significant lesions should be attempted in multivessel disease NSTE-ACS patients, given that it was mandated in trials testing early vs. late intervention171,182,183 and that the prognosis of patients with incomplete revascularization is known to be worse.131,184 In addition, it seems that complete one-stage revascularization is associated with better clinical outcome than multistage PCI.185 The risk of periprocedural complications of PCI defined as MI or myocardial injury, as well as that of long-term ischaemia, remains higher in NSTE-ACS than in stable patients.186,187 For ACS patients who have undergone PCI, revascularization procedures represent the most frequent, most costly, and earliest causes for rehospitalization.188,189 As in ST-elevation myocardial infarction (STEMI), routine treatment of non-culprit lesions during the primary intervention by PCI is harmful in NSTE-ACS patients with cardiogenic shock, as shown by the recently published CULPRIT-SHOCK (Culprit Lesion Only PCI versus Multivessel PCI in Cardiogenic Shock) trial (see section 7.3).190
6.3.2 CORONARY ARTERY BYPASS GRAFTING
Approximately 5-10% of NSTE-ACS patients require CABG,191 and they represent a challenging subgroup given their high-risk characteristics compared with patients undergoing elective CABG.192 In the absence of randomized data, optimal timing for non-emergent CABG in NSTE-ACS patients should be determined individually. The risk of ischaemic events possibly related to suboptimal antiplatelet therapy while awaiting surgery is <0.1%, while that of perioperative bleeding complications associated with platelet inhibitors is >10%.193 In patients with ongoing ischaemia or haemodynamic instability with an indication for CABG, emergency surgery should be performed and not postponed as a consequence of antiplatelet treatment exposure.
6.3.3 PERCUTANEOUS CORONARY INTERVENTION VS. CORONARY ARTERY BYPASS GRAFTING
There is no randomized comparison of PCI vs. CABG in the specific setting of NSTE-ACS. The currently available evidence indirectly suggests that the criteria applied to patients with SCAD to guide the choice of revascularization modality should be applied to stabilized patients with NSTE-ACS.100,121,150,194 Recent individual-patients data analysis from the BEST, PRECOMBAT, and SYNTAX studies compared the outcome of CABG with that of PCI in 1246 patients with stabilized NSTE-ACS and multivessel or LM disease.194 The 5 year incidence of the primary outcome – the composite of death, MI, or stroke – was significantly lower with CABG than with PCI (13.4 vs. 18%, P=0.036). The findings of this meta-analysis were consistent with the main findings of the studies included, thus supporting the concept that the principles of SCAD should apply to stabilized patients with NSTE-ACS as well.
For complex cases, Heart Team discussion and use of the SYNTAX score are recommended,195 given its ability to predict death, MI, and revascularization in patients with NSTE-ACS and multivessel disease undergoing PCI. In patients with multivessel disease and diabetes in particular, recent evidence suggests a greater benefit of CABG vs. PCI.196
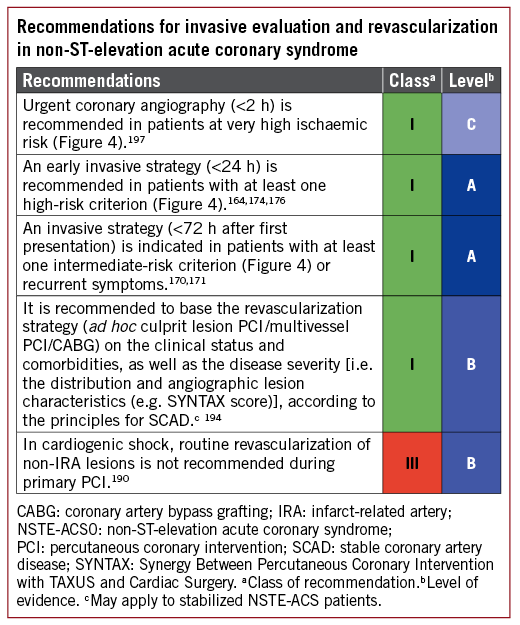
6.4 GAPS IN THE EVIDENCE
In the setting of NSTE-ACS, there are no dedicated prospective studies on the revascularization strategy with multivessel disease. Thus, current recommendations on the choice of lesions to be treated and treatment modality (PCI or CABG) are based on an analogy to findings obtained in SCAD or STEMI. Likewise, the prognostic role of FFR and iwFR in guiding myocardial revascularization needs additional clarification.
7 Revascularization in ST-segment elevation myocardial infarction
Myocardial revascularization in patients with STEMI is addressed by the 2017 ESC Guidelines on STEMI. After reviewing the subsequent literature, the current Task Force endorses most recommendations of these Guidelines.198
7.1 TIME DELAYS
Delays in the timely implementation of reperfusion therapy are key issues in the management of STEMI. Detailed recommendations on timelines, logistics, and pre-hospital management have been provided in the recent ESC STEMI Guidelines (Figure 5).198
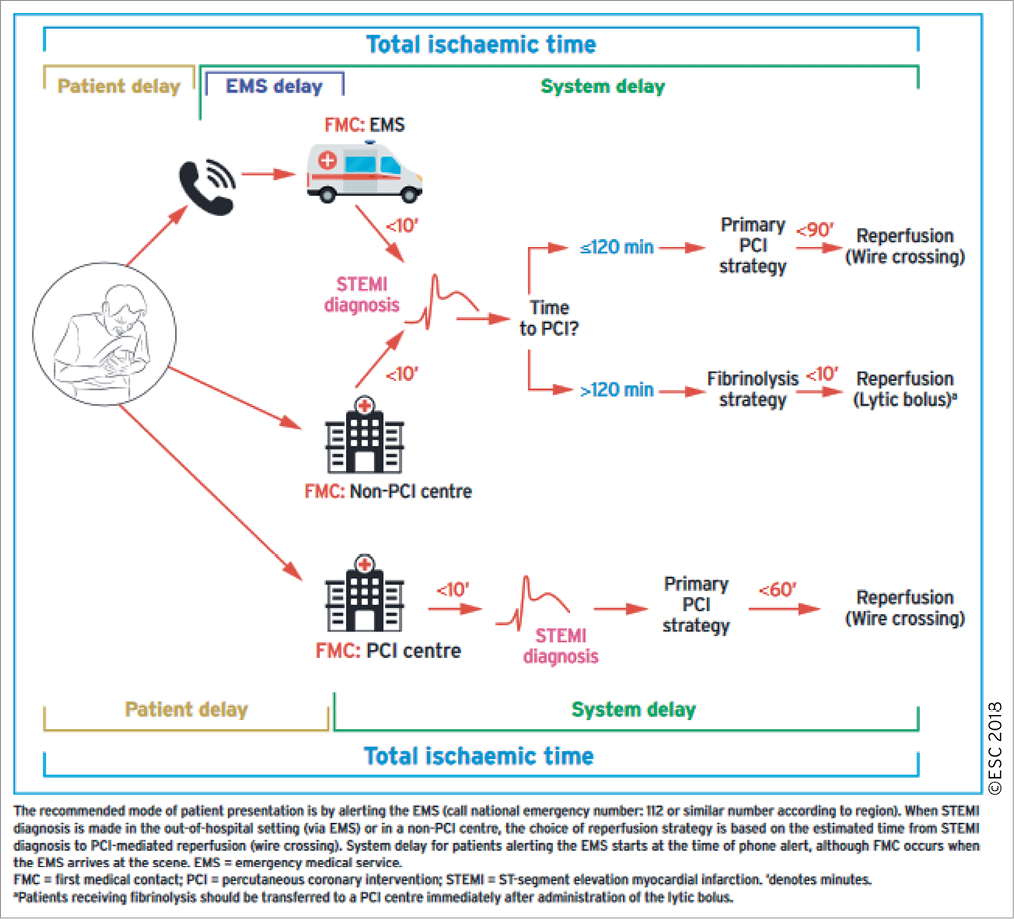
Figure 5. Modes of patient’s medical contact, components of ischaemia time, and flowchart for reperfusion strategy selection.
A recent analysis of 12 675 STEMI patients in the FITT-STEMI (Feedback Intervention and Treatment Times in ST-Elevation Myocardial Infarction) trial emphasizes the strong impact of time delays on mortality, particularly in STEMI patients with cardiogenic shock or out-of-hospital cardiac arrest.199 In shock without out-of-hospital cardiac arrest, every 10 min treatment delay between 60-180 min from the first medical contact resulted in 3.3 additional deaths per 100 PCI-treated patients, and in 1.3 additional deaths after out-of-hospital cardiac arrest without cardiogenic shock. In stable STEMI patients, time delays were substantially less relevant (0.3 additional deaths per 100 PCI-treated patients for every 10 min delay between 60-180 min from the first medical contact). Thus, high-risk STEMI patients with cardiogenic shock or out-of-hospital cardiac arrest are those who benefit most from expediting all steps of the care pathway.
7.2 SELECTION OF REPERFUSION STRATEGY
Primary PCI, defined as percutaneous catheter intervention in the setting of STEMI without previous fibrinolysis, is the preferred reperfusion strategy. It has replaced fibrinolysis in patients with STEMI, provided it can be performed in a timely manner in high-volume PCI centres with experienced operators and 24 h/7 days a week catheterization laboratory activation.198,200,201 In settings where primary PCI cannot be performed in a timely fashion, fibrinolysis should be administered as soon as possible. If first medical contact (FMC) is out-of-hospital, lysis should be implemented pre-hospital (e.g. in the ambulance) (Figure 5).202-206 It should be followed by transfer to PCI-capable centres for routine coronary angiography in all patients, and should be performed without delay for rescue PCI in the case of unsuccessful fibrinolysis or within 2-24 h after bolus administration.198 Emergency CABG may be indicated in selected STEMI patients unsuitable for PCI.
7.3 PRIMARY PERCUTANEOUS CORONARY INTERVENTION
Key points for optimizing and guiding primary PCI are summarized below.
The infarct-related artery (IRA) should be systematically treated during the initial intervention. Patients with extensive CAD in vessels remote from the IRA have an adverse prognosis following primary PCI.207 Staged PCI in patients with multivessel disease and no haemodynamic compromise is an independent predictor of survival, and more frequent ischaemic events have been reported in direct vs. staged revascularization of STEMI patients with multivessel disease.208-210
Four major randomized trials-PRAMI (Preventive Angioplasty in Acute Myocardial Infarction),211 CvLPRIT (Complete Versus Lesion-Only Primary PCI trial),212 DANAMI-3-PRIMULTI (The Third DANish Study of Optimal Acute Treatment of Patients with ST-segment Elevation Myocardial Infarction: PRImary PCI in MULTIvessel Disease),213 and Compare-Acute214-have consistently shown a benefit of complete revascularization (performed immediately or staged) as compared with IRA-only PCI in patients with STEMI and multivessel disease (for details see the Supplementary Data). A recent meta-analysis of 10 trials has shown that complete revascularization was associated with a lower risk of MACE (RR 0.57, 95% CI 0.42-0.77), due to a lower risk of urgent revascularization (RR 0.44, 95% CI 0.30-0.66), with no significant difference in mortality (RR 0.76, 95% CI 0.52-1.12) or MI (RR 0.54, 95% CI 0.23-1.27).215 This meta-analysis did not include Compare-Acute. Yet, similar to earlier studies, the benefit of complete revascularization over culprit-only revascularization seen in Compare-Acute was driven by a lower need for unplanned reintervention, whereas the incidences of death and recurrent MI were similar between the two strategies.214
Most of the studies support the concept of full revascularization either during the initial hospital stay for STEMI or a staged admission,215 but it remains to be determined how clinicians can identify lesions that should be revascularized beyond the culprit lesion and whether complete revascularization should be performed in single- or multi-stage procedures. Moreover, there is a lack of evidence on the optimal timing of staged procedures. In most of the studies, staged procedures were performed during the initial hospital stay. At present, one-stage multivessel PCI during STEMI without cardiogenic shock should be considered in patients in the presence of multiple, critical stenoses or highly unstable lesions (angiographic signs of possible thrombus or lesion disruption), and if there is persistent ischaemia after PCI on the supposed culprit lesion.
In patients with multivessel disease and AMI with cardiogenic shock, the recently published CULPRIT-SHOCK trial showed that a strategy with PCI of the culprit lesion only with possible staged revascularization determined a lower 30 day risk of the composite of all-cause mortality or severe renal failure compared with immediate multivessel PCI.190 This was driven by a significant risk reduction in 30 day all-cause mortality by the culprit lesion-only strategy compared with immediate multivessel PCI (43.3 vs. 51.6%; HR 0.84, 95% CI 0.72-0.98, P=0.03). These findings need to be interpreted in light of a low 12.5% (43 out of 344 patients) crossover rate from culprit lesion-only to immediate multivessel PCI based on physicians’ judgment. Based on these findings, culprit lesion-only PCI is recommended as the default strategy in patients with AMI with cardiogenic shock. A more detailed discussion of the revascularization strategy in MI patients with cardiogenic shock is found in the Supplementary Data.
In patients with STEMI, DES (in particular new-generation DES) have demonstrated better efficacy as compared with BMS and should be used as the default strategy in STEMI patients, even when DAPT cannot be sustained beyond 1 month.177,178,216-218 (see section 16.1.2). As discussed in section 16.4, radial access is preferred over femoral access.
Delaying stenting in primary PCI has been investigated as an option to reduce microvascular obstruction (MVO) and preserve microcirculatory function in two small trials with conflicting results.219,220 More recently, in the larger deferred vs. conventional stent implantation in patients with STEMI [The Third DANish Study of Optimal Acute Treatment of Patients with ST-segment Elevation Myocardial Infarction: DEFERred stent implantation in connection with primary PCI (DANAMI 3-DEFER)] trial in 1215 STEMI patients, there was no effect on the primary clinical outcome (composite of death, non-fatal MI, or ischaemia-driven revascularization of non-IRA lesions) over a median follow-up of 42 months.221 Routine deferred stenting was associated with a higher risk of TVR.
Thrombus aspiration has been proposed as an adjunct during primary PCI to further improve epicardial and myocardial reperfusion by the prevention of distal embolization of thrombotic material and plaque debris.222 Two landmark RCTs, which were adequately powered to detect the superiority of routine manual thrombus aspiration vs. conventional PCI, showed no benefit on clinical outcomes of the routine aspiration strategy overall or in any subgroup of patients indicating high thrombotic risk.223-226 A safety concern emerged in TOTAL (Trial of Routine Aspiration Thrombectomy with PCI versus PCI Alone in Patients with STEMI) trial with an increase in the risk of stroke.225,227 Taken together, these results suggest that the routine use of thrombus aspiration is not indicated. In the high-thrombus burden subgroup, the trend towards reduced cardiovascular death and increased stroke/transient ischaemic attack (TIA) provides a rationale for future trials of improved thrombus aspiration technologies in this high-risk subgroup (although statistical tests did not support significant subgroup interaction).228
7.4 PERCUTANEOUS CORONARY INTERVENTION AFTER THROMBOLYSIS AND IN PATIENTS WITH LATE DIAGNOSIS
The benefits of early, routine PCI after thrombolysis were seen in the absence of an increased risk of adverse events (stroke or major bleeding). Based on data from the four most recent trials, all of which had a median delay between the start of thrombolysis and angiography of 2-6 h, a time frame of 2-24 h after successful lysis is recommended.206,229-231 In cases of failed fibrinolysis, or if there is evidence of re-occlusion or reinfarction with recurrence of ST-segment elevation, the patient should undergo immediate coronary angiography and rescue PCI.232 Patients presenting between 12 and 48 h after the onset of symptoms, even if pain free and with stable haemodynamics, may still benefit from early coronary angiography and possibly PCI.233,234 In patients presenting days after the acute event with a completed MI, only those with recurrent angina or documented residual ischaemia – and proven viability on non-invasive imaging in a large myocardial territory – may be considered for revascularization when the infarct artery is occluded. Routine late PCI of an occluded IRA after MI in stable patients has no incremental benefit over medical therapy.235
7.5 GAPS IN THE EVIDENCE
Patients undergoing primary PCI benefit from full revascularization, but the optimal timing of treatment of the non-culprit lesion is not known. More studies evaluating the assessment of non-culprit lesions by FFR or iwFR at the time of acute PCI, and studies investigating whether intravascular imaging guidance of primary PCI can improve the outcomes of STEMI patients, are needed. Future trials of improved thrombus aspiration technologies may address the role of this strategy in patients with high-risk features, such as large thrombus burden.228
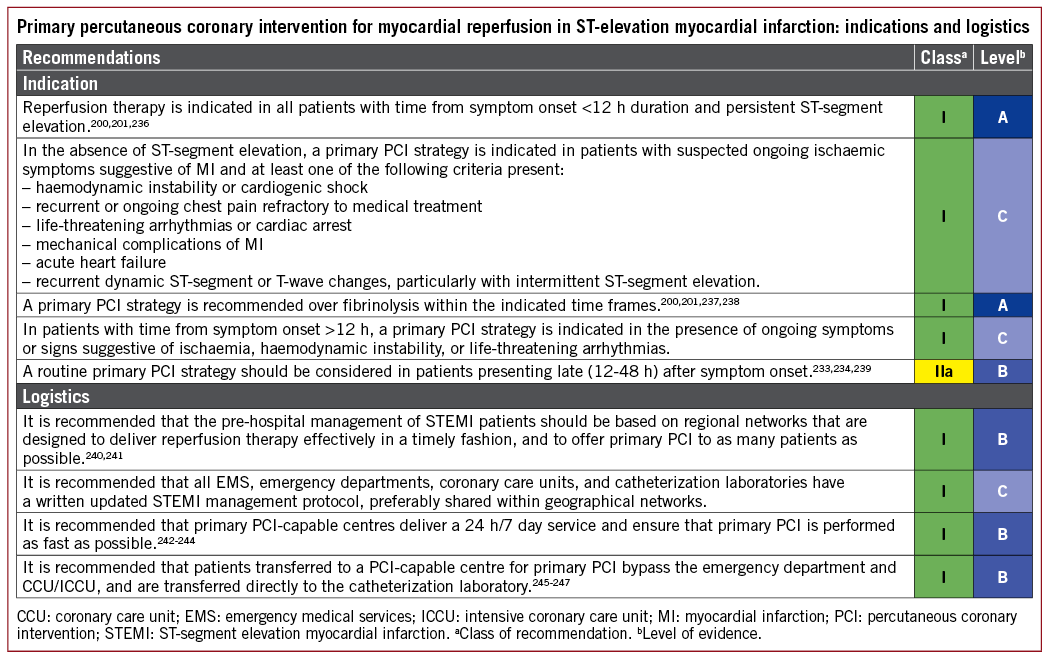
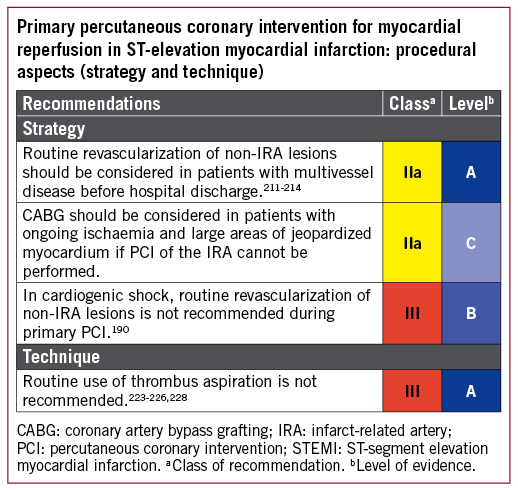
8 Myocardial revascularization in patients with heart failure
8.1 CHRONIC HEART FAILURE
8.1.1 RECOMMENDATIONS FOR MYOCARDIAL REVASCULARIZATION IN PATIENTS WITH CHRONIC HEART FAILURE
When compared with medical therapy alone, coronary revascularization is superior in improving survival in patients with HF of ischaemic origin and is recommended in clinical practice.81,248 However, the optimal revascularization strategy is not defined. The choice between CABG and PCI should be made by the Heart Team after careful evaluation of the patient’s clinical status and coronary anatomy, expected completeness of revascularization (see section 5.3.1.3), myocardial viability, coexisting valvular disease, and comorbidities. Considerations relating to the need for viability testing prior to revascularization are discussed in section 3.
Randomized clinical trial data comparing revascularization with medical therapy exists only for CABG in the setting of the STICH trial.81 One analysis from this trial showed that CABG can be performed with acceptable 30 day mortality rates (5.1%) in patients with LV dysfunction (LVEF ≤35%).249 Extended follow-up in the STICH Extension Study (STICHES) supports a significant survival benefit of CABG combined with medical therapy vs. medical therapy alone in a 10 year observation period.81
There are currently no dedicated randomized clinical trials comparing PCI vs. medical therapy in patients with HF with reduced EF (HFrEF). In addition, CABG vs. PCI randomized trials have excluded patients with severe HF. In one prospective registry including 4616 patients with multivessel disease and severe HFrEF, propensity score-matched comparison revealed similar survival (mean follow-up 2.9 years) with PCI (using EES) vs. CABG.250 PCI was associated with a higher risk of MI, particularly in patients with incomplete revascularization, and repeat revascularization. CABG was associated with a higher risk of stroke. The conclusion of the study was that multivessel PCI can be a valuable option in HF patients if complete revascularization is possible. A systematic review of studies comparing revascularization with medical therapy in patients with an EF ≤40% showed that there was a significant mortality reduction with CABG (HR 0.66, 95% CI 0.61-0.72, P <0.001) and PCI (HR 0.73, 95% CI 0.62-0.85, P<0.001) vs. medical therapy, though these finding are limited by the predominantly observational nature of the included studies and missing information on the completeness of revascularization.248
A recent observational study investigated outcomes with PCI or CABG for multivessel CAD and LV dysfunction in 1738 propensity-matched patients with diabetes mellitus.251 Similar to the findings in the absence of LV dysfunction, when CABG was compared with PCI it was associated with a significantly lower risk of MACE, which included a significant reduction in mortality. Event curves separated early during the first year and continued to separate out to 12 years.
PCI should be considered in older patients without diabetes in whom complete revascularization can be achieved, whereas CABG is preferred in younger patients with more extensive CAD or those with diabetes. In patients with diabetes and LV moderate or severe dysfunction (EF <50%), CABG is associated with better long-term survival and reduced incidence of MACCE.250,251
8.1.2 VENTRICULAR RECONSTRUCTION AND ANEURYSM RESECTION
The aim of surgical ventricular reconstruction (SVR) is to restore physiological volume, and achieve an elliptical shape of the LV, by scar resection and LV wall reconstruction on a mannequin of predefined size. The aim of ventricular aneurysmectomy is to remove fibrous scars in cases of severe dilatation, thrombus formation, or as a source of life-threatening ventricular arrhythmias.
The STICH trial revealed no difference in the primary outcome (total mortality or cardiac hospitalization) between patients randomly allocated to CABG vs. combined CABG and SVR.252 Subgroup analyses of patients with a less dilated LV and better LVEF showed benefit from SVR.253 In the STICH trial, a post-operative LV end-systolic volume index ≤70 mL/m2, after CABG plus SVR, resulted in improved survival compared with CABG alone.252,254 In experienced centres, SVR may be done at the time of CABG if HF symptoms are more predominant than angina, and if myocardial scar and moderate LV remodelling are present.
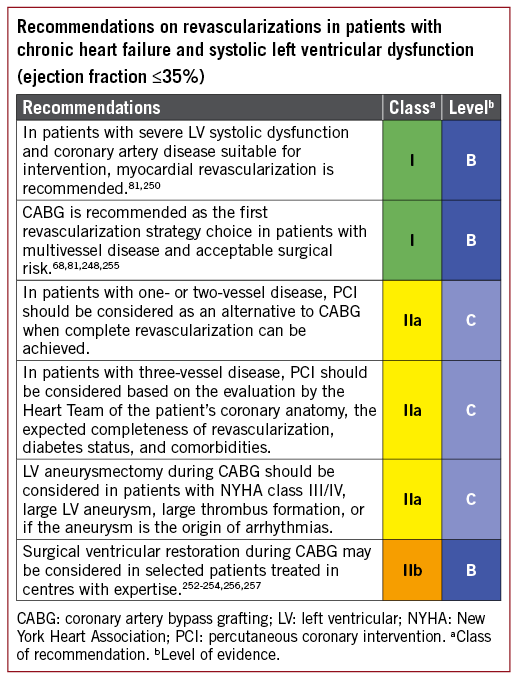
8.2 ACUTE HEART FAILURE AND CARDIOGENIC SHOCK
Acute myocardial ischaemia in the setting of AMI is the antecedent event for the majority of patients with cardiogenic shock undergoing percutaneous revascularization. Mechanical complications – such as papillary muscle rupture with severe mitral valve regurgitation, ventricular septal defect, or free wall rupture – are additional precipitating causes.
8.2.1 REVASCULARIZATION
The SHOCK (Should We Emergently Revascularize Occluded Coronaries for Cardiogenic Shock) trial demonstrated that in patients with cardiogenic shock complicating AMI, emergency revascularization with PCI or CABG improved long-term survival when compared with initial intensive medical therapy. All-cause mortality at 6 months was lower in the group assigned to revascularization than in the medically treated patients (50.3 vs. 63.1%, respectively; RR 0.80, 95% CI 0.65-0.98, P=0.03).258
The revascularization strategy for patients with cardiogenic shock and multivessel disease is addressed in section 7.
A subanalysis of the SHOCK trial comparing patients treated with CABG or PCI showed similar survival rates between the two subgroups.259 There were more patients with diabetes (48.9 vs. 26.9%; P=0.02), three-vessel disease (80.4 vs. 60.3%; P=0.03), and LM coronary disease (41.3 vs. 13.0%; P=0.001) in the CABG group. The findings of this non-randomized comparison suggest that CABG should be considered in patients with cardiogenic shock who have suitable anatomy, particularly if successful PCI is not feasible.
8.2.2 MECHANICAL CIRCULATORY SUPPORT
Short-term MCS devices that are currently available are the intra-aortic balloon pump (IABP), veno-arterial extracorporeal membrane oxygenation (ECMO), and percutaneous left ventricular assist devices (pLVADs). Short-term MCS may be considered in refractory cardiogenic shock depending on patient age, comorbidities, neurological function, and the prospects for long-term survival and quality of life.
8.2.2.1 Intra-aortic balloon pump
IABPs are low-cost devices that are easy to insert and remove. They moderately increase cardiac output and coronary and cerebral perfusion, while decreasing ventricular workload. In patients with cardiogenic shock complicating acute MI, the IABP-SHOCK II (Intraaortic Balloon Pump in Cardiogenic Shock II) randomized trial (600 patients) showed that the use of IABPs did not reduce 30 day mortality and that there was no evidence of long-term benefit.260,261 A recent Cochrane review of seven trials (790 patients) showed that IABPs may have a beneficial effect on some haemodynamic parameters but did not result in survival benefits.262 Thus, the routine use of IABPs in patients with cardiogenic shock complicating acute MI is not recommended.
8.2.2.2 Extracorporeal membrane oxygenation
Veno-arterial ECMO (VA-ECMO), also known as extracorporeal life support (ECLS), in its current form is a modified form of cardiopulmonary bypass. It decompresses the venous system; increases coronary, cerebral, and peripheral perfusion; and also provides supplementary blood oxygenation. When performed percutaneously, it does not allow for LV decompression and leads to increasing LV afterload.
In patients with cardiac arrest, evidence from observational trials supports better survival in patients treated with VA-ECMO compared with those without.263 When compared with IABP, VA-ECMO provides superior circulatory support.264,265 Moreover, a meta-analysis of observational studies suggested that in patients with cardiogenic shock post-ACS, VA-ECMO showed a 33% higher 30 day survival compared with IABP [95% CI 14-52%, P <0.001; number needed to treat (NNT) 13].263 However, the low number of patients included in the analysed studies and the non-random treatment allocation are important limitations.
8.2.2.3 Percutaneous left ventricular assist devices
The majority of clinical experience with currently available pLVADs is limited to two types of device: (i) a transaortic microaxial pump (Impella) that directly unloads the LV providing 2.5-5 L/min blood flow and (ii) a transseptal centrifugal assist device (TandemHeart) that unloads the LV via a cannula introduced into the left atrium through a transseptal puncture.
A recent meta-analysis on MCS in cardiogenic shock included four randomized trials investigating the efficacy and safety of pLVADs vs. IABP, and demonstrated similar short-term mortality despite initial beneficial effects on arterial blood pressure and peripheral perfusion, measured by serum lactate levels.266 In all trials, a higher rate of bleeding from vascular access sites and a significantly higher incidence of limb ischaemia following pLVAD was noted. Similar outcomes were noted in an RCT of high-risk PCI in patients with impaired LV function. The 30 day incidence of major adverse events was not different for patients with pLVAD vs. IABP.267
In summary, the evidence for pLVAD is insufficient to provide a recommendation on its clinical use in cardiogenic shock.
8.2.2.4 Surgically implanted left ventricular assist devices
There are limited data on surgically-implanted LV assist device (LVAD) therapy in patients with AMI and cardiogenic shock. One multicentre registry showed that despite being more critically ill prior to implantation, patients with acute MI managed with LVAD had outcomes similar to other LVAD populations.268
A suggested algorithm for the management of patients with cardiogenic shock is shown in Figure 6.
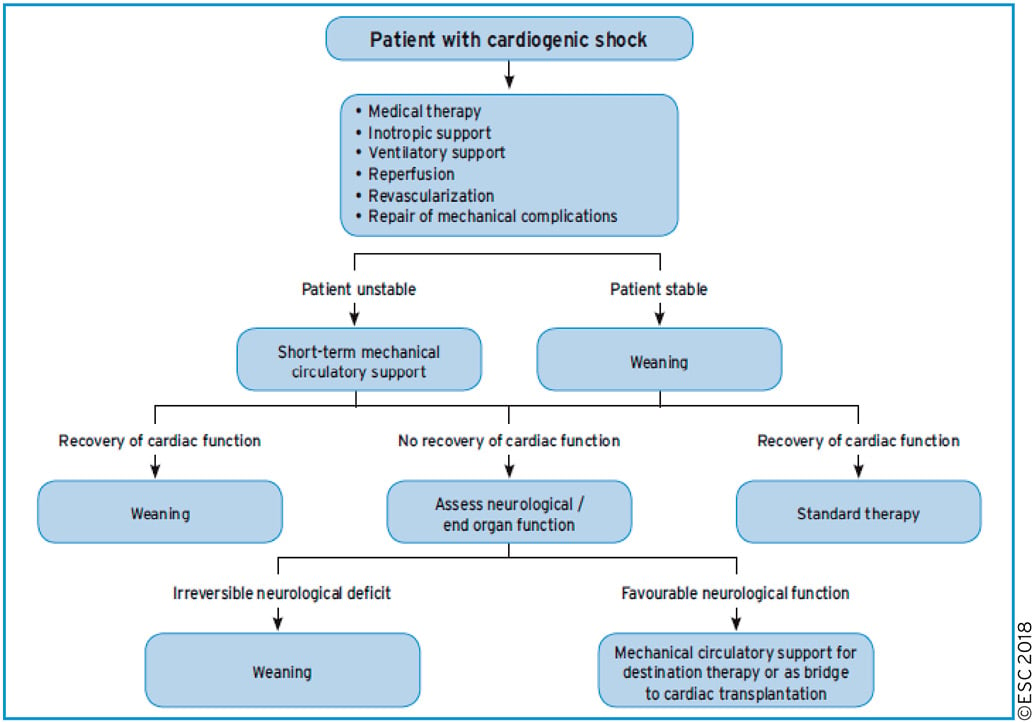
Figure 6. Algorithm for the management of patients with cardiogenic shock.
8.3 GAPS IN THE EVIDENCE
There is no RCT comparing revascularization with PCI vs. CABG in patients with HF.
There is limited evidence on the role of active MCS in patients with cardiogenic shock compared with standard therapy.
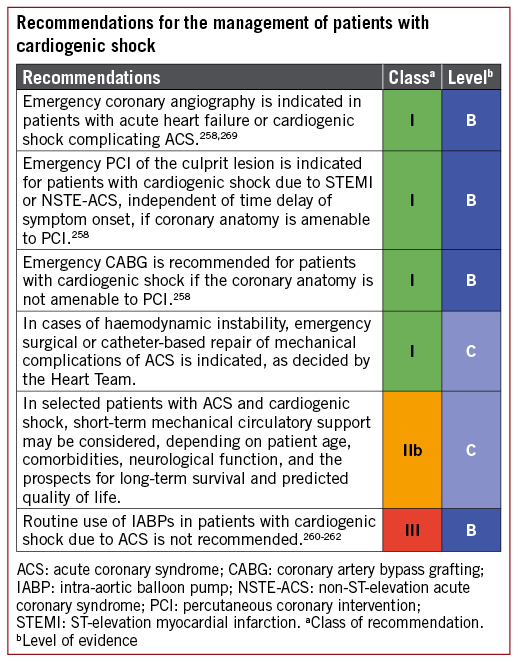
9 Revascularization in patients with diabetes
Patients with diabetes mellitus have a higher prevalence of CAD, which often manifests earlier in life and confers a substantially worse prognosis than for patients without diabetes.270 Patients with diabetes who have suffered an MI have a worse prognosis, particularly those requiring treatment with insulin, and the presence of diabetes amplifies the risk of any cardiovascular event.271 Diabetes mellitus is present in 25-30% of patients admitted with ACS and in up to 40% of patients undergoing CABG.272
The anatomical pattern of CAD in patients with diabetes clearly influences their prognosis and response to revascularization. Angiographic studies have demonstrated that patients with diabetes are more likely to have LM disease and multivessel CAD, with more diffuse disease involving smaller vessels.273 In addition, patients with diabetes have a greater atherosclerotic burden and an increased number of lipid-rich plaques, which are prone to rupture,274,275 and those with unstable angina have more fissured plaques and intracoronary thrombi.276 Patients with diabetes undergoing revascularization, either with CABG or PCI, are at greater risk of kidney injury than patients without diabetes.
9.1 EVIDENCE FOR MYOCARDIAL REVASCULARIZATION
In patients with diabetes, the indications for myocardial revascularization are the same as those in patients without diabetes (see sections 5, 6, and 7). A meta-analysis of nine RCTs with 9904 ACS patients did not show an interaction between diabetic status and the benefit from invasive management and revascularization.277 Yet, absolute risk reductions were larger in the diabetic subsets compared with non-diabetic subsets. Consistent with the findings in the absence of diabetes, the adverse impact of incomplete revascularization in patients with diabetes was also demonstrated in the BARI-2D (Bypass Angioplasty Revascularization Investigation 2 Diabetes) trial.278
Data from randomized trials on revascularization in patients with diabetes are summarized in Supplementary Table 5.
9.2 TYPE OF MYOCARDIAL REVASCULARIZATION
The selection of the optimal myocardial revascularization strategy for patients with diabetes and multivessel CAD requires particular consideration. The recommendations are provided in section 5.
9.2.1. RANDOMIZED CLINICAL TRIALS
The FREEDOM trial compared elective revascularization with CABG or PCI with first-generation DES (94%) in 1900 patients with diabetes (6% of the screened population) with multivessel disease but without LM stenosis.150 The primary endpoint of any-cause death, non-fatal MI, or stroke at 5 years occurred in 26.6% in the PCI group, compared with 18.7% in the CABG group (absolute difference 7.9%, 95% CI 3.3-12.5%, P=0.005). The incidences of death (16.3% in the PCI group vs. 10.9% in the CABG group; absolute difference 5.4%, 95% CI 1.5-9.2%, P=0.049) and MI (13.9% in the PCI group vs. 6.0% in the CABG group, P <0.001) were higher in the PCI group, but the incidence of stroke was lower (2.4 vs. 5.2%; P=0.03). Within the FREEDOM trial at 5 years, patients with diabetes treated with insulin had higher event rates, but there was no significant interaction of treatment and insulin requirement for the primary endpoint (Pinteraction=0.40), even after adjusting for SYNTAX score: the NNT with CABG vs. PCI to prevent one event was 12.7 for insulin-treated patients and 13.2 in those not requiring insulin.279
VACARDS (Veterans Affairs Coronary Artery Revascularization in Diabetes Study) compared CABG with PCI in patients with diabetes and extensive CAD in the USA.154 Only 198 patients with diabetes were randomized due to early termination of the study. The combined risk of death or non-fatal MI was 18.4% for the CABG arm and 25.3% for the PCI arm (HR 0.89, 95% CI 0.47-1.71, P <0.05).154
In the CARDia (Coronary Artery Revascularization in Diabetes) trial, 510 patients with diabetes and multivessel or complex single-vessel CAD were randomly assigned to either CABG or PCI, with the use of either BMS or DES and routine use of abciximab.156 There were no differences between CABG and PCI for the primary endpoint of 1 year composite of death, MI, or stroke, but the trial was underpowered to detect these differences. However, repeat revascularization was more likely to occur in patients treated with PCI (P <0.001).156
In the subset of 452 patients with diabetes and multivessel CAD who were enrolled in the SYNTAX trial, there were no differences in the composite safety endpoint of all-cause death, stroke, and MI at 5 year follow-up.155 However, the need for repeat revascularization (HR 2.01, 95% CI 1.04-3.88, P <0.001) was significantly more frequent in patients with diabetes treated with PCI than in those who underwent CABG.155,275
Patients with diabetes had a higher rate of repeat revascularization after PCI when compared with CABG in the low (≤22) (38.5 vs. 18.5%, respectively, P=0.014) and intermediate (23-33) (27 vs. 13.4%, respectively, P=0.049) SYNTAX score tertiles. Further analyses according to treatment with either oral hypoglycaemic agents or insulin showed that the MACCE rate was significantly greater after PCI in both the oral hypoglycaemic agent group (PCI 40.4 vs. CABG 26.4%, P=0.022) and the insulin-dependent group (PCI 56.2 vs. CABG 32.6%, P=0.002). A higher incidence of cardiac death was noted in the insulin-dependent patients treated with PCI (PCI 18.8 vs. CABG 7.1%, P=0.023).
In the SYNTAX trial, diabetes was not an independent predictor of outcomes once the SYNTAX score was entered into the multivariable model.127 Consequently, the SYNTAX 2 score does not include diabetes as one of the eight variables that impacts on the preferential selection of revascularization modality.127 Conflicting data were seen in a patient-level pooled analysis of 6081 patients treated with stents (75% newer generation DES), stratified according to diabetes status and SYNTAX score.157 After Cox regression adjustment, SYNTAX score and diabetes were both associated with MACE (P <0.001 and P=0.0028, respectively). At 2 years, patients with diabetes had higher MACE (HR 1.25, 95% CI 1.03-1.53, P=0.026) and TVR, and similar death and MI rates.157
In the BEST trial, patients with diabetes treated with PCI had a higher rate of the primary endpoint of death, MI, or TVR compared with CABG (EES: n=177; CABG: n=186) (19.2 vs. 9.1%, P=0.007) (see section 5).105
9.2.2 META-ANALYSIS OF CORONARY ARTERY BYPASS GRAFTING VS. PERCUTANEOUS CORONARY INTERVENTION IN PATIENTS WITH DIABETES
A meta-analysis – restricted to four RCTs covering 3052 patients-compared PCI with the use of early-generation DES vs. CABG in patients with diabetes and multivessel CAD. It suggested a higher risk of death and MI with revascularization by early-generation DES (RR 1.51, 95% CI 1.09-2.10; P <0.01), but a lower risk of stroke (2.3 vs. 3.8%; RR 0.59, 95% CI 0.39- 0.90; P <0.01).152 A sensitivity analysis revealed that this superiority of CABG over early-generation DES for the endpoint MACCE was most pronounced among patients with a high SYNTAX score. A network meta-analysis had suggested that the survival benefit of CABG over PCI in patients with diabetes might be lost when using EES,151 though this was not confirmed in a subsequent meta-analysis that also included the direct comparison between EES and CABG in the subset of BEST.153
In a collaborative, individual patient data pooled analysis of 11518 patients with multivessel or LM disease randomized to CABG or PCI with stents, all-cause death was significantly different after CABG (9.2%) and PCI (11.2%) (P=0.0038), which was evident in patients with diabetes (10.7 vs. 15.7%, respectively; P=0.0001) but not in patients without diabetes (8.4 vs. 8.7%, respectively; P=0.81) (Pinteraction=0.0077).124 Similar results were found in the subgroup of 7040 patients with multivessel disease (Pinteraction=0.0453), while the interaction with diabetes was not significant in the 4478 patients with LM disease (Pinteraction=0.13).
A recent population-based analysis has confirmed the benefit of CABG compared with PCI in patients with diabetes when patients present with an ACS.196 Consequently, overall current evidence continues to favour CABG as the revascularization modality of choice for patients with diabetes and multivessel disease. When patients present with a comorbidity that increases surgical risk, the choice of revascularization method is best decided by multidisciplinary individualized risk assessment.
9.3 REVASCULARIZATION WITH THE USE OF PERCUTANEOUS CORONARY INTERVENTION
For the reasons discussed above, PCI in patients with diabetes is often more complex than PCI in the absence of diabetes. Nevertheless, irrespective of diabetic status, the same principles apply as discussed in section 16. Placement of a new-generation DES is the default strategy.
9.4 ANTITHROMBOTIC PHARMACOTHERAPY
In the current context of the use of oral P2Y12-inhibitors, there is no indication that antithrombotic pharmacotherapy should differ between diabetics and patients without diabetes who are undergoing revascularization. For detailed discussion refer to section 17.
9.5 METFORMIN
There is a theoretical risk of lactic acidosis and deteriorating renal function in patients treated with metformin who are exposed to iodinated contrast media.280 Consequently, it is generally recommended that in elective cases, metformin should be withheld before angiography or PCI for 48 h, as the plasma half-life of metformin is 6.2 h,280 and reintroduced 48 h later. However, clinical experience suggests that the actual risk of lactate acidosis is very small, and that checking renal function after angiography in patients on metformin and withholding the drug when renal function deteriorates appears to be an acceptable alternative.280 In patients with renal failure, metformin should be stopped before the procedure. Accurate recognition of metformin-associated lactic acidosis based on arterial pH <7.35, blood lactate >5 mmol/L (45 mg/dL), and detectable plasma metformin concentration should prompt the initiation of haemodialysis.
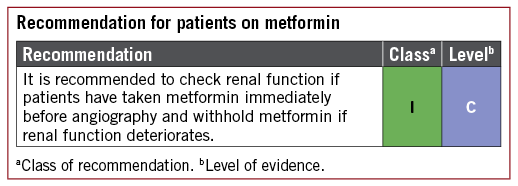
9.6 GAPS IN THE EVIDENCE
Following successful revascularization, the rate of events during follow-up remains high in patients with diabetes, independent of the mode of revascularization. Future research should be focused on identifying new disease-modifying therapies to influence the progression of vascular disease in this high-risk cohort.
10 Revascularization in patients with chronic kidney disease
10.1 EVIDENCE BASE FOR REVASCULARIZATION AND RECOMMENDATIONS
Myocardial revascularization in patients with chronic kidney disease (CKD), specifically National Kidney Foundation stage 3 or higher, is addressed by the 2014 ESC/EACTS Guidelines on myocardial revascularization. After reviewing the subsequent literature, the current Task Force has not found any evidence to support a major update. A recent post hoc analysis of the SYNTAX trial on patients with CKD confirms the principles for allocating patients to PCI or CABG,281 as discussed in section 5 of this document.
10.2 PREVENTION OF CONTRAST-INDUCED NEPHROPATHY
The risk of contrast-induced nephropathy (CIN) depends on patient-related factors, such as CKD, diabetes mellitus, congestive HF, haemodynamic instability, reduced plasma volume, female sex, advanced age, anaemia, and periprocedural bleeding, as well as on the type and volume of contrast administered.282-288 When the ratio of total contrast volume (in mL) to glomerular filtration rate (in mL/min) exceeds 3.7, the risk of CIN increases significantly.287,288
Adequate hydration remains the mainstay of CIN prevention.289-294 High-dose statins, as indicated for secondary prevention irrespective of the risk of CIN are also beneficial.293 All other strategies for the prevention of CIN do not have sufficient evidence to justify a recommendation in favour or against.293,294 For more detailed discussion refer to the Supplementary Data.
10.3 GAPS IN THE EVIDENCE
Thus far, patients with CKD have been excluded from randomized trials on myocardial revascularization, hence current data are based on observational studies only. A randomized trial on optimal long-term revascularization strategies in patients with moderate-to-severe stress-induced ischaemia and severe CKD is currently ongoing (ISCHEMIA-CKD, https://clinicaltrials.gov/ct2/show/NCT01985360). Moreover, additional randomized evidence on optimal strategies for CIN prevention is needed.
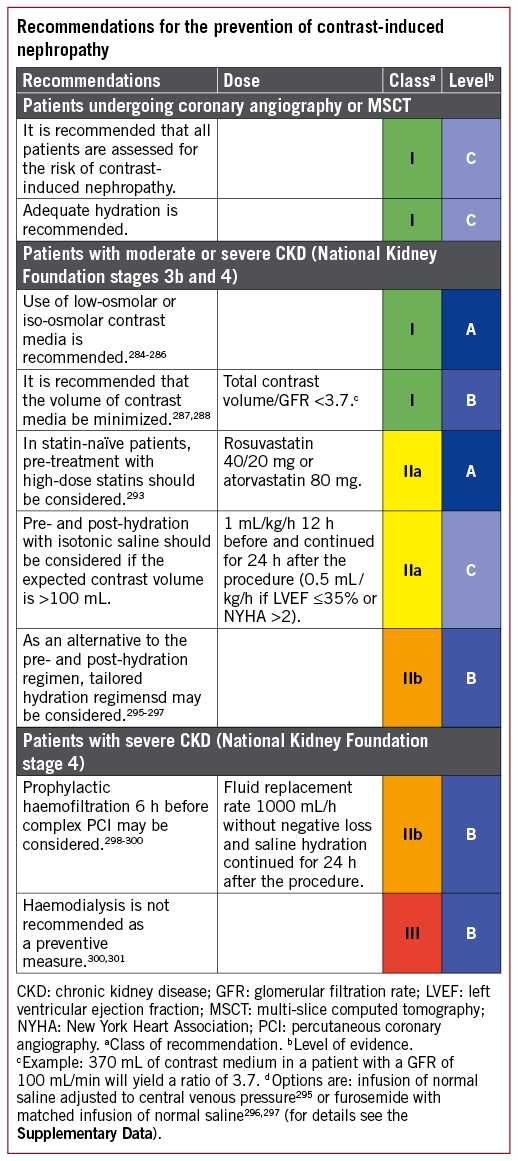
11 Revascularization in patients requiring valve interventions
11.1 PRIMARY INDICATION FOR VALVE INTERVENTIONS
Myocardial revascularization in patients undergoing primary valve interventions, either by surgery or transcatheter routes, is addressed by the 2014 ESC/EACTS Guidelines on myocardial revascularization. After reviewing the subsequent literature, the current Task Force endorses the recommendations of the 2014 Guidelines and has not found any evidence to support a major update. These recommendations are included below for ease of reference. Of note, the available evidence on invasive functional assessment of CAD (with FFR or iwFR) in patients with severe aortic stenosis (AS) is limited to a few small-scale observational studies. These studies support the feasibility of FFR and iwFR in this setting.302-304 Notwithstanding, the available evidence is insufficient to support the use of invasive functional assessment of coronary lesions in patients with AS, particularly in consideration of the altered haemodynamic condition related to the presence of AS. Therefore, the Task Force is in consensus that indications for myocardial revascularization based on angiographic assessment of CAD should be maintained, consistent with the 2014 ESC/ EACTS Guidelines on myocardial revascularization and the 2017 ESC/EACTS Guidelines for the management of valvular heart disease.305
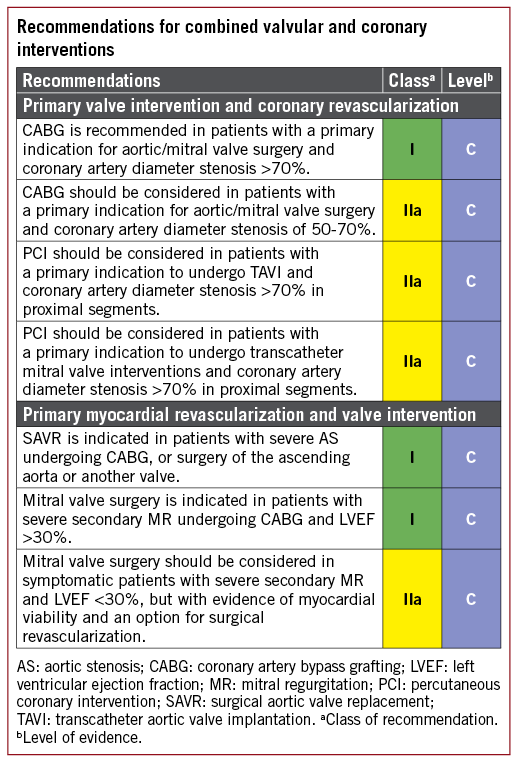
11.2 PRIMARY INDICATION FOR MYOCARDIAL REVASCULARIZATION
11.2.1 AORTIC VALVE DISEASE
The recommendations for patients undergoing CABG for the clinically leading problem of CAD, who also have coexisting severe aortic stenosis or regurgitation, remain unchanged from those of the 2014 Guidelines and support replacement of the aortic valve.305 However, in the current era of rapid developments in transcatheter valve implantation technologies, a decision regarding replacement of the aortic valve for moderate stenosis/regurgitation should be carefully considered on a case-by-case basis in discussion with the Heart Team. The patient’s age, type of prosthesis, pathogenesis of aortic stenosis/regurgitation, aortic annular size, predicted size of implanted valve, transcatheter aortic valve implantation (TAVI) access routes, and technical feasibility of a TAVI procedure in the future in case of disease progression should all be taken into account.306
11.2.2 MITRAL VALVE DISEASE
Patients with concomitant severe primary mitral regurgitation (MR) should undergo mitral valve repair at the time of CABG in keeping with guidance for the surgical repair of primary MR.305 There is also consensus based on expert opinion on the surgical repair of severe secondary MR at the time of CABG.305,307 However, considerable controversy exists about the treatment of moderate secondary or ischaemic MR in patients undergoing CABG. Until the publication of 2 year outcomes of the CTSN (Cardiothoracic Surgical Trials Network) randomized trial on treatment of `moderate’ ischaemic MR, the literature in this field was limited to small single-centre randomized trials, observational studies, and case series, and failed to provide clear direction. The CTSN trial showed that addition of surgical mitral valve repair to CABG made no significant difference to survival, overall reduction of adverse events, or LV reverse remodelling at 2 years.308,309 Increased length of intensive care and hospital stay and perioperative morbidity, including neurological complications and supraventricular arrhythmias, were reported in the CTSN and other randomized trials in this group of patients.308-310 Because the CTSN trial used a very broad definition of moderate MR, including an effective regurgitant orifice area (EROA) ≤0.2 cm2 plus additional criteria, no firm conclusions can be drawn concerning patients with an EROA >0.2 cm2. Observational data suggest that in secondary MR, an EROA >0.2 cm2 and regurgitant volume >30 mL indicates greater risk of cardiovascular events.311,312 In the absence of dedicated trials in this setting, the decision to combine mitral valve surgery with CABG in patients with an EROA >0.2 cm2 and regurgitant volume >30 mL needs to be made on a case-by-case basis by the Heart Team. For a more detailed discussion of this issue, please refer to the Supplementary Data.
11.3 GAPS IN THE EVIDENCE
In patients with concomitant valvular and coronary disease, the possibility of future transcatheter therapy for the aortic and mitral valves has made a significant impact on decision-making for patients with predominantly coronary disease with moderate valve lesions. However, there is currently little evidence on this topic. The need for and timing of PCI in patients undergoing TAVI is also an area with limited evidence. The long-term outcomes of patients with concomitant surgical repair of ischaemic MR are also awaited.
12 Associated peripheral artery diseases
12.1 PREVENTION OF STROKE ASSOCIATED WITH CAROTID ARTERY DISEASE AND MYOCARDIAL REVASCULARIZATION
The early risk of stroke after myocardial revascularization is higher after CABG than after PCI.313 After 30 days, stroke rates between revascularization techniques were similar in a recent individual patient data meta-analysis of 11 randomized trials.313
Ischaemic stroke after CABG is multifactorial: thrombo-embolism from the aorta, its branches, or the heart; atrial arrhythmias; inflammatory pro-thrombogenic milieu; lower levels of antiplatelet therapy perioperatively; and haemodynamic instability. However, the most consistent predictor of perioperative stroke is previous stroke or TIA. There is no strong evidence that carotid artery stenosis is a significant cause of perioperative stroke except for bilateral severe carotid bifurcation stenosis.314 Therefore, indications for preoperative carotid bifurcation screening by duplex ultrasound are limited.315 Also, there is no evidence that prophylactic revascularization of unilateral asymptomatic carotid stenoses in CABG candidates reduces the risk of perioperative stroke. It may be reasonable to restrict prophylactic carotid revascularization to patients at highest risk of post-operative stroke, i.e. patients with severe bilateral lesions or a history of prior stroke/TIA.316 Hence, the indication for revascularization, and the choice between carotid endarterectomy or carotid artery stenting in these patients, should be made by a multidisciplinary team including a neurologist.
The 2017 Guidelines on the diagnosis and treatment of peripheral arterial diseases in collaboration with the European Society of Vascular Surgery cover the screening for and management of carotid artery disease in patients scheduled for CABG, including screening, indications, and the timing and type of carotid revascularization.317 Its recommendations are reproduced here.
Particularly for patients at high risk for perioperative stroke after CABG, such as elderly patients or patients with previous TIA/stroke, specific preventive measures have been suggested. CT scan screening of the ascending aorta/arch atheroma has been proposed to better assess risk stratification and guide the surgical strategy in elderly patients.318 It is recommended that acetylsalicylic acid is restarted 6 h, or at the latest 24 h, after surgery, and that clopidogrel or ticagrelor are added in patients with ACS. New-onset atrial fibrillation (AF) is associated with a risk of stroke that increased two-to-three times after CABG. Its management is discussed in section 14.
12.2 ASSOCIATED CORONARY AND PERIPHERAL ARTERY DISEASES
Of all patients with CAD, 7-16% have lower extremity artery disease (LEAD), which is associated with a worse prognosis, even if it remains frequently asymptomatic, masked by cardiac symptoms. On the other hand, in patients with LEAD, CAD is present in up to 70% of patients.317 The choice between CABG and PCI is controversial and, in the absence of solid data, it should follow a multidisciplinary approach.127 In patients undergoing CABG, the saphenous vein should be preserved or harvested guided by the results of clinical examination including the ankle-brachial index. In addition, inter-arm blood pressure asymmetry should lead to the investigation of subclavian artery stenosis. Further details are provided in the 2017 peripheral arterial diseases Guidelines.317
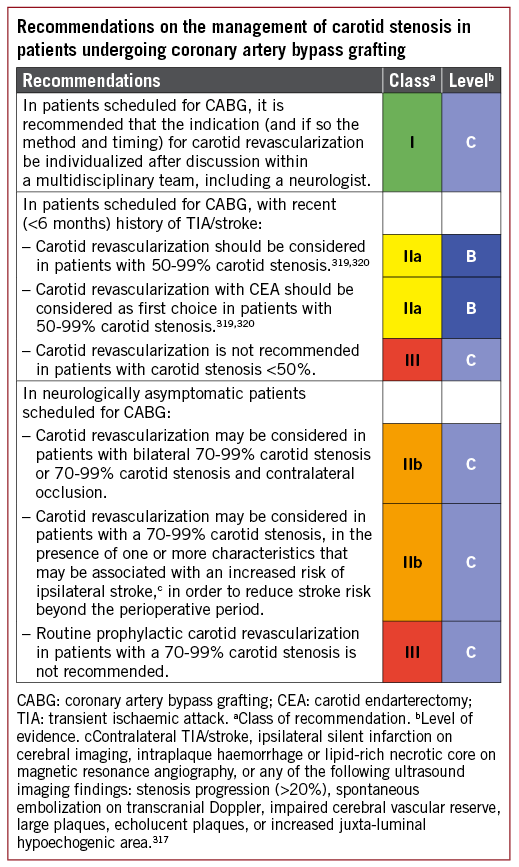
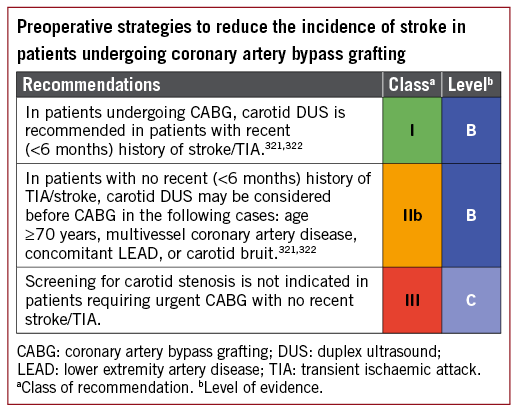
13 Repeat revascularization
13.1 EARLY GRAFT FAILURE
Early graft failure after CABG is reported in up to 12% of grafts, as evaluated by intraoperative angiography.323 However, only a minority (around 3%) are clinically apparent. Graft failure can be due to conduit defects, anastomotic technical errors, poor native vessel run-off, or competitive flow with the native vessel. When clinically relevant, acute graft failure may result in MI with consequently increased mortality and major cardiac events. The suspicion of early graft failure should arise in the presence of ECG signs of ischaemia, ventricular arrhythmias, biomarker changes, new wall motion abnormalities, or haemodynamic instability.324,325 Owing to the low specificity of ECG changes and echocardiographic wall motion abnormalities during the post-operative course, and the delay in the appearance of biomarker changes, a careful assessment of all variables will influence decision-making for angiographic evaluation.
Perioperative angiography is recommended in cases of suspected severe myocardial ischaemia to detect its cause and aid decision-making on the most appropriate treatment.323,325,326 In symptomatic patients, early post-operative graft failure can be identified as the cause of ischaemia in 40-80% of cases.324,326-328 The optimal treatment strategy in patients with acute graft failure should be decided by ad hoc consultation between the cardiovascular surgeon and the interventional cardiologist, on the basis of the patient’s clinical condition and the extent of myocardium at risk. In the case of early post-operative graft failure, emergency ad hoc PCI may limit the extent of infarction, if technically feasible. The target for PCI is the native vessel or the internal mammary artery (IMA) graft, while the acutely occluded saphenous vein graft (SVG) and any anastomotic site should be avoided, if possible, due to concerns regarding embolization or perforation. Redo surgery should be favoured if the anatomy is unsuitable for PCI, if several important grafts are occluded, or in the case of clear anastomotic errors. In asymptomatic patients, repeat revascularization should be considered if the artery is of an appropriate size and supplies a large territory of myocardium.
Further details on the diagnosis and management of perioperative MI are provided in a recent ESC position paper.329
13.2 ACUTE PERCUTANEOUS CORONARY INTERVENTION FAILURE
The need for urgent surgery to manage PCI-related complications is uncommon (<1%) and only required in patients with major complications that cannot be adequately resolved by percutaneous techniques.330,331 The need for emergency CABG is mainly confined to patients with a large, evolving MI due to iatrogenic vessel occlusion that cannot be salvaged percutaneously, or in patients with recurrent cardiac tamponade after pericardiocentesis following PCI-related vessel rupture.330,332,333
13.3 DISEASE PROGRESSION AND LATE GRAFT FAILURE
Ischaemia after CABG may be due to the progression of disease in native vessels or de novo disease of bypass grafts.334 Repeat revascularization in these patients is indicated in the presence of significant symptoms despite medical treatment, and in asymptomatic patients with objective evidence of large myocardial ischaemia (>10% of the LV).32,87
13.3.1 REDO CORONARY ARTERY BYPASS GRAFTING OR PERCUTANEOUS CORONARY INTERVENTION
PCI in patients with prior CABG has worse acute and long-term outcomes than in patients without prior CABG.335,336 Likewise, redo CABG has a two- to four-fold increased mortality compared with first-time CABG, and repeat CABG is generally performed infrequently.334,337-339 There are limited data comparing the efficacy of PCI vs. redo CABG in patients with previous CABG. The proportion of patients undergoing PCI, redo CABG, or conservative treatment differs significantly between studies; in one study, PCI was favoured in 50% of patients with only 22% undergoing redo CABG, while another study favoured CABG in 67% of patients.340,341 In the AWESOME (Angina With Extremely Serious Operative Mortality Evaluation) RCT and registry, overall 3 year mortality was comparable between redo CABG and PCI.341,342 A more recent study also found comparable rates of death and MI between redo CABG and PCI, although there were significantly more repeat revascularizations with PCI.341,343
In view of the higher risk of procedural mortality with redo CABG and the similar long-term outcome, PCI is the preferred revascularization strategy in patients with amenable anatomy.340 PCI via the bypassed native artery should be the preferred approach. If PCI in the native vessel fails or is not an option, PCI in the diseased SVG should be considered. CABG should be considered for patients with extensively diseased or occluded bypass grafts and diffuse native vessel disease, especially in the absence of patent arterial grafts.340 The IMA is the conduit of choice for revascularization during redo CABG if not previously used, or can be salvaged and reused in specific cases.344,345
13.3.2 PERCUTANEOUS CORONARY INTERVENTION FOR SAPHENOUS VEIN GRAFT LESIONS
PCI in SVGs is associated with an increased risk of distal coronary embolization, frequently resulting in periprocedural MI.346 PCI of de novo SVG stenosis is considered a high-risk intervention because SVG atheroma is friable and more prone to distal embolization. Several different approaches have been evaluated to prevent the distal embolization of particulate debris, including distal occlusion/aspiration, proximal occlusion, suction, filter devices, or covered stents. Distal protection devices using filters have shown the most encouraging results. However, although a single randomized trial supports the use of distal embolic protection during SVG PCI, observational studies including data from large-scale registries are conflicting.347-349 Outcomes from studies with other devices used for SVG PCI are not sufficient to recommend its use.350-353
Based on data from a small number of randomized trials, implantation of DES in SVG lesions is associated with a lower risk of repeat revascularization than with BMS at 1 year follow-up.354-356 In the only trial powered for a clinical endpoint – the ISAR-CABG (Is Drug-Eluting-Stenting Associated with Improved Results in Coronary Artery Bypass Grafts) trial354 – the primary endpoint of death, MI, and target lesion revascularization was significantly reduced with DES vs. BMS. However, at 5 year follow-up, the advantage of DES over BMS was lost due to a higher incidence of target lesion revascularization between years 1 and 5 in patients treated with DES.357 Longer-term follow-up of the two smaller trials is available; one suggested sustained superiority of DES over BMS, while the other suggested loss of the efficacy advantage of the DES.358,359
13.4 REPEAT PERCUTANEOUS CORONARY INTERVENTION
Recurrence of symptoms or ischaemia after PCI is the result of restenosis, incomplete initial revascularization, or disease progression.334 Patients may require repeat PCI due to late and very late stent thrombosis.
13.4.1 RESTENOSIS
Restenosis associated with angina or ischaemia should be treated by repeat revascularization, and repeat PCI remains the strategy of choice for most of these patients. In this setting, the results from DES are superior to those obtained with balloon angioplasty, BMS implantation, or brachytherapy.360-364
For restenosis within BMS, drug-coated balloon (DCB) proved superior to plain balloon angioplasty365-367 and comparable to first-generation DES.365,366,368-372 One trial showed inferior angiographic outcomes in comparison to new-generation DES,373 while a second trial showed comparable outcomes.374 For restenosis within DES, DCBs also proved superior to plain balloon angioplasty367,369,371 and comparable to first-generation DES.371 In one study, DCBs were inferior to new-generation DES in terms of the primary angiographic outcome measure.375 In a more recent study, including patients with any type of in-stent restenosis, outcomes between DCB and repeat stenting with new-generation DES were comparable.376 A single randomized trial of patients undergoing DCB for restenosis within DES showed superior angiographic outcomes in patients who underwent lesion preparation with scoring balloons vs. standard angioplasty balloons.377
Network meta-analysis suggests that repeat stenting with new-generation DES (with EES) and DCB are ranked first and second as the highest efficacy treatments.378,379 The superior angiographic antirestenotic efficacy of new-generation DES should be weighed against a possible excess of long-term adverse events with repeat stenting during longer-term follow-up of these trials.380,381 However, observations in relation to clinical events must be interpreted with caution, as none of the trials was powered for clinical endpoints and the comparator stent in studies with long-term follow-up was an early-generation DES.
The use of intracoronary imaging provides unique insights into the underlying mechanisms of in-stent restenosis (see section 16.2). OCT is able to detect the presence of neoatherosclerosis in a significant number of these patients. Underexpanded stents should be aggressively tackled with high-pressure dilatations using non-compliant balloons. The optimization of the final results remains crucial during reinterventions for in-stent restenosis and, in this regard, the use of intracoronary imaging may be particularly helpful. Outcomes of patients with in-stent restenosis after DES are poorer than those in patients with BMS in-stent restenosis, independently of the therapeutic modality.382 In patients with recurrent episodes of diffuse in-stent restenosis in large vessels – and in those with associated multivessel disease, especially in the presence of other complex lesions such as chronic total occlusions – CABG should be considered before a new PCI attempt.
13.4.2 DISEASE PROGRESSION
Patients with symptomatic disease progression after PCI account for up to 50% of reinterventions.383,384 They should be managed using criteria similar to those applied to patients without previous revascularization.
13.4.3 STENT THROMBOSIS
Although stent thrombosis is very rare, particularly since the advent of new-generation DES, it may have devastating clinical consequences. Stent thrombosis usually presents as a large MI and patients should be treated according to the principles outlined in section 8.385 Aggressive, high-pressure balloon dilation should be used to correct underlying, stent-related, predisposing mechanical problems.386,387 Liberal use of intracoronary imaging in order to detect and modify underlying mechanical factors is recommended (Figure 7) (see section 16.2).
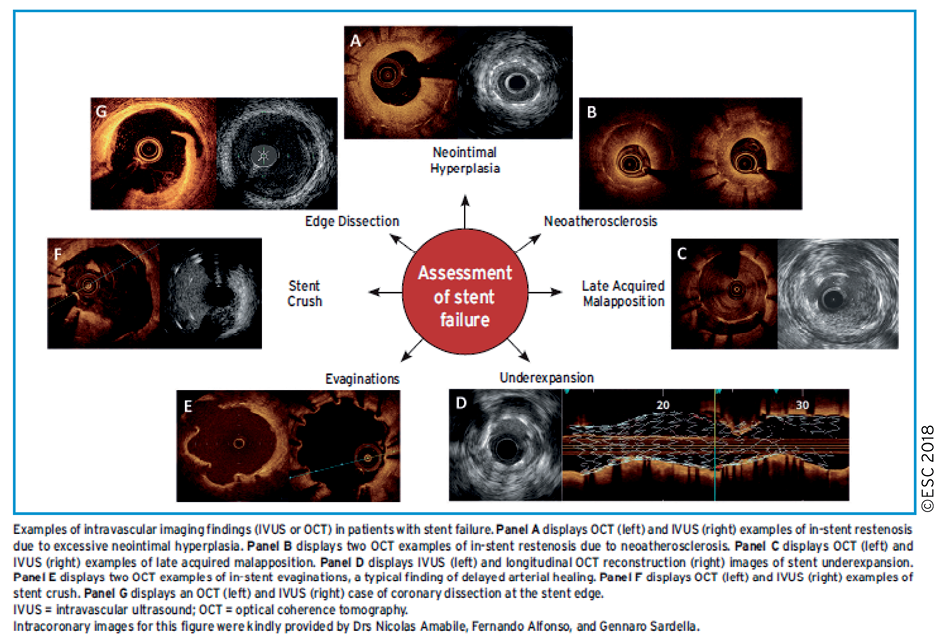
Figure 7. Intracoronary imaging for the assessment of stent failure.
Although repeat stenting in patients with stent thrombosis may be avoided when satisfactory results are obtained with balloon dilation, a new stent may be required to overcome edge-related dissections and adjacent lesions, or to optimize final results.388
There is no evidence that the post-interventional management of patients with stent thrombosis should differ from that of patients with thrombosis of a de novo lesion resulting in STEMI.
14 Arrhythmias
14.1 VENTRICULAR ARRHYTHMIAS
14.1.1 REVASCULARIZATION FOR THE PREVENTION OF SUDDEN CARDIAC DEATH IN PATIENTS WITH STABLE CORONARY ARTERY DISEASE AND REDUCED LEFT VENTRICULAR FUNCTION
Revascularization plays an important role in reducing the frequency of ventricular arrhythmias in patients with normal or mildly reduced LV function,389,390 as well as the risk of sudden cardiac death in patients with CAD and LVEF ≤35%.391 Indirect evidence for a protective effect of revascularization was demonstrated in the MADIT II (Multicenter Automatic Defibrillator Implantation Trial II) and SCD-HEFT studies (Sudden Cardiac Death in Heart Failure Trial), where the efficacy of implantation cardioverter defibrillators (ICDs) was reduced if revascularization was performed prior to implantation.392,393 CABG in patients with reduced EF reduces cardiac and overall mortality for a follow-up of 10 years.78,81 In view of the protective effect of revascularization on ventricular arrhythmias, patients with ischaemic LV dysfunction (LVEF ≤35%) who are considered for primary preventive ICD implantation should be evaluated for ischaemia and/or for potential revascularization targets.
14.1.2 REVASCULARIZATION FOR THE TREATMENT OF ELECTRICAL STORM
Electrical storm is a life-threatening syndrome related to incessant ventricular arrhythmias, which is most frequently observed in patients with ischaemic heart disease, advanced systolic HF, valve disease, corrected congenital heart disease, and genetic disorders such as Brugada syndrome, early repolarization, and long QT syndrome.394 Urgent coronary angiography and revascularization should be part of the management of patients with electrical storm, as well as antiarrhythmic drug therapy and/or ablation of ventricular tachycardia.
14.1.3 REVASCULARIZATION AFTER OUT-OF-HOSPITAL CARDIAC ARREST
Approximately 70% of survivors of out-of-hospital cardiac arrest have CAD, with acute vessel occlusion observed in 50%.395 Multiple non-randomized studies suggest that emergency coronary angiography and, if appropriate, PCI after out-of-hospital cardiac arrest yield a favourable survival rate of ≤60% at 1 year, which is considerably higher than the 25% overall survival rate in patients with aborted cardiac arrest.396,397 More recent data suggest that almost one-quarter of patients, resuscitated from cardiac arrest but without ST-segment elevation, show a culprit lesion (either vessel occlusion or irregular lesion).398-401 Recent large-scale observational studies have shown an impact on mortality of early angiography after out-of-hospital cardiac arrest.402,403 Thus, in survivors of out-of-hospital cardiac arrest, early coronary angiography and PCI, if appropriate, should be performed irrespective of the ECG pattern if no obvious non-cardiac cause of the arrhythmia is present.404
14.2 ATRIAL ARRHYTHMIAS
The management of AF in patients with ischaemic heart disease is addressed by the 2016 ESC Guidelines for the management of AF developed in collaboration with EACTS.405 After reviewing the subsequent literature, the current Task Force endorses the recommendations of the 2016 Guidelines and has not identified a need for any major update. Accordingly, the recommendation tables are taken from the 2016 Guidelines. For a detailed discussion, we refer to the previous Guidelines.405
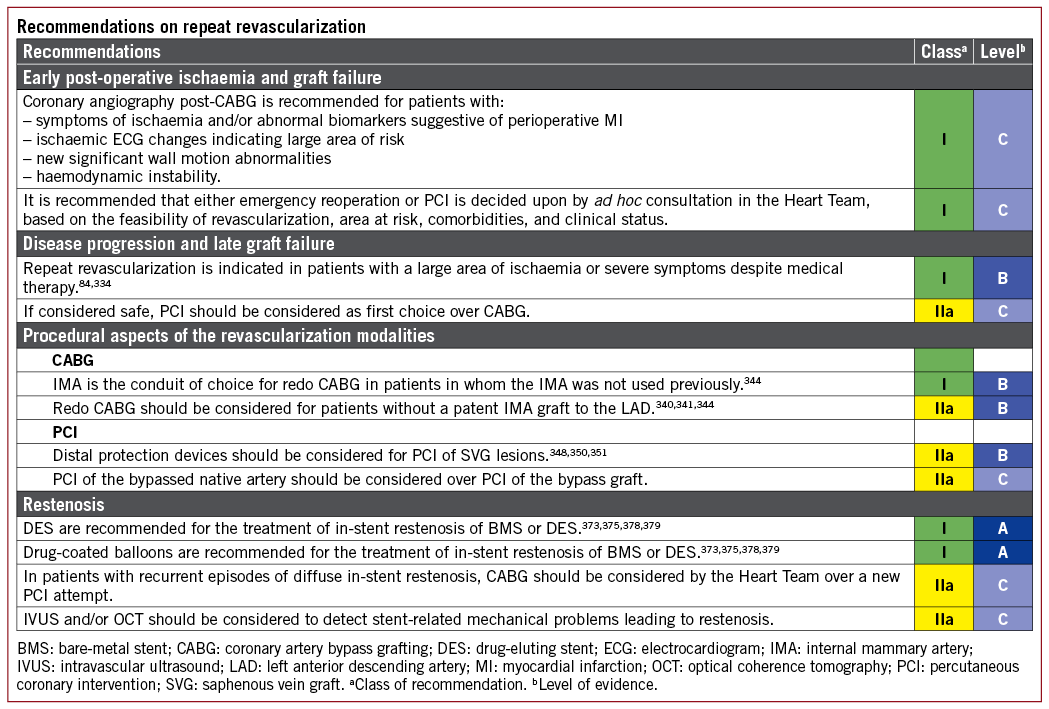
14.2.1 ATRIAL FIBRILLATION COMPLICATING PERCUTANEOUS CORONARY INTERVENTION
New-onset AF in patients undergoing PCI occurs in 2-6% of procedures and increases with age, pre-existing HF, AMI, and arterial hypertension.406-409 Notably, new-onset AF [defined as change from sinus rhythm (SR) at admission to AF during/after PCI] typically occurs during the first 4 days after AMI, and is associated with impaired prognosis and a more than doubling of the risk of death, congestive HF, and stroke.403
The use of oral anticoagulation (OAC) for stroke prevention in patients with AF occurring during or after PCI should follow the ESC Guidelines on Atrial Fibrillation for antithrombotic treatment of AF that occurs outside the setting of PCI,405 although prospective studies are scarce. The combination and duration of anticoagulation and antiplatelet therapy should be assessed according to the clinical situation, as outlined in section 17 as well as in the ESC Guidelines on Atrial Fibrillation405 and the ESC Focused Update on Dual Antiplatelet Therapy.410
14.2.2 ATRIAL FIBRILLATION COMPLICATING CORONARY ARTERY BYPASS GRAFTING
Post-operative AF affects one-third of patients undergoing cardiac surgery.411-414 The main risk factor for post-operative AF is age, and it is associated with an increased immediate risk of stroke, increased morbidity, and 30 day mortality.415-417 In the long-term, patients with an episode of post-operative AF have a two-fold increase in cardiovascular mortality, and a substantially increased risk of future AF and ischaemic stroke compared with patients who remain in SR after surgery.416,418-422
Post-operative AF is a common complication, in which prophylactic treatment has a moderate effect. Pre-operative anti-arrhythmic drug treatment may be initiated but will have to be weighed against side effects. Beta-blockers decrease the incidence of post-operative AF after CABG.412,423-429
14.2.3 POST-OPERATIVE ATRIAL FIBRILLATION AND STROKE RISK
Patients with post-operative AF have an increased stroke risk post-operatively as well as during follow-up,419,430 and warfarin medication at discharge has been associated with a reduced long-term mortality.431 To date, there are no studies indicating that post-operative AF is less harmful than any other form of AF, and good quality data are needed. Anticoagulation treatment with warfarin or non-vitamin K antagonist oral anticoagulants (NOACs) for stroke prevention in patients with post-operative AF should therefore follow the guidelines for the antithrombotic treatment of AF occurring outside the setting of CABG using the CHA2DS2-VASc [Cardiac failure, Hypertension, Age ≥75 (Doubled), Diabetes, Stroke (Doubled) - Vascular disease, Age 65-74 and Sex category (Female)] score. The duration and timing of OAC in post-operative AF patients should be assessed individually.
Whether or not surgical left atrial appendage (LAA) obliteration reduces stroke risk has been studied in smaller trials and registry studies with conflicting results,432-434 and is currently under investigation in a large randomized trial.435 Removal or closure of the LAA should be considered as an adjunct to anticoagulation and not as an alternative until more data are available.
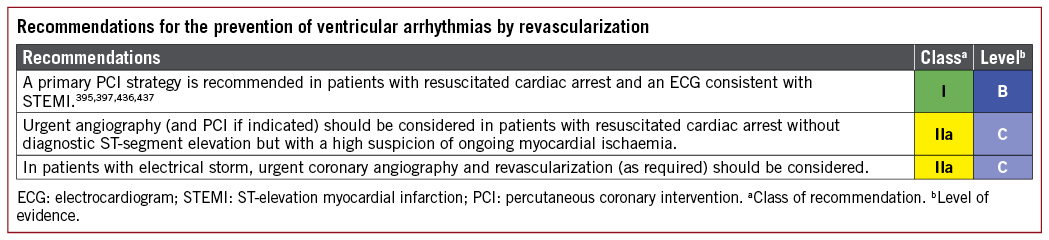
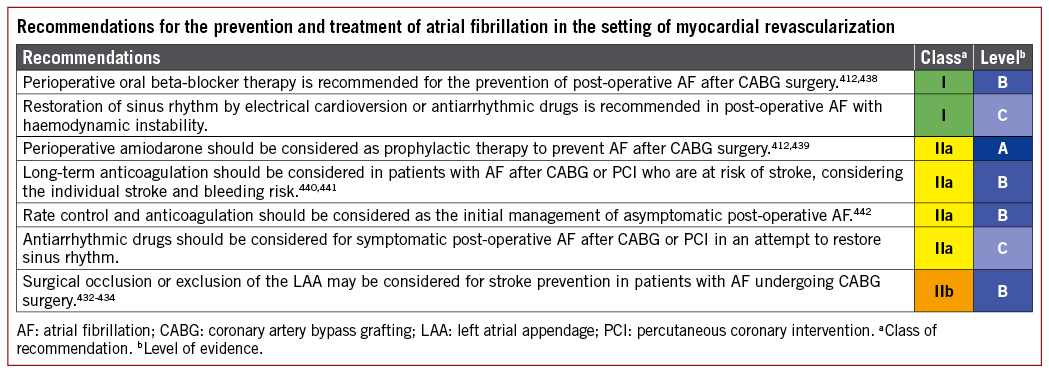
14.3 GAPS IN THE EVIDENCE
The duration of anticoagulation and their combination with antiplatelet therapy in patients with new-onset AF after PCI or CABG has not been studied sufficiently. Likewise, the role of routine left atrial exclusion at surgery for the prevention of stroke is currently unclear.
15 Procedural aspects of coronary artery bypass grafting
CABG remains the most common cardiac surgical procedure, and the techniques have been refined during 50 years of evolution.443 Perioperative medication and blood management are covered in separate Guidelines.410,444
15.1 SURGICAL TECHNIQUES
15.1.1 COMPLETENESS OF REVASCULARIZATION
Current surgical practice is largely based on an anatomical definition of complete revascularization, and aims to bypass all epicardial vessels with a diameter exceeding ≥1.5 mm and a luminal reduction of ≥50% in at least one angiographic view.131 Depending on the definition of completeness of revascularization, the outcome after CABG in patients with incomplete revascularization was either similar445-449 or inferior131,132,449-451 to that of patients with complete revascularization. Certainly, in some patients with a stenosis in small vessels with little myocardium at risk, complete revascularization may not be necessary.
FFR-guided surgical revascularization has been associated with improved graft patency, but more studies are needed to investigate whether it improves clinical outcomes.28,452 Further discussion of FFR-guided revascularization is provided in sections 3.2.1.1 and 5.3.1.3.
15.1.2 CONDUIT SELECTION
In addition to patient-related factors, the outcome following CABG is related to the long-term patency of grafts and therefore is maximized with the use of arterial grafts, specifically the IMA.453,454 Except in rare circumstances, all patients should receive at least one arterial graft – the left IMA (LIMA) – preferentially to the LAD.453,455 SVG patency rates for non-LAD targets have been reported to be suboptimal.456 Bilateral IMA (BIMA) and radial artery for non-LAD targets have been shown to provide better patency rates than SVG, particularly for the left coronary artery system.457 Therefore, a second arterial graft should be considered depending on the patient’s life expectancy, risk factors for sternal wound complications, coronary anatomy, degree of target vessel stenosis, graft quality, and surgical expertise.
Whether or not the use of additional arterial grafts can translate into prolonged survival remains debatable. Data from non-randomized studies suggest that the use of BIMA over single IMA (SIMA) use is associated with improved long-term survival, as well as fewer non-fatal events such as MI, recurrent angina, and the need for re-operation.458-465 However, observational studies are subject to selection bias, despite propensity matching, and the effect of prolonged survival with additional arterial grafts has not been confirmed in randomized trials.466
The ART trial (Arterial Revascularization Trial) has been designed to answer the question of whether BIMA can improve 10 year survival when compared with SIMA. Interim analysis showed no difference at 5 years in the rate of death or the composite of death, MI, or stroke, and 10 year results are warranted to draw final conclusions.467 Limitations of the ART trial include a high crossover rate from the BIMA arm to the SIMA arm and a high rate of radial artery use in the SIMA arm that may have diluted the benefit of BIMA.468-470 The use of BIMA grafting is associated with an increase in sternal dehiscence, and an increased rate of mediastinitis in obese patients and patients with diabetes.458,464,471-475 In the ART trial, the use of BIMA was associated with a 1.0-1.5% absolute risk increase in the need for sternal would reconstruction, and a subsequent subanalysis has found that this risk is minimized with skeletonized harvesting.476 While we await the 10 year data of the ART trial, BIMA grafting should be considered in patients with a reasonable life expectancy and a low risk of sternal wound complications.
The radial artery constitutes an alternative as the second arterial graft in patients in whom BIMA grafting is not feasible, patients with a high risk of sternal wound complications, or as a third arterial graft. There is a strong, adverse influence on radial artery patency when the native coronary artery stenosis is <70%, and therefore its use should be limited to coronary artery stenosis >70% and ideally >90%.477 Use of the radial artery as the second conduit of choice has been linked to improved survival in registry studies.478-480 Available RCTs testing the radial artery vs. saphenous vein graft used angiographic patency as the primary endpoint, and none was powered to detect differences in clinical outcomes.481 A recently published patient-level meta-analysis pooling six RCTs comparing radial artery vs. saphenous vein graft showed that the use of the radial artery was associated with a lower rate of the primary endpoint (composite of death, myocardial infarction, and repeat revascularization) at mean follow-up of 50 months, mainly driven by a significantly strong reduction of need for reintervention and a more modest reduction in subsequent MI.482 Despite a significantly lower risk of occlusion at follow-up angiography, no difference in all-cause mortality was found.
15.1.3 MAMMARY ARTERY HARVESTING
While the skeletonized technique of harvesting the IMA has a higher theoretical potential for injury, the potential benefits include a longer conduit, more versatility (sequential anastomosis), higher blood flow, and fewer wound-healing problems.471,483-488 Therefore, in patients at higher risk of sternal wound complications, skeletonization is recommended.
15.1.4 RADIAL ARTERY HARVESTING
Radial artery harvesting is associated with negligible morbidity if preceded by assessment of the hand’s collateral circulation. Endoscopic radial harvesting is possible, but robust evidence concerning its safety and efficacy is scarce.489,490 Use of the radial artery after recent coronary angiography with radial access should be discouraged due to potential endothelial damage.491 Harvesting of the whole radial artery pedicle, together with the intraluminal and subadventitial injection of vasodilators, are useful steps to prevent spasm.
15.1.5 SAPHENOUS VEIN HARVESTING
Saphenous vein harvesting can be accomplished using open and minimally invasive techniques, which include interrupted incisions and partial or full endoscopic procedures. Endoscopic vein graft harvesting leads to a reduced rate of leg wound complications,492-495 but the short- and long-term patency of endoscopically harvested vein grafts, compared with openly harvested grafts, has been challenged.456,496-498 Although there is no unequivocal evidence concerning patency rates, most data from meta-analyses and randomized and non-randomized trials do not demonstrate inferior clinical outcomes with endoscopic vein harvest.492,493,499,500 If an endoscopic vein graft harvest is performed, it should be undertaken by experienced surgeons or physician assistants with appropriate training and reasonable caseloads.501-503 If an open technique is used, the `no-touch’ technique has shown superior patency rates in multiple randomized trials,504-507 with a patency rate >80% after 16 years.507
15.1.6 CROSS-CLAMPING
A single cross-clamp technique may be preferred to multiple manipulations of the aorta, with the aim of reducing atheroembolic events, but a strict no-touch technique most effectively reduces embolization of atherosclerotic material.508-510 In cases of off-pump surgery, devices that allow a clampless procedure may help reduce the incidence of cerebral vascular complications.511,512
15.1.7 INTRAOPERATIVE QUALITY CONTROL
Besides continuous ECG monitoring and transoesophageal echocardiography immediately after revascularization, intraoperative quality control may also include graft flow measurement to confirm or exclude a technical graft problem.513 Transit-time flow measurement is the most frequently used technique for graft assessment and has been able to detect 2-4% of grafts that require revision.513,514 In observational studies, the use of intraoperative graft assessment has been shown to reduce the rate of adverse events and graft failure, although interpretation can be challenging in sequential and T-graft configurations.513,515-517
15.1.8 ON-PUMP AND OFF-PUMP PROCEDURES
Two large, international randomized trials have shown no difference in 30 day or 1 year clinical outcomes between on- and off-pump surgery when performed by experienced surgeons.518-520 There is also evidence to conclude that, for most patients and surgeons, on-pump surgery provides excellent short- and long-term outcomes.518,520-523 For some surgeons, off-pump surgery is associated with inferior early and late graft patency rates, and possibly compromised long-term survival; however, aortic no-touch/clampless off-pump procedures in the hands of highly trained teams appear to be associated with a reduced risk of early morbidity, such as stroke, and fewer transfusions.508-510,524-528 In the subgroup of patients with end-stage CKD, there is some evidence that off-pump surgery is associated with lower in-hospital mortality and less need for new renal replacement therapy.529
A summary of these technical aspects can be found in Figure 8.
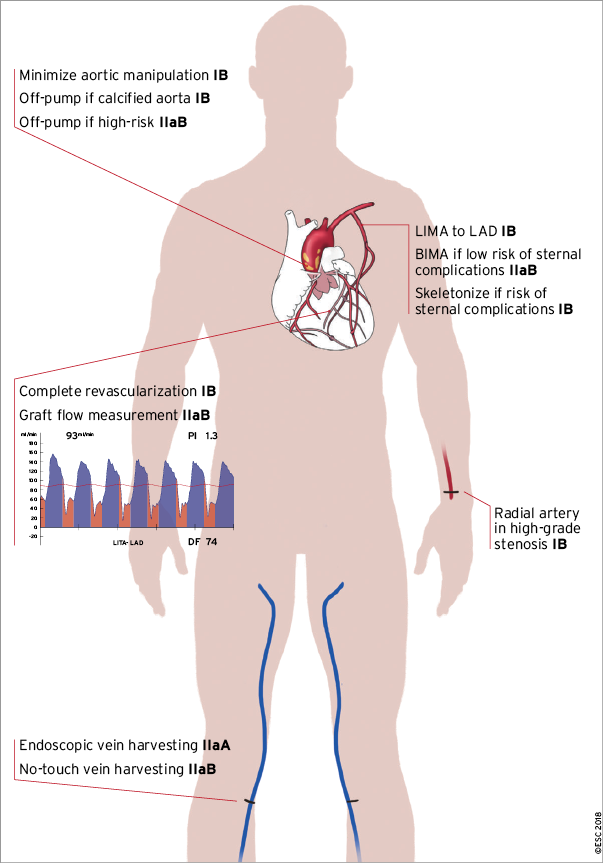
Figure 8. Technical aspects of CABG. BIMA: bilateral internal mammary artery; CABG: coronary artery bypass grafting; IMA: internal mammary artery; LAD: left anterior descending coronary artery.
15.1.9 MINIMALLY INVASIVE AND HYBRID PROCEDURES
Minimally invasive coronary surgery with LIMA, harvested either directly or under video-assisted vision, may represent an attractive alternative to a sternotomy.530 It has a similar safety and efficacy profile to conventional on-pump and off-pump procedures, with a markedly reduced post-operative length of stay and an early quality of life benefit, although spreading of the ribs is associated with increased post-operative pain.531-533 It has been shown to be safe and effective in the treatment of proximal LAD stenosis or chronically occluded LAD arteries.144 Moreover, when compared with PCI in a setting of single-vessel proximal LAD disease, minimally invasive coronary surgery was associated with less need for coronary reintervention.143,534,535 When combined with PCI to non-LAD vessels, it provides the opportunity for hybrid coronary revascularization to be performed in selected patients with multivessel disease.536
Hybrid revascularization can be performed consecutively in a hybrid operating room, or sequentially on separate occasions in the conventional surgical and PCI environments.537-540 In a small randomized trial of 200 patients, 1 year and 5 year rates of death, MI, stroke, and major bleeding or repeat revascularization were not significantly different between hybrid revascularization and CABG.536,541 Heart Team discussion and the prospective planning of a joint strategy are critical for the success of a hybrid revascularization strategy.542
15.2 REPORTING PERIOPERATIVE OUTCOMES
Perioperative reporting of outcomes after CABG procedures should be done on a risk-adjusted basis. The early risk period after CABG extends up to 3 months, is multifactorial, and depends on the interface between technical variability and patient comorbidity.543
15.3 GAPS IN THE EVIDENCE
The role of FFR and iwFR in guiding surgical revascularization needs further investigation into whether it improves clinical outcomes. Likewise, there are insufficient data on the impact of intraoperative assessment of graft flow on outcomes.
In view of the limitations of observational studies comparing BIMA with SIMA and the limitations of the ART trial, the ROMA (Randomization of Single vs. Multiple Arterial Grafts) trial is recruiting to answer the question of whether the use of additional arterial conduits (either BIMA or radial artery) translates into superior clinical outcomes when compared with SIMA supplemented by SVG only.
Hybrid procedures, which combine minimally invasive arterial grafting with PCI, proved feasible and safe. However, multicentre studies are required to prove the efficacy and superiority of this approach in stable, multivessel coronary disease.
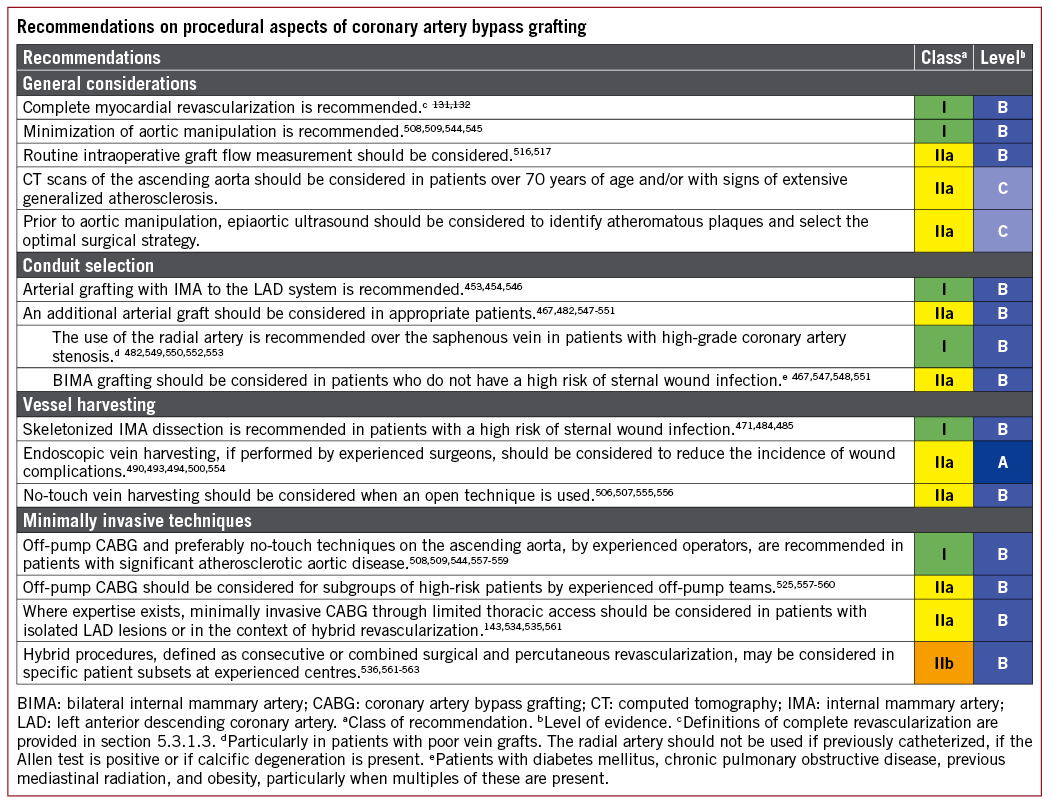
16 Procedural aspects of percutaneous coronary intervention
16.1 PERCUTANEOUS CORONARY INTERVENTION DEVICES
16.1.1 BALLOON ANGIOPLASTY
Plain balloon angioplasty has been superseded in the treatment of de novo coronary lesions after demonstration of the superiority of stenting in terms of the requirement for repeat revascularization.564 Balloon angioplasty might be considered for the treatment of selected patients in whom implantation of stents is not technically feasible, or in a vessel that is considered to be too small to be stented. Balloon angioplasty is no longer preferred to stenting with DES for patients who require urgent non-cardiac surgery as short-duration DAPT may be reasonable with both strategies.565,566
16.1.2 CHOICE OF CORONARY STENTS
Stenting with BMS results in an approximately 30% lower rate of restenosis in comparison with plain balloon angioplasty.564 Although many efforts have been made to further reduce restenosis by the modification of stent designs and materials, reducing the thickness of stent struts has been the only proven modification capable of reducing restenosis of BMS.567,568
A major reduction in the risk of restenosis has been achieved with DES technology. Early-generation DES released sirolimus569 or paclitaxel570 from a permanent polymer matrix coating on a relatively thick-strut (120-140 mm) stainless steel backbone. These devices reduced angiographic and clinical restenosis by approximately 50-70%, but increased the risk of very late stent thrombosis compared with BMS.336,571
Early-generation DES have now been supplanted by new-generation DES. These stents represented an iterative development of early generation technology, including polymers with enhanced biocompatibility (permanent or biodegradable), exclusively sirolimus-analogue active drugs, and stent backbones with thin struts (50-100 mm) composed of stainless steel, cobalt chromium, or platinum chromium.572-577 New-generation DES have higher efficacy and safety in comparison with both early-generation DES and BMS.336,571,578 Although stenting with new-generation DES confers a similar risk of death or MI at mid- to long-term follow-up in comparison with BMS,579 the risk of subacute and late stent thrombosis is significantly lower.579,580 Moreover, the risk of very late stent thrombosis is at least comparable to that of BMS and lower than that of early-generation DES.336,571,579,580 These observations were confirmed in a recent trial enrolling patients aged 75 years or older and demonstrating superior outcomes (composite of all-cause mortality, MI, stroke, or ischaemia-driven target lesion revascularization) with DES as compared with BMS with similar duration of intended DAPT (1 month or 6 months) in both treatment arms.581 Similarly, there is no clear evidence of a difference between DES and BMS on the risk of stent thrombosis following unplanned disruption of DAPT.565 Accordingly, new-generation DES should be preferred to BMS for routine use.
A large number of new-generation DES have received approval for use and CE mark in Europe.578 Supplementary Table 6 displays a list of new-generation DES with the CE mark and evidence from large-scale clinical trials powered for clinical primary endpoints.
Biodegradable polymer and polymer-free DES offer the potential to reduce late adverse events after PCI by eliminating inflammatory reactions to permanent polymer coatings. A number of large-scale trials showed comparable efficacy and safety compared with permanent polymer stents.575,576,582-590 However, at the moment, there is no evidence of differential efficacy with new-generation biodegradable polymer DES in comparison with new-generation permanent polymer DES in large-scale randomized trials with follow-up out to 5 years.591-594
Regarding polymer-free DES, two large-scale trials with different devices showed comparable results with new-generation DES and superior results to BMS.173,577 Long-term follow-up from randomized trials vs. new-generation permanent polymer DES is only available for a single device and shows comparable outcomes between the devices.591
The high clinical efficacy and safety of new-generation DES support their preferred use in patients with an indication for PCI, including patients with diabetes, CKD, multivessel and LMS disease, AMI, vein grafts, restenotic lesions, and chronic total occlusions. New-generation DES should therefore be considered as the default stent type for PCI regardless of clinical presentation, lesion subtype, concomitant therapies, or comorbidities.
16.1.3 BIORESORBABLE SCAFFOLDS
Completely bioresorbable scaffolds (BRS), which degrade to predominantly inert end products after fulfilling their scaffold function in the lesion site of the coronary vessel, have been developed with the goa
The safety and efficacy profile of the Absorb BVS has been compared with contemporary DES in several trials. Findings of these trials as well as meta-analyses consistently indicate the inferior efficacy and safety of Absorb BVS compared with contemporary DES during long-term follow-up. Specifically, the Absorb BVS is associated with a significantly increased risk of target lesion revascularization and device thrombosis, with numbers needed to harm of 40-60.596,597 Of note, commercial use of the Absorb BVS was stopped in 2017 (for additional details see the Supplementary Data).
Available evidence on the magnesium scaffold is limited to small observational studies. Initial results appear encouraging, but further evaluation is needed. Therefore, the Task Force endorses the recommendation of the recent ESC/European Association for Percutaneous Cardiovascular Interventions (EAPCI) document on bioresorbable scaffolds that any BRS should not be used outside well-controlled clinical studies. In patients who have been treated with BRS, prolonged-duration DAPT for 3 years or longer may be considered.
16.1.4 DRUG-COATED BALLOONS
The rationale for using DCBs is based on the concept that with highly lipophilic drugs, even short contact times between the balloon surface and the vessel wall are sufficient for effective drug delivery. There are various types of DCB that are approved for use in Europe and their main characteristics are listed in Supplementary Table 8. Although specifically designed comparative randomized trials are lacking, a class effect for all DCBs cannot be assumed.598 Randomized trial data supporting the use of DCB angioplasty are limited to the treatment of in-stent restenosis (see section 13.4). In terms of the use of DCB angioplasty for de novo disease, a number of small randomized trials have been reported with somewhat conflicting results.599-601 At present, there are no convincing data to support the use of DCB angioplasty for this indication.
16.1.5 DEVICES FOR LESION PREPARATION
Lesion preparation is critical for successful PCI. In addition to plain balloon angioplasty (with standard or non-compliant balloons), cutting or scoring balloon angioplasty or rotational atherectomy may be required in selected lesions – particularly those with heavy calcification – in order to adequately dilate lesions prior to stent implantation. However, studies investigating the systematic use of these adjunctive technologies, such as rotational atherectomy, have failed to show clear clinical benefit.602
16.2 INVASIVE IMAGING TOOLS FOR PROCEDURAL GUIDANCE
16.2.1 INTRAVASCULAR ULTRASOUND
The majority of the existing clinical trial data relate to the use of IVUS guidance during PCI. In the BMS era, several RCTs addressed the potential of IVUS in reducing restenosis and adverse events after stenting, with somewhat conflicting results. Findings from one meta-analysis of randomized trials suggested better outcomes with IVUS guidance in terms of acute procedural results and reduced angiographic restenosis, repeat revascularization, and MACE, with no effect on death and MI.603,604 In the DES era, meta-analysis of randomized and observational studies also suggests better clinical outcomes with IVUS-guided vs. angiography-guided PCI.605,606 However, the contribution of findings from observational studies must be weighed against the likelihood of considerable residual confounding due to treatment selection bias. Similarly, findings of improved outcome in patients undergoing LM stem PCI with IVUS-guided PCI vs. angiography-guided PCI from a propensity score matched analysis must be interpreted with caution.35
In cases of stent failure, including restenosis and stent thrombosis, the use of IVUS should be considered in order to identify and correct underlying mechanical factors (see section 13).386
16.2.2 OPTICAL COHERENCE TOMOGRAPHY
A number of studies have assessed OCT imaging for PCI guidance. Two observational studies show that while OCT imaging changes operator behaviour, its impact on clinical outcomes is unclear.607,608 Indeed, OCT is more accurate than angiography or IVUS in detecting subtle morphological details including malapposition, residual thrombus, plaque prolapse, and residual dissections, although many of these additional findings may have a benign course.609,610 A single randomized trial compared OCT with IVUS and coronary angiography, and showed that OCT-guided PCI was safe and resulted in a similar minimum stent area to that of IVUS-guided PCI.611 However, OCT guidance was not superior to either IVUS or angiography alone. An additional randomized trial that enrolled patients with NSTE-ACS compared OCT-guided PCI with angiography-guided PCI and found no signal of impact on clinical outcomes.612
A number of observational studies have shown that OCT is feasible and safe in the assessment of stent failure due to thrombosis, and may yield information that may be clinically useful.386,387,613,614 Likewise, in cases of in-stent restenosis, intrastent neointimal tissue may be characterized by OCT, enabling for example the detection of neoatherosclerosis.386,615,616 In cases of stent failure, the use of OCT should be considered in order to identify and correct underlying mechanical factors (see section 13).
16.3 SPECIFIC LESION SUBSETS
16.3.1 BIFURCATION STENOSIS
A number of RCTs have investigated the optimal intervention strategy in patients with bifurcation lesions and showed no benefit for the systematic two-stent approach vs. main branch-only stenting with provisional stenting of the side branch in terms of clinical outcomes.617 A recent pooled analysis of two RCTs showed lower 5 year survival in patients randomized to a systematic two-stent approach.618 In addition, procedure time, contrast volume, radiation exposure, and cost are higher with a two-stent approach.618 The EBC TWO (European Bifurcation Coronary TWO) trial found no difference between a provisional T-stent strategy and a systematic two-stent strategy (culotte technique) in terms of the composite endpoint of death, MI, and TVR at 12 months among 200 patients with large-calibre true bifurcation lesions (side branch diameter ≥2.5 mm) and significant ostial disease length (≥5 mm).619 Thus, main branch-only stenting with provisional stenting of the side branch should be the preferred approach for most bifurcation lesions. Exceptions to this rule, where upfront side branch stenting may be preferable, include the presence of a large side branch (≥2.75 mm) with a long ostial side branch lesion (>5 mm) or anticipated difficulty in accessing an important side branch after main branch stenting, and true distal LM bifurcations. Recently, a multicentre trial conducted in China directly compared a double-kissing crush two-stent strategy with provisional stenting of the main branch in 482 patients with distal LM bifurcation disease. Double-kissing crush resulted in a lower risk of the primary endpoint target lesion failure at 1 year compared with provisional stenting.620
When a two-stent strategy is necessary, which two-stent technique should be preferred is debated. The three most widely used contemporary two-stent techniques are culotte, crush (classic or double-kissing crush), and T and protrusion (TAP).621,622 Several RCTs have compared these techniques. In non-LM bifurcation lesions, there is no compelling evidence that one technique is superior to the others in terms of major clinical endpoints.621,622 In LM true bifurcation lesions, double-kissing crush has the most favourable outcome data.623
Final `kissing’ balloon dilation is generally recommended when two stents are eventually required, with no advantage from final kissing with the one-stent technique.624,625 Several stents, designed specifically for the treatment of bifurcation lesions, have undergone extensive evaluation with promising angiographic and clinical results, though RCTs against current recommended therapy are limited.626 Further technical details relating to bifurcation PCI are described in the consensus document of the European Bifurcation Club.627
16.3.2 CHRONIC TOTAL CORONARY OCCLUSION
Dedicated RCTs examining the outcomes of patients with chronic total occlusion (CTO) allocated to revascularization or conservative therapy are scarce. One trial randomized patients with STEMI and CTO in a non-culprit vessel to CTO-PCI vs. conservative therapy, and found no difference in the primary endpoint of LVEF and LV end-diastolic volume at 4 months.628 More recently, the prospective randomized EUROCTO (Randomized Multicentre Trial to Compare Revascularization With Optimal Medical Therapy for the Treatment of Chronic Total Occlusions) trial showed symptomatic improvement by PCI of CTO.629 This trial included 396 patients who were randomly assigned to PCI of CTO with optimal medical therapy, or optimal medical therapy alone. During the 12 month follow-up, the primary endpoint-the change in health status assessed by the Seattle angina questionnaire-showed significantly greater improvement of angina frequency and quality of life with CTO PCI as compared with optimal medical therapy alone. Yet, MACE were comparable between the two groups. A systematic review of 25 observational studies showed that at median follow-up of 3 years, successful CTO-PCI was associated with improved clinical outcomes in comparison with failed revascularization, including overall survival, angina burden, and the requirement for bypass surgery.630 Broadly speaking, the treatment of CTOs may be considered analogous to the treatment of non-CTO lesions (see recommendations in section 5). In cases of regional wall motion abnormalities in the territory of the CTO, objective evidence of viability should be sought. The decision to attempt CTO-PCI should be considered against the risk of greater contrast volume, longer fluoroscopy time, and higher MACE rates in comparison with non-CTO PCI patients.631 Ad hoc PCI is generally not recommended for CTOs, although it may be necessary in selected cases (e.g. acute bypass graft failure not amenable to recanalization of the bypass graft).
Recent developments in catheter and wire technology, and increasing operator expertise with both antegrade and retrograde approaches as well as wire escalation and dissection/re-entry techniques, have translated into increasing success rates of CTO-PCI with low rates of MACE.631-633 Success rates are strongly dependent on operator skills, depending on experience with specific procedural techniques, and the availability of dedicated equipment, and vary from 60-70% to >90%.631-633
16.3.3 OSTIAL LESIONS
In ostial coronary lesions, additional judgement and caution is essential before proceeding to PCI. In particular, a catheter-induced coronary spasm must be rigorously excluded. Lesion assessment with IVUS may be helpful, particularly in LM ostial stenosis. FFR measurement may also be valuable in the assessment of ostial lesions of borderline significance,634 taking special care to avoid a wedge position of the guiding catheter and using i.v., rather than intracoronary, adenosine. When performing an intervention, due to interaction between the guide catheter and the proximal stent edge, the risk of longitudinal stent deformation must be considered635 and avoided with careful catheter manipulation. The accurate positioning of the stent, precisely in the coronary ostium, may be technically challenging and some specialized techniques that may help to achieve optimal stent placement have been described.636,637
16.4 VASCULAR ACCESS
A number of RCTs have compared radial access with femoral access for diagnostic angiography and PCI. The two largest were RIVAL (Radial versus femoral access for coronary angiography and intervention in patients with acute coronary syndromes) and MATRIX (Minimizing Adverse Haemorrhagic Events by Transradial access Site and Systemic Implementation of AngioX).172,638 In the RIVAL trial, which enrolled 7021 patients, the primary outcome of death, MI, stroke, or non-CABG-related major bleeding at 30 days occurred at a similar rate in radial vs. femoral access (HR 0.92, 95% CI 0.72-1.17, P=0.50).638 In the MATRIX trial, 8404 ACS patients were randomly allocated to radial or femoral access.172 In terms of the first co-primary endpoint of 30 day MACE, there was no significant difference between radial access and femoral access (RR 0.85, 95% CI 0.74-0.99, two-sided P=0.031; non-significant at a pre-specified a of 0.025). The second co-primary outcome of 30 day net adverse clinical events [MACE or non-CABG BARC (Bleeding Academic Research Consortium (major bleeding] was significantly lower with radial access (RR 0.83, 95% CI 0.73-0.96; P=0.009). Major BARC 3 or 5 bleeding was significantly reduced in the radial group (1.6 vs. 2.3%; RR 0.67, 95% CI 0.49-0.92; P=0.013), and radial access was associated with a lower risk of all-cause mortality (1.6 vs. 2.2%; RR 0.72, 95% CI 0.53-0.99, P=0.045). However, the benefit of radial over femoral access depends upon the operator’s expertise in the radial technique.639
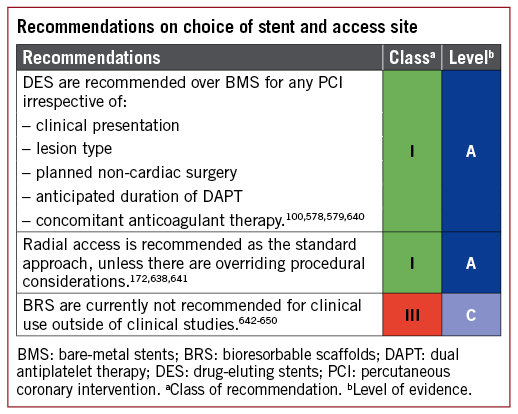
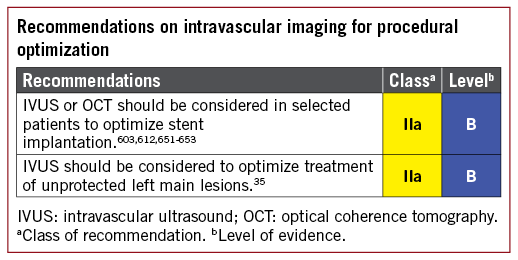
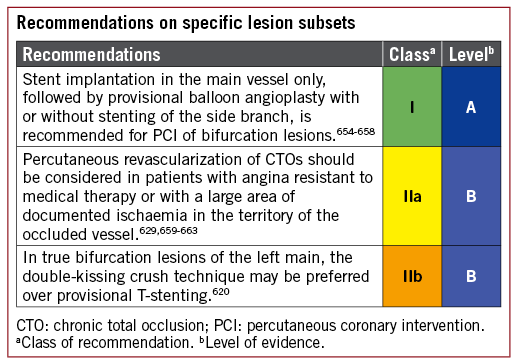
Treatment of restenotic and saphenous vein graft lesions are discussed in section 13.3.
17 Antithrombotic treatments
Antithrombotic treatment is mandatory in CAD patients undergoing myocardial revascularization. The choice of treatment, the combination, the time point of initiation, and the duration depend on the patient’s characteristics, comorbidities, the clinical setting (elective revascularization vs. ACS), and the mode (PCI vs. CABG) of revascularization. Both ischaemic and bleeding events significantly influence the outcome of CAD patients and their overall mortality risk during and after myocardial revascularization.664 Thus, the choice of treatment should reflect the ischaemic and bleeding risk. The recommended drugs (Figure 9) and doses (Table 7) for anticoagulant and antiplatelet drugs used in conjunction with myocardial revascularization are summarized below.
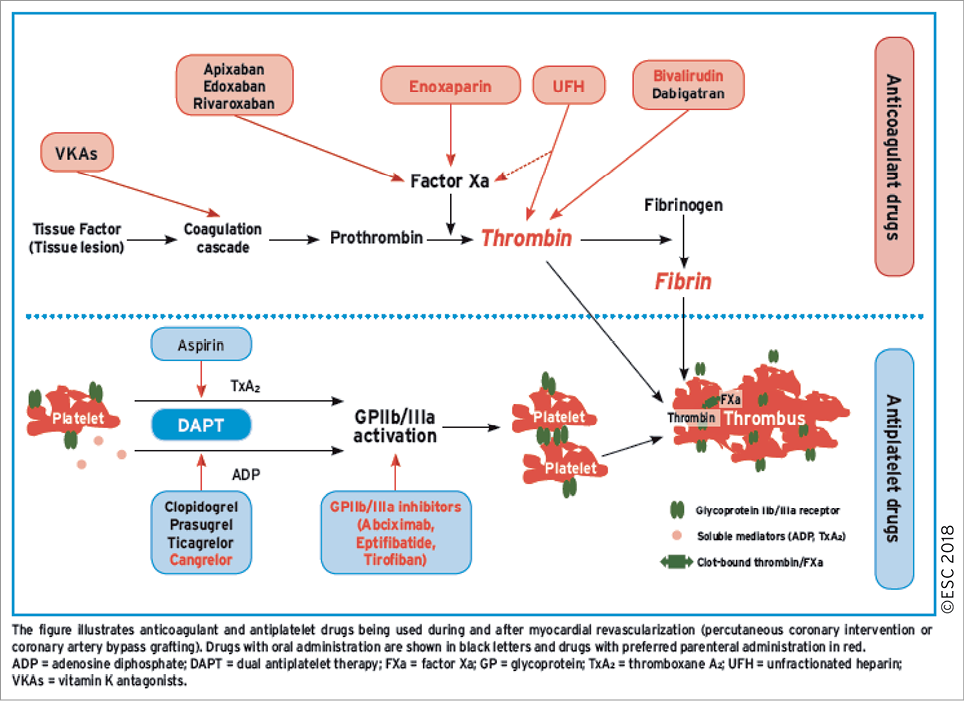
Figure 9. Antithrombotic treatment for myocardial revascularization and its pharmacological targets.
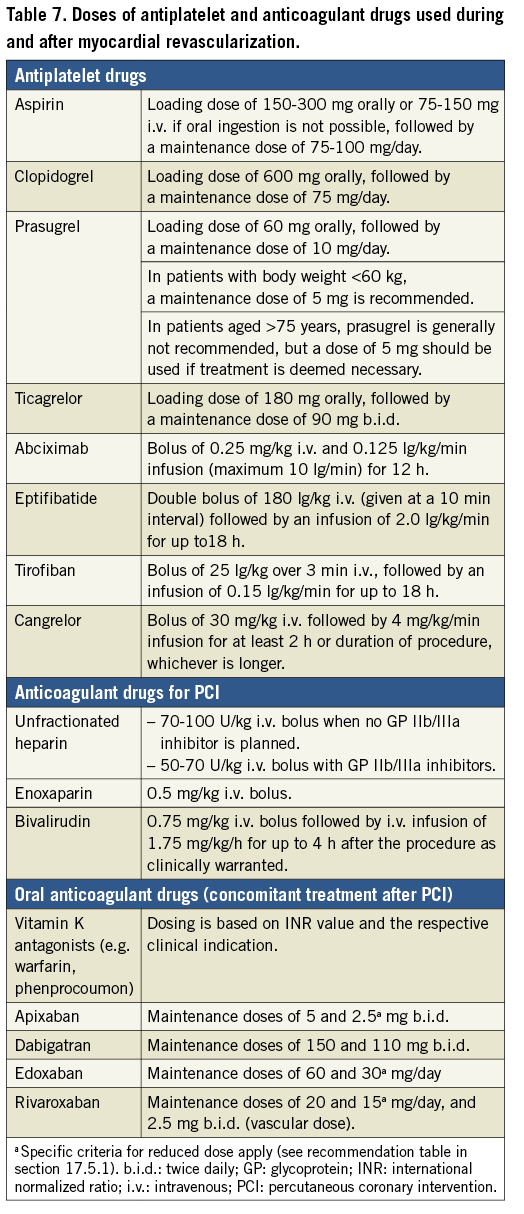
17.1 PERCUTANEOUS CORONARY INTERVENTION IN STABLE CORONARY ARTERY DISEASE
17.1.1 CHOICE OF TREATMENT AND PRE-TREATMENT
DAPT consisting of aspirin and a P2Y12 receptor inhibitor represents the cornerstone of treatment in patients undergoing elective PCI.665 The P2Y12 receptor inhibitor clopidogrel is recommended for elective stenting procedures. For routine clopidogrel pre-treatment (administration of the drug when the coronary anatomy is unknown), there is no compelling evidence for a significant clinical benefit in SCAD patients.666-668 Thus, pre-treatment may only be an option in selected patients with high probability of PCI or before staged PCI procedures. Figures 9 and 10 summarize the commonly used antiplatelet and anticoagulant drugs in SCAD patients undergoing PCI.
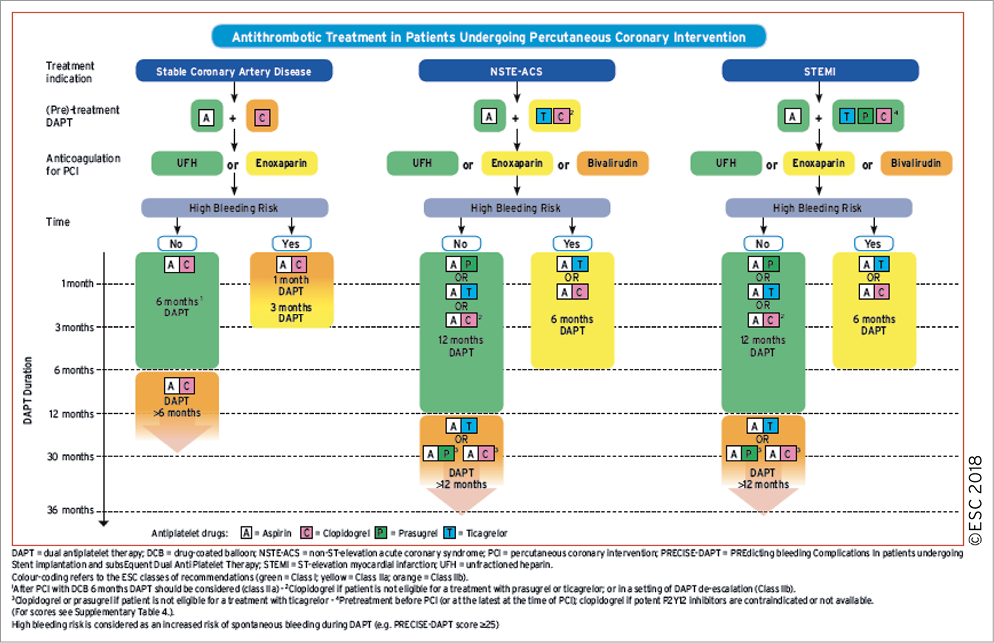
Figure 10. Algorithm for the use of antithrombotic drugs in patients undergoing percutaneous coronary intervention. High bleeding risk is considered as an increased risk of spontaneous bleeding during DAPT (e.g. PRECISE-DAPT score ≥25). Colour-coding refers to the ESC classes of recommendations (green: class I; yellow: class IIa; and orange: class IIb).
17.1.2 PERI-INTERVENTIONAL TREATMENT
While aspirin and clopidogrel are indicated for elective stenting procedures, prasugrel or ticagrelor may only be considered in selected patients for specific high-risk situations of elective stenting (e.g. complex PCI procedures such as LM stenting and CTO procedures) or in patients with a history of stent thrombosis on clopidogrel treatment.
In parallel with antiplatelet treatment, the use of anticoagulants is standard of care during elective PCI to inhibit thrombin generation and activity. Different agents, including unfractionated heparin (UFH) and bivalirudin, have been evaluated for their use in clinical practice. The REPLACE-2 (Randomised Evaluation in PCI Linking Angiomax to Reduced Clinical Events 2) trial demonstrated that the outcome with bivalirudin and provisional glycoprotein (GP) IIb/IIIa blockade is similar to that of UFH plus planned GP IIb/IIIa inhibition during elective PCI.669 The ISAR-REACT (Intracoronary Stenting and Antithrombotic Regimen Rapid Early Action for Coronary Treatment) 3 trial also showed a similar outcome for bivalirudin vs. UFH treatment.670 In ISAR REACT 3A,671 evaluating a lower dose of 100 U/kg UFH, this lower dose showed net clinical benefit compared with the historical control cohort and this benefit was mostly driven by a reduction in bleeding events. In view of the primary endpoint results of the RCTs and in view of a trend towards a lower risk of MI, UFH remains the standard anticoagulant for elective PCI. Based on the results of the STEEPLE (Safety and Efficacy of Intravenous Enoxaparin in Elective Percutaneous Coronary Intervention Randomised Evaluation) trial, enoxaparin should be considered as an alternative anticoagulant drug.672
Drugs for parenteral antiplatelet treatment include cangrelor and GP IIb/IIIa inhibitors. Cangrelor is a direct reversible, short-acting P2Y12-inhibitor that has been evaluated during PCI for SCAD and ACS in clinical trials comparing cangrelor with clopidogrel, administered before PCI [CHAMPION (Cangrelor versus Standard Therapy to Achieve Optimal Management of Platelet Inhibition) PCI] or after PCI (CHAMPION PLATFORM and CHAMPION PHOENIX).673 A meta-analysis showed a benefit with respect to major ischaemic endpoints that is counter-balanced by an increase in relevant bleeding.673 Moreover, the benefit of cangrelor with respect to ischaemic endpoints was attenuated in CHAMPION PCI with upfront administration of clopidogrel. Nevertheless, due to its proven efficacy in preventing intraprocedural and post-procedural stent thrombosis in P2Y12-inhibitor naïve patients, cangrelor may be considered in P2Y12-inhibitor naïve patients undergoing PCI (for more detailed discussion see the Supplementary Data).
Available GP IIb/IIIa inhibitors include abciximab, eptifibatide, and tirofiban. In a setting of elective PCI, clinical trials did not demonstrate an additional benefit of GP IIb/IIIa inhibitor administration in SCAD patients in a setting of DAPT treatment that includes loading with clopidogrel.674,675 A meta-analysis on this topic revealed no mortality benefit of GP IIb/IIIa treatment and while non-fatal MIs were reduced, (minor) bleeding events were significantly higher when utilizing these agents.676 Thus, GP IIb/IIIa inhibitors may only be considered in specific `bail-out’ situations including high intraprocedural thrombus burden, slow flow, or no-flow with closure of the stented coronary vessel.
An algorithm for the use of antithrombotic drugs in patients undergoing PCI is shown in Figure 10.
17.1.3 POST-INTERVENTIONAL AND MAINTENANCE TREATMENT
Following elective stenting, DAPT consisting of clopidogrel in addition to aspirin is generally recommended for 6 months, irrespective of the stent type. In specific clinical scenarios, this standard DAPT duration can be shortened (<6 months) or extended (>6-12 months). For a more detailed description of the pertinent clinical trials in the field of DAPT duration, we refer the reader to the 2017 ESC Focused Update on Dual Antiplatelet Therapy in Coronary Artery Disease.410 Following DAPT, a life-long single antiplatelet therapy (usually with aspirin) is recommended, and patients should be advised not to prematurely discontinue oral antiplatelet therapy after stenting due to the risks of stent thrombosis and recurrent MI.677 Recently, the value of a vascular dose of rivaroxaban (2.5 mg b.i.d.) in conjunction with aspirin was demonstrated in the large-scale COMPASS (Rivaroxaban for the Prevention of Major Cardiovascular Events in Coronary or Peripheral Artery Disease) trial.678 However, its utilization in SCAD patients is a matter of secondary prevention and is not linked to myocardial revascularization procedures.
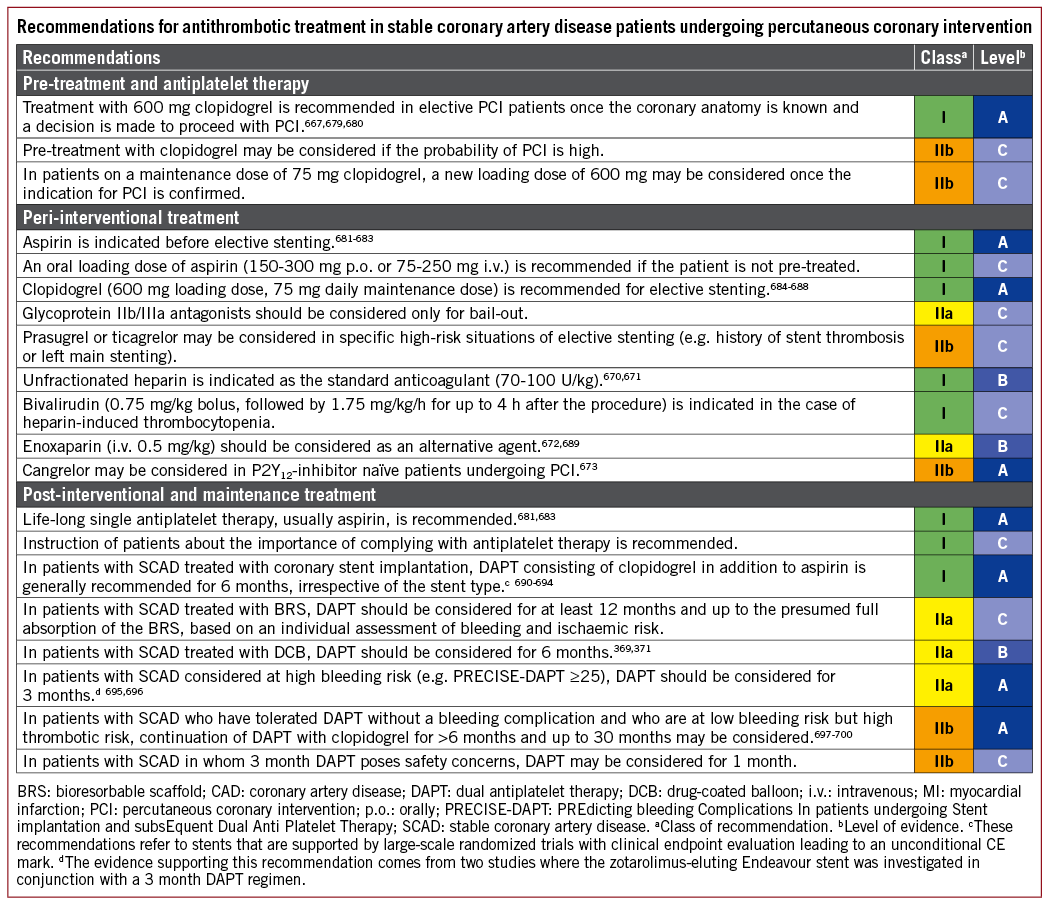
17.2 NON-ST-SEGMENT ELEVATION ACUTE CORONARY SYNDROME
The activation of blood platelets and the coagulation cascade plays a key role in the initial phase and evolution of an ACS. Hence, sufficient platelet inhibition and anticoagulation is essential during ACS, and especially in ACS patients undergoing PCI.
17.2.1 CHOICE OF TREATMENT AND PRE-TREATMENT
For NSTE-ACS patients, DAPT including aspirin and a potent P2Y12 receptor inhibitor (prasugrel or ticagrelor) is recommended (see the Supplementary Data).701,702 Clopidogrel should only be used when prasugrel or ticagrelor are not available or are contraindicated. Based on the results of the ACCOAST (Comparison of Prasugrel at the Time of Percutaneous Coronary Intervention or as Pretreatment at the Time of Diagnosis in Patients with Non-ST Elevation Myocardial Infarction) trial,165 it is not recommended that prasugrel is administered in patients in whom coronary anatomy is not known. Nevertheless, pre-treatment with ticagrelor was part of the PLATO trial (Study of Platelet Inhibition and Patient Outcomes) and was associated with an early benefit over clopidogrel.702 For these reasons, pre-treatment with ticagrelor can be used, although there is no direct evidence from head-to-head comparisons between pre-treatment strategies.
17.2.2 PERI-INTERVENTIONAL TREATMENT
Anticoagulation is recommended for all patients in addition to antiplatelet therapy during PCI for NSTE-ACS.703 In general, a crossover between anticoagulants should be avoided [especially between UFH and low-molecular-weight heparin (LMWH)], with the exception of adding UFH to fondaparinux when a patient proceeds to PCI.704,705 The respective agents should be discontinued after PCI except for specific clinical settings, such as the presence of an LV aneurysm with thrombus or AF requiring anticoagulation.
A number of trials have compared bivalirudin with UFH in ACS patients undergoing PCI (see the Supplementary Data). Some of these trials pursued a balanced use of adjunctive GP IIb/IIIa inhibitors with both bivalirudin and heparin, whereas others, predominantly the older ones, had selective use of GP IIb/IIIa inhibitors in the heparin arm. These trials have been reviewed extensively in a number of meta-analyses.706-708 A meta-analysis that included the MATRIX trial but not VALIDATE-SWEDEHEART (Bivalirudin versus Heparin in ST-Segment and Non-ST-Segment Elevation Myocardial Infarction in Patients on Modern Antiplatelet Therapy on the Swedish Web-system for Enhancement and Development of Evidence-based care in Heart disease Evaluated According to Recommended Therapies) showed no significant benefit of bivalirudin compared with UFH with respect to death, MACE, and MI.708 Nevertheless, bivalirudin was associated with a significant increase in the risk of stent thrombosis and a significant decrease in the risk of bleeding. However, the reduction of bleeding risk was linked to unbalanced use of GP IIb/IIa inhibitors predominantly with UFH. Recently, the VALIDATE-SWEDEHEART study709 compared UFH vs. bivalirudin in a background of radial access and limited use of GP IIb/IIIa inhibitors. The study demonstrated similar risk patterns for both ischaemia and bleeding when comparing the two drugs. Of note, while prior studies reported a reduced bleeding risk with bivalirudin vs. UFH, this was not confirmed in VALIDATE-SWEDEHEART and in a contemporary setting of preferred radial access and selective use of GP IIb/IIIa inhibitors. More recently, a meta-analysis updated for the results of VALIDATE-SWEDEHEART confirmed that bivalirudin compared with heparin was associated with a similar incidence of all-cause death and ischaemic events after PCI for ACS.710 A significant association of bivalirudin with decreased risk of bleeding was only found with unbalanced use of GP IIb/IIIa inhibitors in conjunction with heparin. In summary and based on the above-mentioned trials, UFH is primarily recommended as an anticoagulant for PCI. Due to its short half-life and favourable results in some of the studies, bivalirudin may be considered as an alternative to UFH in selected cases.
Patients may undergo cardiac catheterization after a conservative treatment phase and these patients are commonly treated with fondaparinux during the conservative treatment phase. This regimen is based on the OASIS-5 (Optimal Antiplatelet Strategy for Interventions 5) trial.711 Of note, catheter thrombus formation was an issue with fondaparinux and therefore full-dose UFH must be added to prevent thrombus formation when the patient proceeds to PCI. Enoxaparin should be considered as anticoagulant for PCI in patients pre-treated with subcutaneous enoxaparin. A benefit of enoxaparin over UFH in reducing mortality and bleeding complications was recently reported in a meta-analysis including NSTE-ACS patients.689 Yet, this meta-analysis did not include a dedicated randomized study in NSTE-ACS and was largely based on non-randomized comparisons.
Most of the trials evaluating GP IIb/IIIa inhibitors in PCI-treated patients pre-date the era of routine oral DAPT treatment. These early trials demonstrated a reduction in the incidence of ischaemic events in favour of GP IIb/IIIa treatment in combination with UFH compared with UFH alone, primarily through a reduction in MI.712 However, coronary angiography and PCI were delayed compared with what is recommended now, and a consistent major bleeding risk was observed. Overall, there is no compelling evidence for an additional benefit of routine upstream use of GP IIb/IIIa inhibitors in NSTE-ACS patients scheduled for coronary angiography and receiving DAPT treatment.713,714 In a setting of potent platelet inhibition with ticagrelor or prasugrel, where randomized data on GP IIb/IIIa inhibitor use is limited, routine use of these agents cannot be recommended. Nevertheless, it should be considered for bail-out situations or thrombotic complications, and may be used for high-risk PCI in patients without pre-treatment with P2Y12-inhibitors. The available evidence on cangrelor suggests that the potential benefit is independent of the clinical presentation. Thus, similar to SCAD patients, cangrelor may be considered in specific settings in P2Y12-naïve patients undergoing PCI.
17.2.3 POST-INTERVENTIONAL AND MAINTENANCE TREATMENT
Following PCI for NSTE-ACS, DAPT consisting of a P2Y12 receptor inhibitor in addition to aspirin is generally recommended for 12 months, irrespective of the stent type. Recently, the SMART-DATE (Smart Angioplasty Research Team-safety of 6-month duration of Dual Antiplatelet Therapy after percutaneous coronary intervention in patients with acute coronary syndromes) prospective multicentre randomized trial supported this notion in the setting of contemporary interventional practice. The study randomly assigned 2712 patients undergoing PCI for NSTE-ACS or STEMI to either 6 month DAPT or 12 month or longer DAPT. Although the primary endpoint – a composite of all-cause death, MI, or stroke – did not confirm the benefit of prolonged DAPT over 6 month DAPT (cumulative event rate 4.7 vs. 4.2%; absolute risk difference 0.5%; upper limit of one-sided 95% CI 1.8%; Pnon-inferiority=0.03 with a predefined non-inferiority margin of 2.0%), MI occurred more frequently in the 6 month DAPT group than in the prolonged DAPT group (1.8 vs. 0.8%; P=0.02). The rate of BARC type 2-5 bleeding was not significantly affected by prolonged DAPT (HR 0.69, 95% CI 0.45-1.05, P=0.09). The authors stated that the increased risk of MI with 6 month DAPT and the wide non-inferiority margin prevented them from concluding that short-term DAPT was safe in this setting, and suggested that prolonged DAPT should remain the standard of care in patients with ACS without excessive risk of bleeding.715
In specific clinical scenarios, this standard DAPT duration can be shortened (<12 months) or extended (>12 months). Further on, switching and especially a de-escalation of DAPT (switching from potent P2Y12-inhibitors to clopidogrel) was subject to a number of randomized clinical trials.716,717 Triggers for DAPT de-escalation include clinical (bleeding events or presumed high bleeding risk) and socio-economic factors.716 Based on recent results from the randomized TROPICAL-ACS (Testing responsiveness to platelet inhibition on chronic antiplatelet treatment for acute coronary syndromes) trial717, an approach of DAPT de-escalation guided by platelet function testing may be considered in ACS patients (NSTE-ACS and STEMI) as an alternative to 12 months potent platelet inhibition, especially for patients deemed unsuitable for maintained potent platelet inhibition. For a more detailed description of the pertinent clinical trials in the field of DAPT duration and switching antiplatelet drugs, we refer the reader to the International Expert Consensus document on Switching Platelet P2Y12 Receptor-Inhibiting Therapies718 and the 2017 ESC Focused Update on Dual Antiplatelet Therapy in Coronary Artery Disease.410 Following DAPT, lifelong single antiplatelet therapy (usually with aspirin) is recommended and patients should be advised not to prematurely discontinue oral antiplatelet therapy after stenting.677,719
Based on the results of the ATLAS-ACS 2-TIMI 51 (Anti-Xa Therapy to Lower cardiovascular events in Addition to Standard therapy in subjects with Acute Coronary Syndrome-Thrombolysis In Myocardial Infarction 51) trial in NSTE-ACS and STEMI patients,720 low-dose rivaroxaban may be considered after discontinuation of parenteral anticoagulation for patients with no prior stroke/TIA, and at high ischaemic risk as well as low bleeding risk, receiving aspirin and clopidogrel. Of note, rivaroxaban has not been investigated in a background of potent P2Y12-inhibitors.
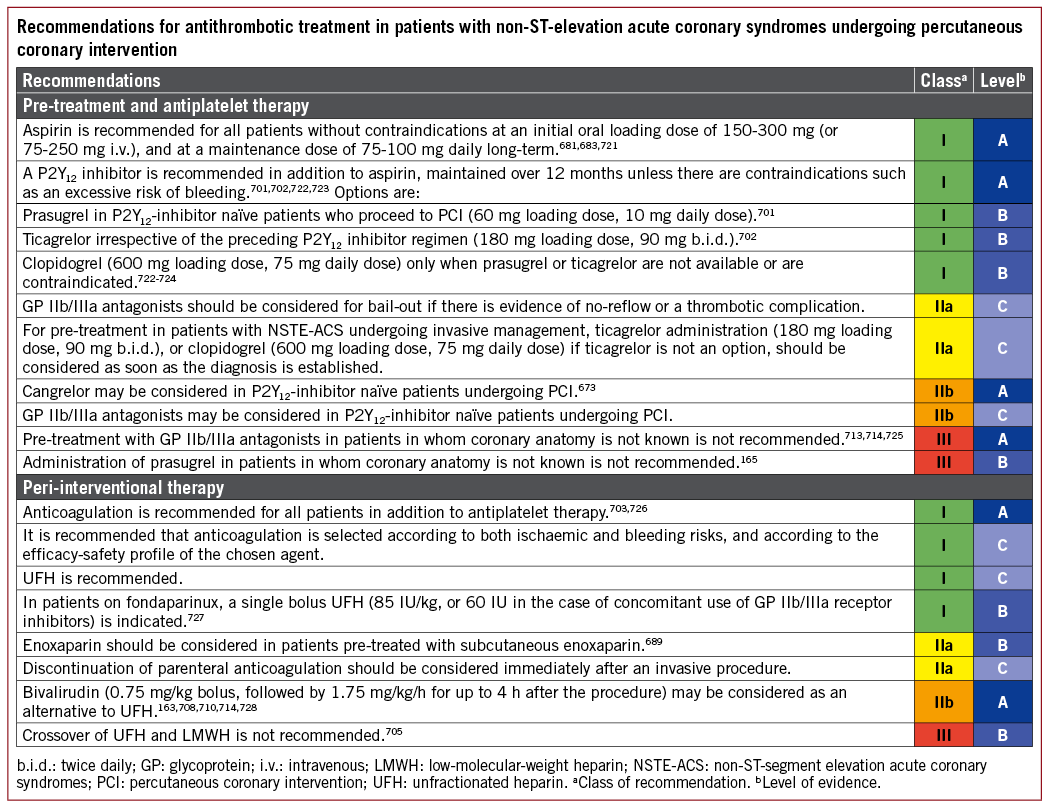
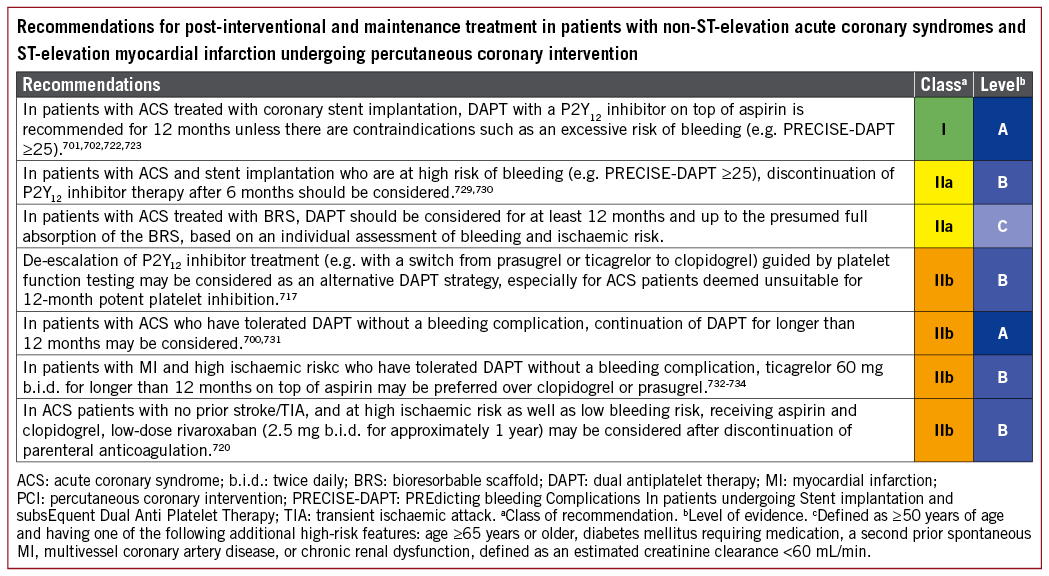
17.3 ST-SEGMENT ELEVATION MYOCARDIAL INFARCTION
17.3.1 CHOICE OF TREATMENT AND PRE-TREATMENT
STEMI patients undergoing primary PCI should receive aspirin and a P2Y12 receptor inhibitor as soon as the diagnosis of STEMI is established. In line with the treatment recommendations for NSTE-ACS patients, DAPT is the cornerstone of treatment for STEMI patients and includes aspirin and a potent P2Y12 receptor inhibitor (prasugrel or ticagrelor).701,702 For both antiplatelet drugs, published subgroup analyses on STEMI patients are available (see the Supplementary Data). Randomized data on a comparison of ticagrelor vs. prasugrel in STEMI patients are limited, but the recently published randomized PRAGUE-18 (Comparison of Prasugrel and Ticagrelor in the Treatment of Acute Myocardial Infarction) trial735 with limited statistical power found similar safety and efficacy profiles of ticagrelor and prasugrel in a setting of primary PCI. When potent P2Y12 receptor inhibitors are contraindicated or are not available, clopidogrel should be given for primary PCI instead.724 The value of pre-treatment with ticagrelor was addressed in the ATLANTIC (Administration of Ticagrelor in the Cath Lab or in the Ambulance for New ST-Elevation Myocardial Infarction to Open the Coronary Artery) trial.736 No significant differences were observed in the levels of the two co-primary surrogate endpoints measured before PCI (thrombolysis in Myocardial Infarction flow and ST-segment resolution). Likewise, the incidence of a combined ischaemic endpoint (death, MI, stroke, stent thrombosis, and urgent revascularization) did not differ between the two treatment arms. Nevertheless, in both the TRITON (TRial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet InhibitioN with Prasugrel-Thrombolysis In Myocardial Infarction) and PLATO trials, pre-treatment was part of the therapeutic regimen in STEMI.
17.3.2 PERI-INTERVENTIONAL TREATMENT
Immediate and sufficient anticoagulation is mandatory in the setting of primary PCI for STEMI and available options include UFH, bivalirudin, and enoxaparin. A number of RCTs compared bivalirudin vs. UFH in different settings and with different utilization of GP IIb/IIIa inhibitors (see the Supplementary Data). The primary recommendation of UFH, reserving bivalirudin for selected cases, is essentially the same for primary PCI as for PCI in NSTE-ACS, and is mostly based on the same clinical trials706,709 (see section 17.2.2).
Enoxaparin was compared with UFH in the randomized open-label ATOLL (Acute STEMI Treated with primary PCI and intravenous enoxaparin Or UFH to Lower ischaemic and bleeding events at short- and Long-term follow-up) trial,737 and based on the trial results, enoxaparin should be considered as an alternative to UFH treatment in STEMI patients.
A number of clinical trials, performed at a time when pre-treatment and potent platelet inhibition was not part of routine clinical practice, have documented clinical benefits of GP IIb/IIIa inhibitors as an adjunct to primary PCI performed with UFH.738,739 A meta-analysis showed a significant survival benefit, especially in high-risk STEMI patients, but also a higher risk of bleeding with GP IIb/IIIa administration.740 Dedicated trials have investigated the value of upstream treatment in the past.741,742 Based upon the available evidence, the routine use of i.v. or intracoronary GP IIb/ IIIa inhibitor administration – regardless of whether treatment starts upstream or in the catheterization laboratory – cannot be recommended. Especially in a setting where potent P2Y12-inhibitors like prasugrel or ticagrelor are used, the value of GP IIb/IIIa inhibitors remains uncertain as these agents exhibit a fast onset of action (usually <1 h). GP IIb/IIIa inhibitors remain an option as bail-out therapy or in high-risk PCI without pre-treatment with P2Y12-inhibitors. Of note, the bail-out scenarios have never been addressed in randomized controlled trials. For reasons discussed above (see sections 17.1 and 17.2), cangrelor may be considered in specific settings in P2Y12-naïve patients undergoing PCI.
17.3.3 POST-INTERVENTIONAL AND MAINTENANCE TREATMENT
Following PCI for STEMI, DAPT consisting of a P2Y12 receptor inhibitor in addition to aspirin is generally recommended for 12 months. Recommendations for maintenance DAPT treatment are generally consistent with those for NSTE-ACS patients and are detailed in section 17.2.3.
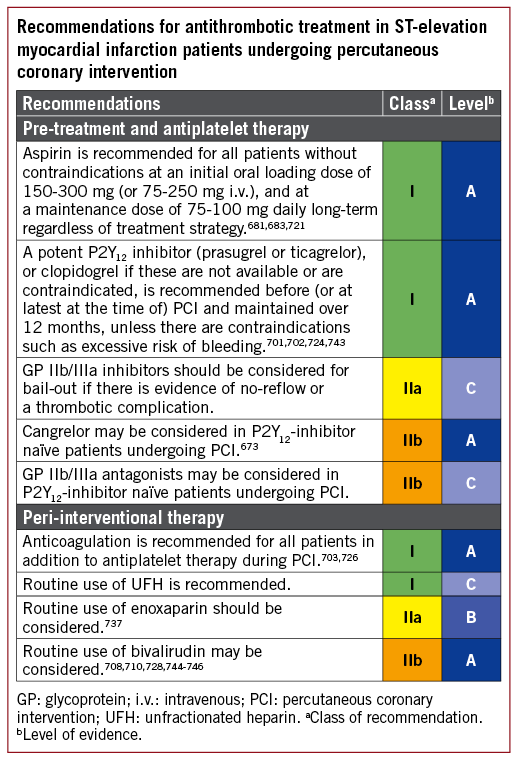
17.4 CORONARY ARTERY BYPASS GRAFTING
Antithrombotic treatment before and after CABG is addressed in the 2017 ESC Focused Update on Dual Antiplatelet Therapy in Coronary Artery Disease.410 After reviewing the subsequent literature, the current Task Force endorses the recommendations of the update on DAPT and does not identify a need for any major update. Accordingly, the recommendation tables in this section are taken from the Focused Update. For a detailed discussion, we refer the reader to the Focused Update.
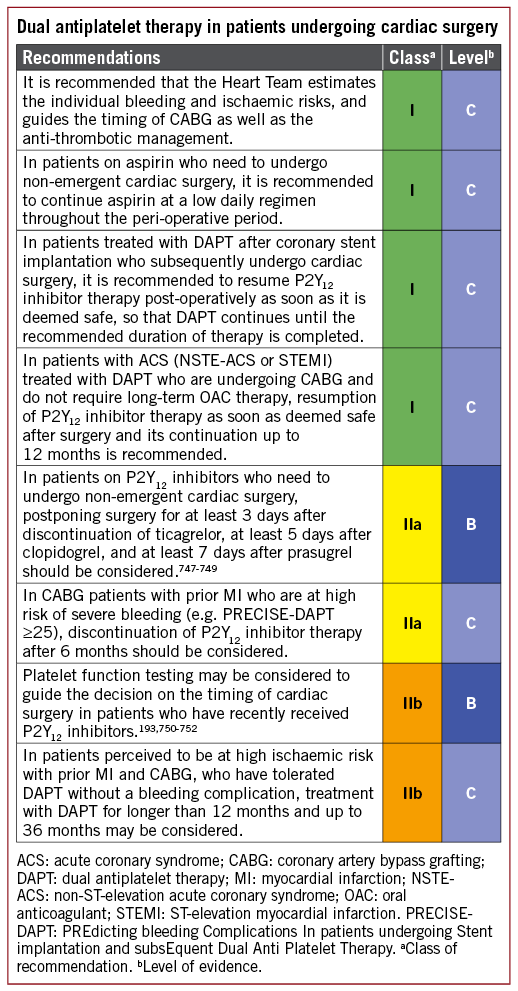
17.5 SPECIAL CONDITIONS
17.5.1 ANTITHROMBOTIC THERAPY AFTER PERCUTANEOUS CORONARY INTERVENTION IN PATIENTS REQUIRING ORAL ANTICOAGULATION
Compared with OAC therapy alone, the addition of DAPT to OAC therapy results in a two- to three-fold increase in bleeding complications, suggesting that every effort should be undertaken to avoid bleeding (Table 8).753 Assessing the balance of ischaemic and bleeding risks of relatively short (i.e. ≤6 months) triple therapy duration compared with double therapy consisting of clopidogrel and an OAC requires patient-by-patient decisions. Of note, previous randomized studies evaluating the duration of triple therapy or the benefit of NOACs vs. vitamin K antagonists (VKAs) were not adequately powered to assess ischaemic events, and data are lacking on the efficacy of dual therapy in patients at high risk of stroke or recurrent ACS.754-757 In the major trials, there was no interaction between the duration of triple therapy and clinical presentation (ACS vs. no ACS). The rate of bleeding events peaked within the first 30 days of initiation of triple therapy, and was twice as high when compared with the rate of acute coronary events including recurrent MI and stent thrombosis. For these reasons, the duration of triple therapy should be minimized depending on bleeding and ischaemic risks (see Tables 8 to 10 for guidance in decision-making). In stabilized event-free patients, discontinuation of any antiplatelet agent at 1 year after stenting is encouraged, while dual therapy may be continued beyond 1 year according to the stent-driven risk shown in Table 9.
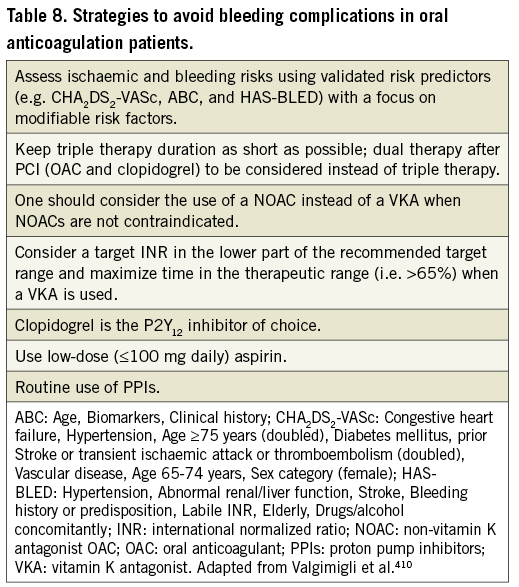

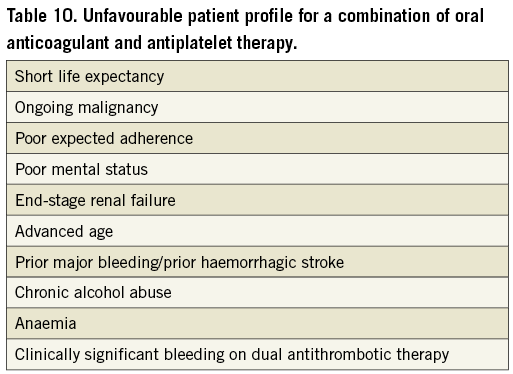
Based on the favourable bleeding risk in the large phase 3 studies, a NOAC should be preferred over a VKA. The PIONEER756 (Prevention of bleeding in patients with AF undergoing PCI) trial and the more recent RE-DUAL (Randomised Evaluation of Dual Antithrombotic Therapy with Dabigatran versus Triple Therapy with Warfarin in Patients with Nonvalvular Atrial Fibrillation Undergoing Percutaneous Coronary Intervention)757 trial compared a NOAC plus single antiplatelet therapy with triple therapy with a VKA plus DAPT and consistently showed significantly lower bleeding risks with the dual antithrombotic regimen. In RE-DUAL, both dosing regimens for dabigatran (150 mg and 110 mg b.i.d.) vs. warfarin triple therapy were associated with a significant reduction of major or clinically relevant bleeding events. However, as compared with triple therapy, an increase in both MI (4.5 vs. 3.0%, P=0.09) and stent thrombosis risk (1.5 vs. 0.8%, P=0.15) was reported for the lower dabigatran dose (110 mg b.i.d.), but not for the higher dabigatran dose (150 mg b.i.d.). Although statistical significance was missed, these findings raise concern about the efficacy of the lower dabigatran dose in combination with single antiplatelet therapy in preventing coronary events. Thus, the 150 mg b.i.d. dose of dabigatran is preferred. At present, evidence for a dual treatment approach is available for VKA,755 rivaroxaban,756 and dabigatran,757 but none of these studies were powered to assess the efficacy of preventing stent thrombosis or thrombo-embolic events and only RE-DUAL used a NOAC dose that was previously shown to be effective in the prevention of thrombo-embolic events. The ongoing AUGUSTUS trial (ClinicalTrials.gov Identifier: NCT02415400) will address the value of apixaban in a similar setting, and with and without aspirin. Edoxaban is currently being investigated in a setting of triple treatment in the ENTRUST-AF-PCI (Evaluation of the safety and efficacy of an edoxaban-based antithrombotic regimen in patients with atrial fibrillation following successful percutaneous coronary intervention) trial (ClinicalTrials.gov Identifier: NCT02866175).
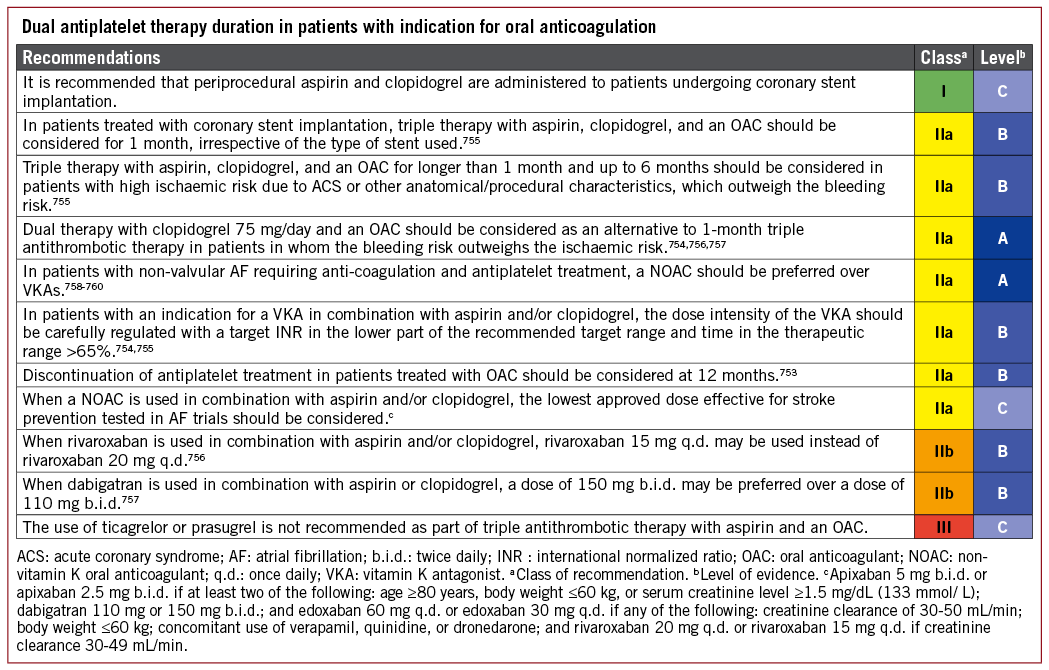
Figure 11 illustrates applicable DAPT algorithms in patients with an indication for OAC undergoing PCI with the respective classes of recommendations for the different treatment regimens. For more details on the pertinent studies in the field of triple treatment (DAPT plus OAC) and the associated issues, we refer the reader to the 2017 ESC Focused Update on Dual Antiplatelet Therapy in Coronary Artery Disease.410
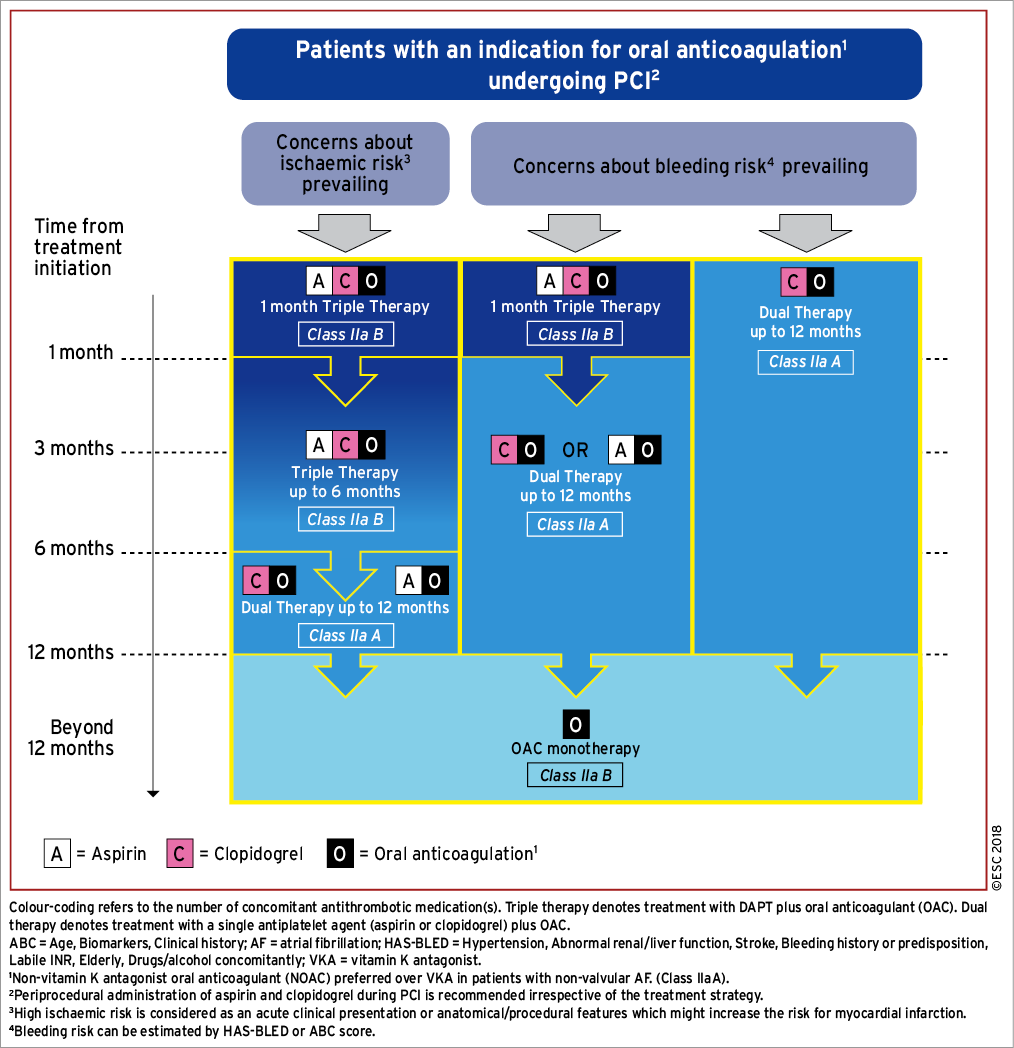
Figure 11. Algorithm for dual antiplatelet therapy in patients with an indication for oral anticoagulation undergoing percutaneous coronary intervention.
17.5.2 REVASCULARIZATION IN PATIENTS WITH RENAL FAILURE
See the Supplementary Data.
17.5.3 MONITORING OF ANTIPLATELET DRUGS (PLATELET FUNCTION TESTING AND GENOTYPING)
See the Supplementary Data.
17.5.4 SURGERY IN PATIENTS ON DUAL ANTIPLATELET THERAPY
See 2017 ESC Focused Update on Dual Antiplatelet Therapy in Coronary Artery Disease.410
17.6 GAPS IN THE EVIDENCE
The value of pre-hospital pre-treatment with prasugrel in STEMI patients, as well as the safety and efficacy of ticagrelor given at hospital admission in NSTE-ACS patients, has not been addressed in dedicated randomized studies.
The safety and efficacy of short-term potent antiplatelet treatment with either prasugrel or ticagrelor in SCAD patients is unknown, and is subject to ongoing clinical trials [the ALPHEUS (Assessment of Loading With the P2Y12 Inhibitor Ticagrelor or Clopidogrel to Halt Ischemic Events in Patients Undergoing Elective Coronary Stenting) trial: NCT02617290 and the SASSICAIA (Comparison of Loading Strategies With Antiplatelet Drugs in Patients Undergoing Elective Coronary Intervention) trial: NCT02548611].
The clinical benefit of a short-term DAPT duration followed by long-term ticagrelor monotherapy (and stopping aspirin) remains unknown. The ongoing GLOBAL LEADERS (Long-term ticagrelor monotherapy versus standard dual antiplatelet therapy followed by aspirin monotherapy in patients undergoing biolimus-eluting stent implantation) and TWILIGHT (Ticagrelor With Aspirin or Alone in High-Risk Patients After Coronary Intervention) trials aim to close this gap in our current knowledge (NCT01813435 and NCT02270242, respectively).
18 Volume outcome relationship for revascularization procedures
Operator experience influences outcomes, particularly in critical, complex situations. Greater total experience of an entire hospital team – consisting of the supporting members in the operating room or catheterization laboratory and those responsible for postoperative care – results in more favourable outcomes.
18.1 CORONARY ARTERY BYPASS GRAFTING
Studies have suggested that the volume of CABG surgery in a hospital significantly impacts in-hospital mortality, although no consistent cut-offs for volume were used in these studies.761-762 This increase in mortality observed in lower volume centres seems to be attributable to so-called `failure to rescue’: although patients operated on at low-volume centres are not at particularly higher risk of suffering a major complication, they are more likely to die from such a complication should it occur.763 Therefore, consideration should be given to the performance of CABG in centres with an annual volume of at least 200 CABG cases. Apart from hospital volume, higher surgeon volume also appears to be inversely related to operative mortality. Birkmeyer et al. provided evidence suggesting that both hospitals and surgeons have some impact on outcomes.764
Several studies suggest that quality measures are more important than volume per se.765,766 Missing quality indicators in hospitals strongly predicted mortality, irrespective of surgeon or hospital case volumes.767 Therefore, it is recommended that such quality measures (as an example see Supplementary Table 9) are adopted and reported to facilitate focused quality improvement.768
18.2 PERCUTANEOUS CORONARY INTERVENTION
Numerous studies have investigated the relationship between the volume of procedures and outcomes of PCI, suggesting a volume-outcome relationship at the operator level, as well as at the institutional level.761,769-773 A population-based study from the PCI reporting system of New York indicated that hospital case volumes <400 PCIs per year and operator case volumes <75 PCIs per year were associated with impaired outcomes.769
Among patients with ACS, particularly STEMI, operator and hospital volumes play important roles. A large study in the USA reported that, in a cohort of 36 535 patients undergoing primary PCI, in-hospital mortality was significantly lower in institutions with higher primary PCI volumes (5.7% in hospitals performing >33 primary PCIs/year vs. 7.7% in hospitals performing <12 primary PCIs/year).774
Operator volume has also been shown to impact outcomes in LM PCI. A single-centre study of 1948 patients who underwent unprotected LM PCI, performed by 25 operators over a 7 year period, showed reduced 30 day and 3 year mortality for patients who had their PCI performed by a high-volume operator (defined as ≥15 LM PCI/year; mean 25/year) vs. a low-volume operator (<15 LM PCI/year).775
An example of quality measures for PCI is provided in Supplementary Table 10.
18.3 TRAINING IN CARDIAC SURGERY AND INTERVENTIONAL CARDIOLOGY FOR MYOCARDIAL REVASCULARIZATION
A European training programme in interventional cardiology has been proposed by the EAPCI in order to ensure the high quality of patient care and clinical excellence.776 The programme should last 1-2 years at high-volume institutions that handle ≥800 PCIs per year and that have an established 24 h/7 day service for the treatment of patients with ACS.
For CABG, no standardized European programme exists at this time. However, the pace at which proficiency reaches certain acceptable standards differs from trainee to trainee. Therefore, although it is recommended that trainees perform ≥200 CABG procedures under supervision before becoming completely independent, a competency-driven residency programme with regular evaluation of progress is recommended over a volume-driven programme.
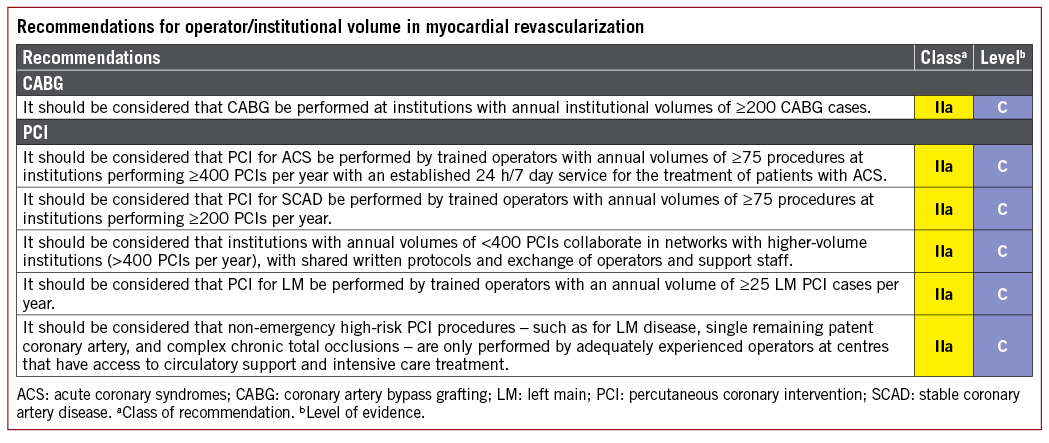
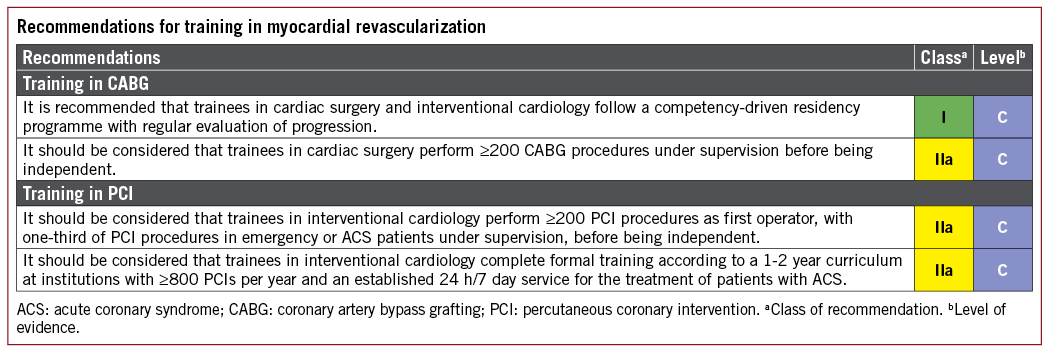
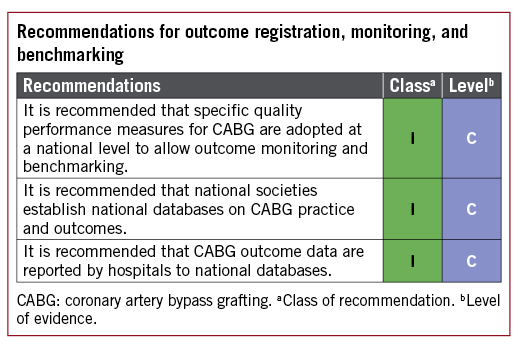
19 Medical therapy, secondary prevention, and strategies for follow-up
Myocardial revascularization must be accompanied by medical therapy and other secondary prevention strategies for risk factor modification and permanent lifestyle changes.42 Secondary prevention and cardiac rehabilitation are an integral part of management after revascularization because such measures reduce future morbidity and mortality in a cost-effective way, and can further improve symptoms. These measures are discussed in detail in the European Guidelines on Cardiovascular Disease Prevention that were published in 2016.42
The need to detect restenosis has reduced in the DES era. Likewise, the durability of CABG results have increased with the use of arterial grafts, and ischaemia stems mainly from SVG attrition and/or progression of CAD in native vessels. Nevertheless, the recurrence of symptoms or ischaemia due to disease progression or restenosis deserves attention.
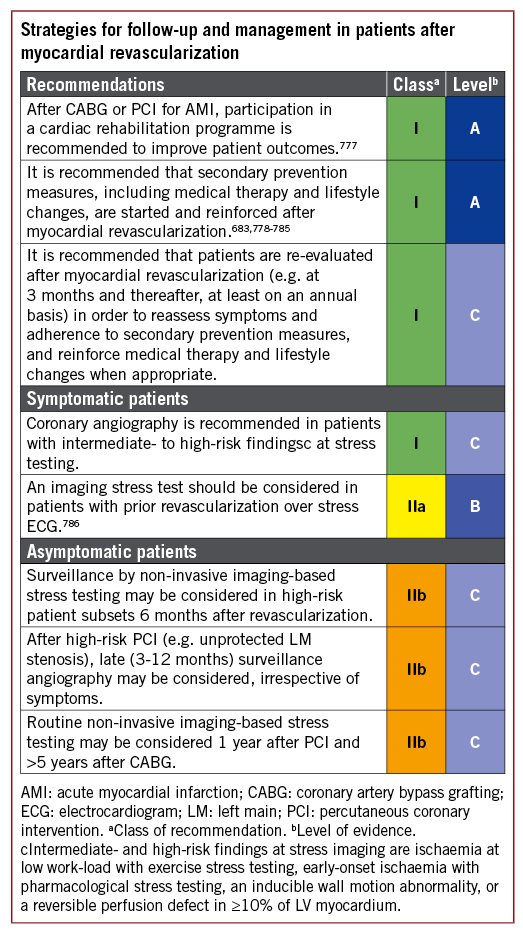
19.1 GAPS IN THE EVIDENCE
In all studies to date on the optimal follow-up after PCI, the gain from discovering patients with restenosis is obscured by the high rate of false positive exercise ECG tests indicating ischaemia. Therefore, simple exercise ECG testing is not recommended for follow-up and a non-invasive imaging approach is preferred. Specific studies to clarify which subset of patients benefits more from a specific follow-up approach are missing. More studies are needed to assess the role of CT angiography in patient surveillance after myocardial revascularization.
20 Key messages
(1) Myocardial revascularization is performed for the relief of symptoms of myocardial ischaemia and the improvement of prognosis. In SCAD, the prognostic benefit is dependent on the extent of myocardium subject to ischaemia.
(2) The prognostic and symptomatic benefits of myocardial revascularization critically depend on the completeness of revascularization. Therefore, the ability to achieve complete revascularization is a key issue when choosing the appropriate treatment strategy.
(3) Apart from issues of individual operative risk and technical feasibility, diabetes mellitus and the anatomical complexity of CAD determine the relative benefits of PCI and CABG.
(4) The SYNTAX score is the recommended tool to gauge the anatomical complexity of coronary disease.
(5) In some instances, both PCI and CABG are equally reasonable, or sometimes even equally problematic, options. This calls for the Heart Team to be consulted to develop individualized treatment concepts, with respect for the preferences of the patient who has been informed about early and late outcomes.
(6) Timely PCI of the culprit lesion remains the mainstay of treatment of ACS.
(7) After PCI of the culprit lesion in ACS, the choice of further revascularization modality should follow the criteria applied to patients with SCAD.
(8) Radial access is preferred for any PCI irrespective of clinical presentation, unless there are overriding procedural considerations.
(9) DES are recommended for any PCI irrespective of clinical presentation, lesion type, anticipated duration of DAPT, or concomitant anticoagulant therapy.
(10) Even though 6 months of DAPT is generally recommended after PCI in SCAD and 12 months of DAPT after ACS, the type and duration of DAPT should be individualized according to the ischaemic and bleeding risks, and appropriately adapted during follow-up. Based on this judgement, treatment durations for DAPT after DES that are as short as 1 month or even as long as lifelong may be reasonable.
(11) Off-pump surgery with no-touch aorta for high-risk patients should be considered when expertise exists.
(12) Multiple arterial grafting should be considered using the radial artery for high-grade stenosis and/or BIMA grafting for patients who do not have an increased risk of sternal wound infection.
21 Evidence-based ‘to do’ and ‘not to do’ messages from the Guidelines
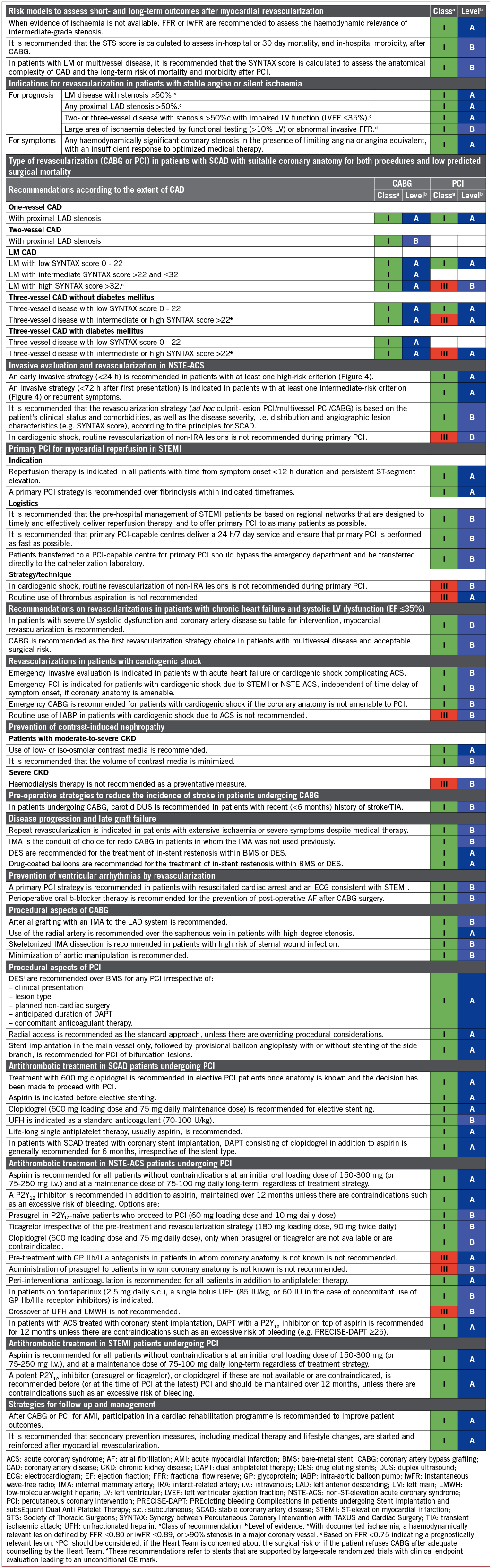
22 Appendix
ESC Committee for Practice Guidelines (CPG): Stephan Windecker (Chairperson) (Switzerland), Victor Aboyans (France), Stefan Agewall (Norway), Emanuele Barbato (Italy), Héctor Bueno (Spain), Antonio Coca (Spain), Jean-Philippe Collet (France), Ioan Mircea Coman (Romania), Veronica Dean (France), Victoria Delgado (The Netherlands), Donna Fitzsimons (UK), Oliver Gaemperli (Switzerland), Gerhard Hindricks (Germany), Bernard Iung (France), Peter Jüni (Canada), Hugo A. Katus (Germany), Juhani Knuuti (Finland), Patrizio Lancellotti (Belgium), Christophe Leclercq (France), Theresa A. McDonagh (UK), Massimo Francesco Piepoli (Italy), Piotr Ponikowski (Poland), Dimitrios J. Richter (Greece), Marco Roffi (Switzerland), Evgeny Shlyakhto (Russia), Miguel Sousa-Uva (Portugal), Iain A. Simpson (UK), Jose Luis Zamorano (Spain).
EACTS Council: (On behalf of the EACTS Council): Domenico Pagano (Secretary General) (UK), Nick Freemantle (UK), Miguel Sousa-Uva (Portugal).
ESC National Cardiac Societies actively involved in the review process of the 2018 ESC/EACTS Guidelines on myocardial revascularization: Algeria: Algerian Society of Cardiology, Mohamed Chettibi; Armenia: Armenian Cardiologists Association, Hamayak Sisakian; Austria: Austrian Society of Cardiology, Bernhard Metzler; Azerbaijan: Azerbaijan Society of Cardiology, Firdovsi _Ibrahimov; Belarus: Belorussian Scientific Society of Cardiologists, Valeriy I. Stelmashok; Bulgaria: Bulgarian Society of Cardiology, Arman Postadzhiyan; Croatia: Croatian Cardiac Society, Bosko Skoric; Cyprus: Cyprus Society of Cardiology, Christos Eftychiou; Czech Republic: Czech Society of Cardiology, Petr Kala; Denmark: Danish Society of Cardiology, Christian Juhl Terkelsen; Egypt: Egyptian Society of Cardiology, Ahmed Magdy; Estonia: Estonian Society of Cardiology, Jaan Eha; Finland: Finnish Cardiac Society, Matti Niemelä; The Former Yugoslav Republic of Macedonia: Macedonian FYR Society of Cardiology, Sasko Kedev; France: French Society of Cardiology, Pascal Motreff; Georgia: Georgian Society of Cardiology, Alexander Aladashvili; Germany: German Cardiac Society, Julinda Mehilli; Greece: Hellenic Society of Cardiology, Ioannis-Georgios Kanakakis; Hungary: Hungarian Society of Cardiology, David Becker; Iceland: Icelandic Society of Cardiology, Thorarinn Gudnason; Ireland: Irish Cardiac Society, Aaron Peace; Italy: Italian Federation of Cardiology, Francesco Romeo; Kosovo: Kosovo Society of Cardiology, Gani Bajraktari; Kyrgyzstan: Kyrgyz Society of Cardiology, Alina Kerimkulova; Latvia: Latvian Society of Cardiology, Ainars Rudzitis; Lebanon: Lebanese Society of Cardiology, Ziad Ghazzal; Lithuania: Lithuanian Society of Cardiology, Aleksandras Kibarskis; Luxembourg: Luxembourg Society of Cardiology, Bruno Pereira; Malta: Maltese Cardiac Society, Robert G. Xuereb; The Netherlands: Netherlands Society of Cardiology, Sjoerd H. Hofma; Norway: Norwegian Society of Cardiology, Terje K. Steigen; Poland: Polish Cardiac Society, Adam Witkowski; Portugal: Portuguese Society of Cardiology, Eduardo Infante de Oliveira; Romania: Romanian Society of Cardiology, Stefan Mot; Russian Federation: Russian Society of Cardiology, Dmitry Duplyakov; San Marino: San Marino Society of Cardiology, Marco Zavatta; Serbia: Cardiology Society of Serbia, Branko Beleslin; Slovakia: Slovak Society of Cardiology, Frantisek Kovar; Slovenia: Slovenian Society of Cardiology, Matjaz Bunc; Spain: Spanish Society of Cardiology, Soledad Ojeda; Sweden: Swedish Society of Cardiology, Nils Witt; Switzerland: Swiss Society of Cardiology, Raban Jeger; Tunisia: Tunisian Society of Cardiology and Cardio-Vascular Surgery, Faouzi Addad; Turkey: Turkish Society of Cardiology, Ramazan Akdemir; Ukraine: Ukrainian Association of Cardiology, Alexander Parkhomenko; United Kingdom: British Cardiovascular Society, Robert Henderson.
23. References

