National Cardiac Societies document reviewers: listed in Addenda
The content of these European Society of Cardiology (ESC) Guidelines has been published for personal and educational use only. No commercial use is authorized. No part of the ESC Guidelines may be translated or reproduced in any form without written permission from the ESC. Permission can be obtained upon submission of a written request to Oxford University Press, the publisher of the European Heart Journal and the party authorized to handle such permissions on behalf of the ESC.
‡ Other ESC entities having participated in the development of this document:
Associations: Acute Cardiovascular Care Association (ACCA), European Association for Cardiovascular Prevention & Rehabilitation (EACPR), European Association of Cardiovascular Imaging (EACVI), European Heart Rhythm Association (EHRA), Heart Failure Association of the ESC (HFA).
Working groups: Working Group on Cardiac Cellular Electrophysiology, Working Group on Cardiovascular Magnetic Resonance, Working Group on Cardiovascular Pharmacology and Drug Therapy, Working Group on Cardiovascular Surgery, Working Group on Coronary Pathophysiology and Microcirculation, Working Group on Nuclear Cardiology and Cardiac Computed Tomography, Working Group on Peripheral Circulation, Working Group on Thrombosis, Working Group on Valvular Heart Disease.
Councils: Council for Cardiology Practice, Council on Cardiovascular Primary Care, Council on Cardiovascular Nursing and Allied Professions.
Disclaimer 2014: The ESC Guidelines represent the views of the ESC and were produced after careful consideration of the scientific and medical knowledge and the evidence available at the time of their dating.
The ESC is not responsible in the event of any contradiction, discrepancy and/or ambiguity between the ESC Guidelines and any other official recommendations or guidelines issued by the relevant public health authorities, in particular in relation to good use of healthcare or therapeutic strategies. Health professionals are encouraged to take the ESC Guidelines fully into account when exercising their clinical judgment as well as in the determination and the implementation of preventive, diagnostic or therapeutic medical strategies; however, the ESC Guidelines do not in any way whatsoever override the individual responsibility of health professionals to make appropriate and accurate decisions in consideration of each patient’s health condition and, where appropriate and/or necessary, in consultation with that patient and the patient’s care provider. Nor do the ESC Guidelines exempt health professionals from giving full and careful consideration to the relevant official, updated recommendations or guidelines issued by the competent public health authorities, in order to manage each patient’s case in light of the scientifically accepted data pursuant to their respective ethical and professional obligations. It is also the health professional’s responsibility to verify the applicable rules and regulations relating to drugs and medical devices at the time of prescription.
ESC Committee for Practice Guidelines: Jose Luis Zamorano (Chairperson) (Spain), Stephan Achenbach (Germany), Helmut Baumgartner (Germany), Jeroen J. Bax (Netherlands), Héctor Bueno (Spain), Veronica Dean (France), Christi Deaton (UK), Çetin Erol (Turkey), Robert Fagard (Belgium), Roberto Ferrari (Italy), David Hasdai (Israel), Arno W. Hoes (Netherlands), Paulus Kirchhof (Germany/UK), Juhani Knuuti (Finland), Philippe Kolh (Belgium), Patrizio Lancellotti (Belgium), Ales Linhart (Czech Republic), Petros Nihoyannopoulos (UK), Massimo F. Piepoli (Italy), Piotr Ponikowski (Poland), Per Anton Sirnes (Norway), Juan Luis Tamargo (Spain), Michal Tendera (Poland), Adam Torbicki (Poland), William Wijns (Belgium), and Stephan Windecker (Switzerland).
EACTS Clinical Guidelines Committee: Miguel Sousa Uva (Chairperson) (Portugal).
Document reviewers: Stephan Achenbach (ESC Review Coordinator) (Germany), John Pepper (EACTS Review Coordinator) (UK), Anelechi Anyanwu (USA), Lina Badimon (Spain), Johann Bauersachs (Germany),
Andreas Baumbach (UK), Farzin Beygui (France), Nikolaos Bonaros (Austria), Marco De Carlo (Italy), Christi Deaton (UK), Dobromir Dobrev (Germany), Joel Dunning (UK), Eric Eeckhout (Switzerland), Stephan Gielen (Germany), David Hasdai (Israel), Paulus Kirchhof (UK/Germany), Heyman Luckraz (UK), Heiko Mahrholdt (Germany), Gilles Montalescot (France), Domenico Paparella (Italy), Ardawan J. Rastan (Germany), Marcelo Sanmartin (Spain), Paul Sergeant (Belgium), Sigmund Silber (Germany), Juan Tamargo (Spain), Jurrien ten Berg (Netherlands), Holger Thiele (Germany), Robert-Jan van Geuns (Netherlands), Hans-Otto Wagner (Germany), Sven Wassmann (Germany), Olaf Wendler (UK), and Jose Luis Zamorano (Spain).
The disclosure forms of the authors and reviewers are available on the ESC website www.escardio.org/guidelines
1. Preamble
Guidelines summarize and evaluate all available evidence, at the time of the writing process, on a particular issue with the aim of assisting health professionals in selecting the best management strategies for an individual patient with a given condition, taking into account the impact on outcome, as well as the risk-benefit ratio of particular diagnostic or therapeutic means. Guidelines and recommendations should help health professionals to make decisions in their daily practice; however, the final decisions concerning an individual patient must be made by the responsible health professional(s), in consultation with the patient and caregiver as appropriate.
A great number of guidelines have been issued in recent years by the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS), as well as by other societies and organisations. Because of their impact on clinical practice, quality criteria for the development of guidelines have been established in order to make all decisions transparent to the user. The recommendations for formulating and issuing ESC/EACTS Guidelines can be found on the ESC web site (http://www.escardio.org/guidelines-surveys/esc-guidelines/about/Pages/rules-writing. aspx). These ESC/EACTS guidelines represent the official position of these two societies on this given topic and are regularly updated.
Members of this Task Force were selected by the ESC and EACTS to represent professionals involved with the medical care of patients with this pathology. Selected experts in the field undertook a comprehensive review of the published evidence for management (including diagnosis, treatment, prevention and rehabilitation) of a given condition, according to the ESC Committee for Practice Guidelines (CPG) and EACTS Guidelines Committee policy. A critical evaluation of diagnostic and therapeutic procedures was performed, including assessment of the risk-benefit ratio. Estimates of expected health outcomes for larger populations were included, where data exist. The level of evidence and the strength of recommendation of particular management options were weighed and graded according to pre-defined scales, as outlined in Tables 1 and 2.
The experts of the writing and reviewing panels completed ‘declarations of interest’ forms which might be perceived as real or potential sources of conflicts of interest. These forms were compiled into one file and can be found on the ESC web site (http://www.escardio.org/guidelines). Any changes in declarations of interest that arise during the writing period must be notified to the ESC/ EACTS and updated. The Task Force received its entire financial support from the ESC and EACTS, without any involvement from the healthcare industry.
The ESC CPG supervises and co-ordinates the preparation of new guidelines produced by Task Forces, expert groups or consensus panels. The Committee is also responsible for the endorsement process of these guidelines. The ESC and Joint Guidelines undergo extensive review by the CPG and partner Guidelines Committee and external experts. After appropriate revisions it is approved by all the experts involved in the Task Force. The finalized document is approved by the CPG/EACTS for simultaneous publication in the European Heart Journal and joint partner journal, in this instance the European Journal of Cardio-Thoracic Surgery. It was developed after careful consideration of the scientific and medical knowledge and the evidence available at the time of their dating.
The task of developing ESC/EACTS Guidelines covers not only the integration of the most recent research, but also the creation of educational tools and implementation programmes for the recommendations. To implement the guidelines, condensed pocket versions, summary slides, booklets with essential messages, summary cards for non-specialists, electronic versions for digital applications (smart phones etc.) are produced. These versions are abridged and thus, if needed, one should always refer to the full-text version, which is freely available on the ESC and EACTS web sites. The national societies of the ESC and of the EACTS are encouraged to endorse, translate and implement the ESC Guidelines. Implementation programmes are needed because it has been shown that the outcome of disease may be favourably influenced by the thorough application of clinical recommendations.
Surveys and registries are needed to verify that real-life daily practice is in keeping with what is recommended in the guidelines, thus completing the loop between clinical research, writing of guidelines, disseminating them and implementing them into clinical practice.
Health professionals are encouraged to take the ESC/EACTS Guidelines fully into account when exercising their clinical judgment, as well as in the determination and the implementation of preventive, diagnostic or therapeutic medical strategies; however, the ESC/ EACTS Guidelines do not, in any way whatsoever, override the individual responsibility of health professionals to make appropriate and accurate decisions in consideration of the condition of each patient’s health and in consultation with that patient and, where appropriate and/or necessary, the patient’s caregiver. It is also the health professional’s responsibility to verify the rules and regulations applicable to drugs and devices at the time of prescription.
2. Introduction
Fifty years of myocardial revascularization
In 2014, coronary artery bypass grafting (CABG) celebrates the 50th anniversary of the first procedures performed in 1964.1 Thirteen years later, the first percutaneous coronary intervention (PCI) was performed.2 Since then both revascularization techniques have undergone continued advances, in particular the systematic use of arterial conduits in the case of CABG, and the advent of stents. In the meantime, PCI has become one of the most frequently performed therapeutic interventions in medicine,3 and progress has resulted in a steady decline of periprocedural adverse events, resulting in excellent outcomes with both revascularization techniques. Notwithstanding, the differences between the two revascularization strategies should be recognized. In CABG, bypass grafts are placed to the mid-coronary vessel beyond the culprit lesion(s), providing extra sources of bloodflow to the myocardium and offering protection against the consequences of further proximal obstructive disease. In contrast, coronary stents aim at restoring normal bloodflow of the native coronary vasculature by local treatment of obstructive lesions without offering protection against new disease proximal to the stent.
Myocardial revascularization has been subject to more randomized clinical trials (RCTs) than almost any other intervention (Figure 1). In order to inform the current Guidelines, this Task Force performed a systematic review of all RCTs performed since 1980, comparing head-to-head the different revascularization strategies –including CABG, balloon angioplasty, and PCI with bare-metal stents (BMS) or with various US Food and Drug Administration-approved drug-eluting stents (DES)– against medical treatment as well as different revascularization strategies, and retrieved 100 RCTs involving 93 553 patients with 262 090 patient-years of follow-up.4
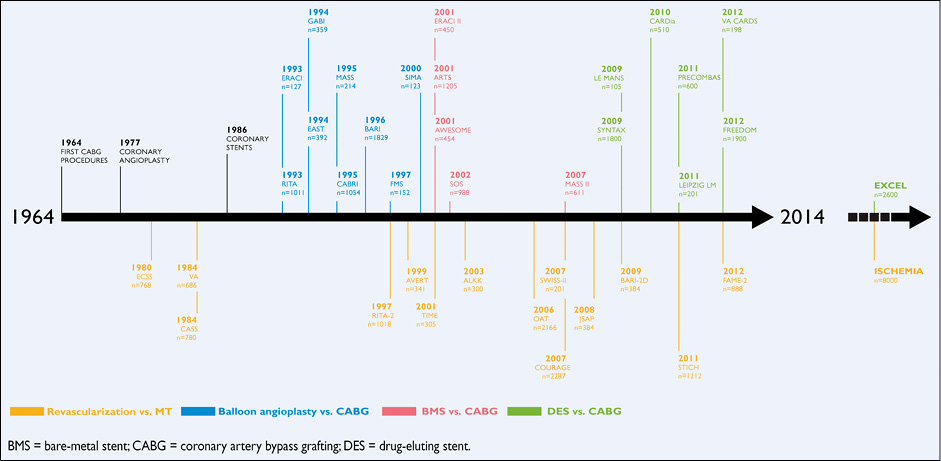
Figure 1 Randomized trials in myocardial revascularization therapy over the past five decades.
Formulation of the best possible revascularization approach, also taking into consideration the social and cultural context, will often require interaction between cardiologists and cardiac surgeons, referring physicians, or other specialists as appropriate. Patients need help with taking informed decisions about their treatment and the most valuable advice will probably be provided to them by the ‘Heart Team’.5 Recognizing the importance of the interaction between cardiologists and cardiac surgeons, the leadership of both the ESC and the EACTS has given this Joint Task Force, along with their respective Guideline Committees, and the reviewers of this document the mission to draft balanced, patient-centred, evidence-driven practice guidelines on myocardial revascularization. The respective Chairpersons of these two associations and CPG Chairperson were also given the task to adapt to the declaration of interest policy and to ensure that their Task Force members followed it throughout the development process of the Guidelines. In the event that any of the Task Force members had a potential conflict of interest to declare, he/she did not participate in the final decision of the Task Force on the given subject.
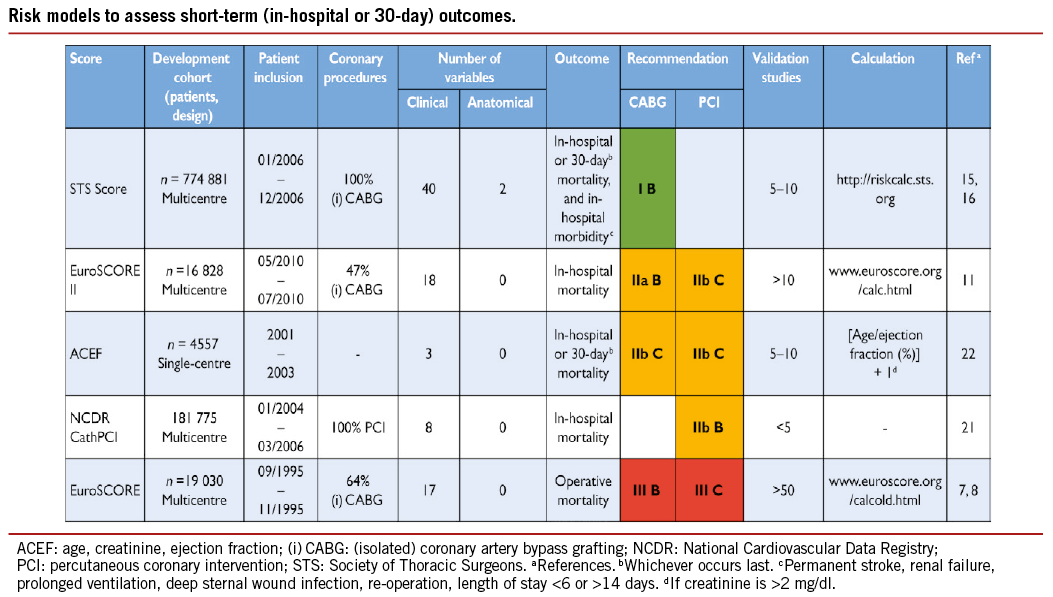
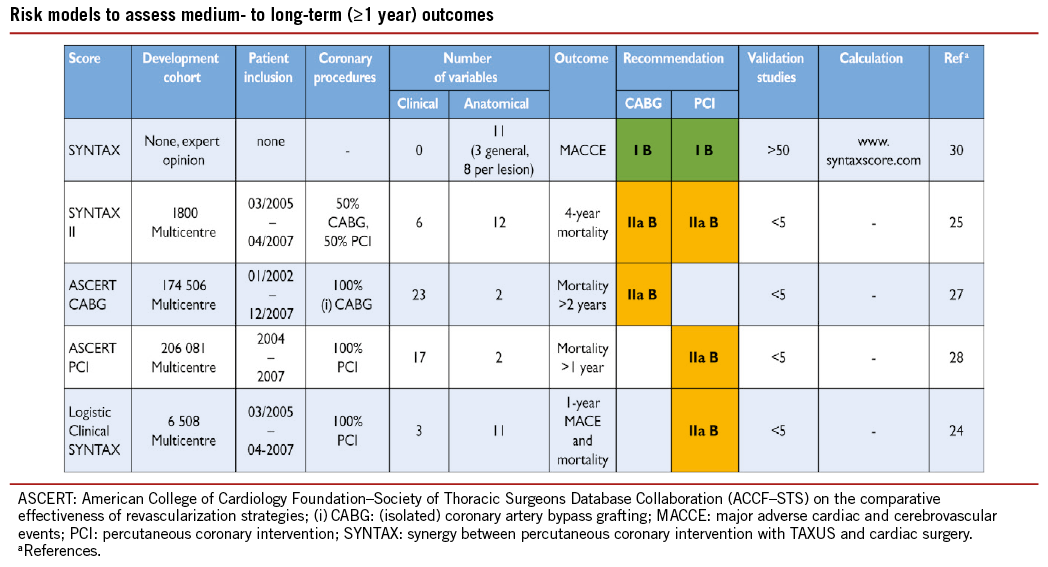
3. Scores and risk stratification
Myocardial revascularization in the elective setting is appropriate when the expected benefits, in terms of survival or health outcomes (symptoms, functional status, and/or quality of life), exceed the expected negative consequences of the procedure. Whether medical therapy, PCI, or CABG is preferred should depend on the risk-benefit ratios of these treatment strategies, weighting the risks of periprocedural death, myocardial infarction and stroke against improvements in health-related quality of life, as well as long-term freedom from death, myocardial infarction or repeat revascu- larization. The Heart Team should take into consideration the coron- ary anatomy, disease, age and comorbidities, patient preference, and hospital/operator experience.
Numerous models have been developed for risk stratification, focussing on anatomical complexity or clinical risk, and have demonstrated their value during decision-making.6 Those models most frequently used in a clinical setting are summarized in the Tables of recommendation [risk models to assess short-term (in-hospital or 30-day) and medium-to-long-term (≥1 year) outcomes].
(1) The EuroSCORE predicts surgical mortality.7,8 It is based on an old data set and has been shown to overestimate the risk of mortality, and should therefore no longer be used.9,10
(2) The EuroSCORE II is an update of the logistic EuroSCORE model and is derived from a more contemporary data set better reflecting current cardiac surgical practice.11 Its value has been demonstrated in specific cohorts of patients undergoing CABG.12 Compared with its original version, the EuroSCORE II may have a better ability to predict mortality.12-14
(3) The Society of Thoracic Surgeons (STS) score is a risk-prediction model, validated in patients undergoing cardiac surgery, with a specific model for CABG surgery and combined CABG and valve surgery.15,16 It can be used to predict in-hospital or 30-day mortality (whichever occurs last) and in-hospital morbidity.
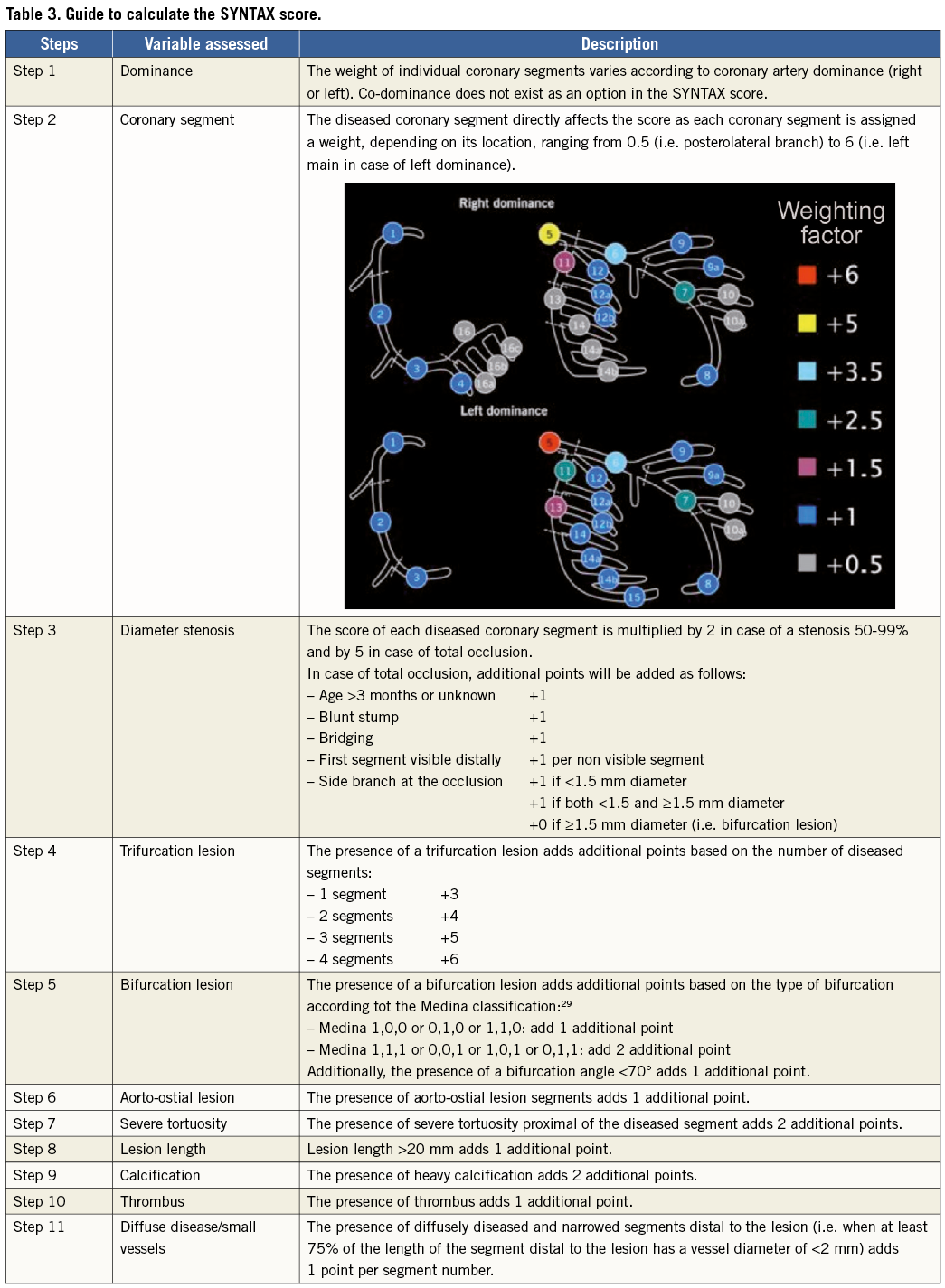
(4) The SYNTAX score (Table 3) was developed to grade the anatomical complexity of coronary lesions in patients with left main or three-vessel disease, and was found to be an independent predictor of long-term major adverse cardiac and cerebrovascular event (MACCE) in patients treated with PCI but not CABG.17,18 It facilitates the selection of optimal treatment by identifying patients at highest risk of adverse events following PCI. The interobserver variability of the Synergy between Percutaneous Coronary Intervention with TAXUS and Cardiac Surgery (SYNTAX) score is significant,19 although development of non-invasive assessments may simplify calculation of the SYNTAX score.20
(5) The National Cardiovascular Database Registry (NCDR CathPCI) risk score has been developed to predict risk in PCI patients and should only be used in this context.21
(6) The age, creatinine, ejection fraction (ACEF) model is a simple score as it contains only three variables, and was developed using data from a cohort of surgical patients.22 ACEF has also been validated to predict mortality in patients undergoing PCI.23
(7) The clinical SYNTAX score is a combination of the ACEF and SYNTAX scores. Originally established as an additive model, the subsequent development of a logistic model has provided more tailored risk assessment.24
(8) The SYNTAX II score is a combination of anatomical and clinical factors [age, creatinine clearance, left ventricular (LV) function, gender, chronic obstructive pulmonary disease, and peripheral vascular disease] and predicts long-term mortality in patients with complex three-vessel or left main (LM) coronary artery disease (CAD).25 It was found to be superior to the conventional SYNTAX score in guiding decision-making between CABG and PCI in the SYNTAX trial, and subsequently validated in the drug-eluting stent for left main coronary artery disease DELTA registry.
(9) For the American College of Cardiology Foundation – Society of Thoracic Surgeons Database Collaboration (ASCERT) study,26 two large datasets from the National Cardiovascular Data Registry (NCDR) and STS were used to develop several models to predict mortality at different time points following CABG and PCI.27,28
Comparative analyses of these models are limited because available studies have largely evaluated individual risk models in different patient populations, with different outcome measures being reported at various time points, and most models are restricted to one type of revascularization. In addition, several important variables, such as frailty, physical independence and porcelain aorta, are not incorporated in current risk scores. An ideal risk-benefit model enables comparison of the short-term benefits of PCI to the long-term benefits of CABG; however, even though risk models may provide useful information for predicting mortality and major adverse events, prediction of which patients will receive benefit in terms of quality of life is so far unavailable.
These limitations restrict the ability to recommend one specific risk model. It is also important to acknowledge that no risk score can accurately predict events in an individual patient. Moreover, limitations exist in all databases used to build risk models, and differences in definitions and variable content can affect the performance of risk scores when they are applied across differing populations. Ultimately, risk stratification should be used as a guide, while clinical judgement and multidisciplinary dialogue (The Heart Team) remain essential.25
4. Process for decision-making and patient information
4.1 PATIENT INFORMATION AND INFORMED CONSENT
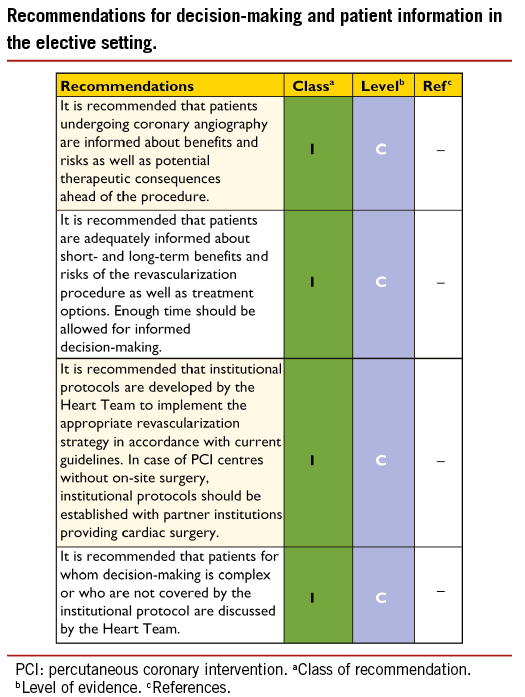
The process of medical decision-making and patient information is guided by the ‘four principles’ approach to healthcare ethics: autonomy, beneficence, non-maleficence, and justice.31 The informed consent process should not be regarded as a necessary legal requirement but as an opportunity to optimize decision-making. Patient-related factors, institutional factors and referral patterns may impact the decision-making process.
Informed consent requires transparency, especially if there is controversy over various treatment options. Collaborative care requires the pre-conditions of communication, comprehension, and trust. Treatment decisions should not be based solely on research results and the physician’s appraisal of the patient’s circumstances, since active patient participation in the decision-making process may yield better outcomes. Patients are subject to bias by labels when considering coronary revascularization,32 and patient preference may sometimes contradict evidentiary best practice. Patients may have limited understanding of their disease and sometimes unreasonable expectations with regard to the outcomes of a proposed intervention. As many as 68% of patients are not aware of an alternative revascularization strategy.33 Short-term procedure-related and long-term risks and benefits –such as survival, relief of angina, quality of life, potential need for late re-intervention, and uncertainties associated with different treatment strategies– should be thoroughly discussed. Patients can only weigh this information in the light of their personal values and cultural background and must therefore have the time to reflect on the trade-offs imposed by the outcome estimates.
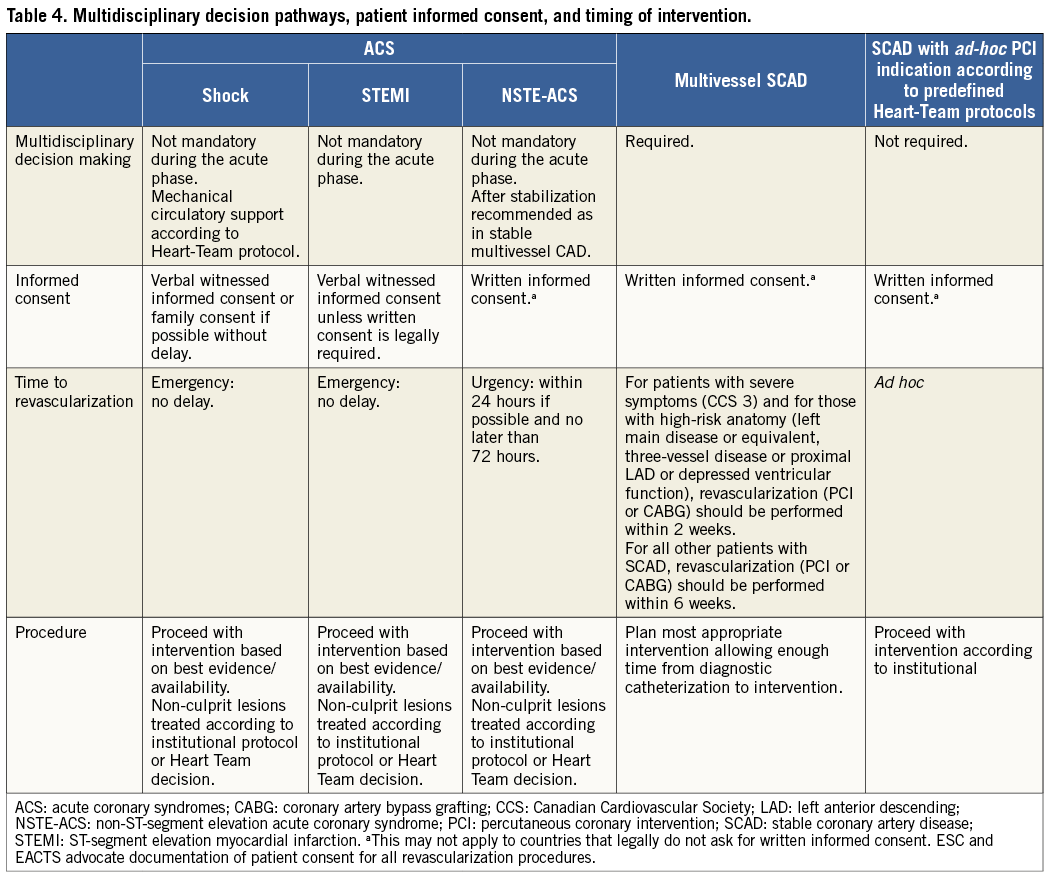
In order to seek a second opinion or to discuss the findings and consequences with referring physicians, enough time should be allowed –up to several days, as required– between diagnostic catheterization and intervention. Patient information needs to be unbiased, evidence-based, up-to-date, reliable, accessible, relevant, and consistent with legal requirements. Consistent use of terminology, that the patient understands, is essential. A written patient information document is needed. These recommendations pertain to patients in stable condition, for whom various treatment options exist and who can make a decision without the constraints of an urgent or emergency situation (Table 4).
Anonymous treatment should be avoided. The patient has the right to obtain information on the level of expertise of the operator, the workload of the centre and whether all treatment options including surgery are available on site. Patients considered for revascularization should also be clearly informed of the continuing need for medical therapy, as well as lifestyle modification and other secondary prevention strategies (section 20).
4.2 MULTIDISCIPLINARY DECISION-MAKING (HEART TEAM)
The Heart Team, made up of clinical or non-invasive cardiologists, cardiac surgeons and interventional cardiologists, provides a balanced, multidisciplinary decision-making process.5 Additional input may be needed from other specialties involved in the care of the patient. The Heart Team should meet on a regular basis to analyse and interpret the available diagnostic evidence, put into context the clinical condition of the patient, determine the need –or otherwise– for an intervention and the likelihood of safe and effective revascularization with either PCI or CABG. Ad hoc meetings of the Heart Team should facilitate and support efficient clinical workflows.
The demand for an interdisciplinary approach is underlined by reports on (i) underuse of revascularization procedures in 18-40% of patients with CAD,34 and (ii) inappropriate use of revascularization strategies and a lack of case discussions.35 The large variability between European countries in PCI-to-CABG ratios (ranging from 2.0 to 8.6 in 2007) has raised concerns regarding the appropriate selection of revascularization in Europe.36 Rates for the inappropriate use of PCI (11-15%) or doubt over the appropriateness of PCI (40-50%)5,37 and, to a lesser degree for CABG (1-2% and 0-9%, respectively) are reported.5,38 The increasing underuse of CABG is in part explained by PCI treatment in patients with indications for surgery.39,40 Multidisciplinary decision-making in a Heart Team can minimize specialty bias and prevent self-referral from interfering with optimal patient care.32,41 Standard evidence-based, interdisciplinary, institutional protocols may be used for common case scenarios, to avoid the need for the systematic case-by-case review of all diagnostic angiograms, but complex cases should be discussed individually. In these cases, revascularization should not be performed at the time of diagnostic angiography, to allow sufficient time to assess all available information, and clearly explain and discuss the findings with the patient.41 The rationale for a decision and consensus on the optimal revascularization treatment should be documented on the patient’s chart. In hospitals without a cardiac surgical unit or in an ambulatory setting, protocols should be designed in collaboration with an expert interventional cardiologist and a cardiac surgeon. Decisions made by a Heart Team seem to be reproducible.42
4.3 TIMING OF REVASCULARIZATION AND AD HOC PERCUTANEOUS CORONARY INTERVENTION
Studies of patients scheduled for revascularization have revealed that considerable morbidity and mortality are associated with extended delay of treatment.43,44 The waiting period for diagnostic catheterization should therefore be minimal. Once the decision for revascularization has been reached after diagnostic coronary angiography, the Task Force recommends that patients with severe symptoms Canadian Cardiovascular Society (CCS) Class 3 and those with high-risk anatomy [left main disease or equivalent; three-vessel disease or proximal left anterior descending (LAD) or depressed ventricular function] preferably undergo revascularization (PCI or CABG) within 2 weeks. For all other patients with stable coronary artery disease (SCAD) and an indication for revascularization, it is desirable to perform revascularization (PCI or CABG) within 6 weeks (Table 4).44
Ad hoc PCI is defined as a therapeutic intervention performed within the same procedure as the diagnostic coronary angiography. Ad hoc PCI is convenient, associated with fewer access site complications, and often cost-effective and safe.45 In the USA, however, up to 30% of patients undergoing ad hoc PCI are potential candidates for CABG. Although this number may be lower in Europe,35 ad hoc PCI should not be applied as a default approach.45,46 Ad hoc PCI in stable patients is only justified after adequate information given to the patient (see section 4.1) and if a full diagnostic work-up, including functional testing (section 5) is available. Institutional protocols developed by the Heart Team in accordance with current guidelines should define specific anatomical criteria and clinical subsets that may be –or should not be– treated ad hoc. Complex pathologies in stable patients, including lesions of the LM or proximal LAD and three-vessel disease, should in general not be treated ad hoc, but discussed by the Heart Team.
5. Strategies for diagnosis: functional testing and imaging
Exercise testing and cardiac imaging are used to confirm the diagnosis of CAD, to document ischaemia in patients with stable symptoms, to risk-stratify patients, and to help choose treatment options and evaluate their efficacy as explained in detail in the ESC Guidelines on the management of stable coronary artery disease.47
Another indication for non-invasive imaging before revascularization is the detection of myocardial viability in patients with poor LV function.
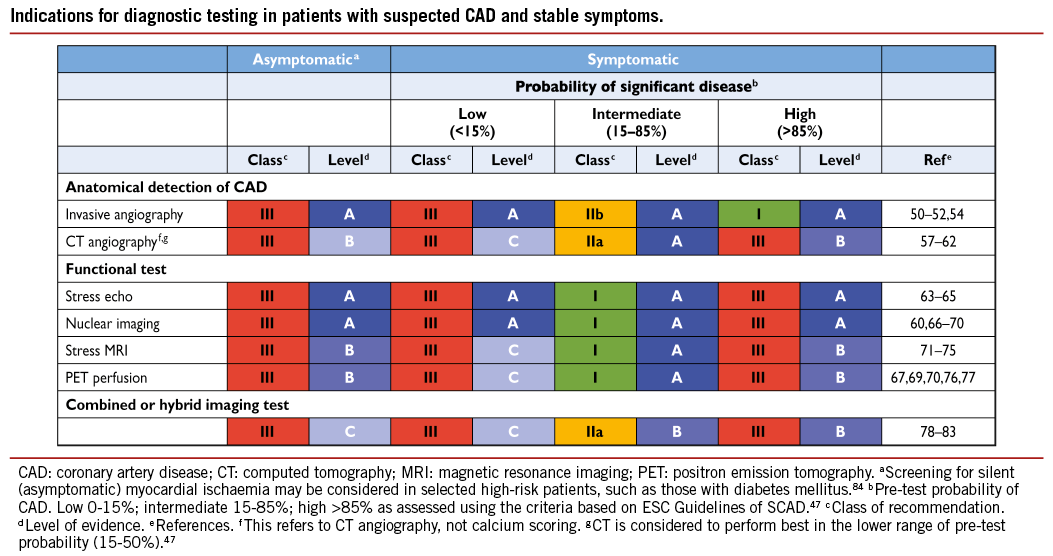
5.1 NON-INVASIVE TESTS
The documentation of ischaemia using functional testing is recommended in patients with suspected SCAD before elective invasive procedures, preferably using non-invasive testing before invasive angiography. Although several tests can be used, it is important to avoid unnecessary diagnostic steps. The current evidence supporting the use of various tests for the detection of CAD is based on meta-analyses and multicentre studies, and using only anatomical evaluation of invasive coronary angiography as the reference standard.47 The risks of exercise, pharmacological stressors, contrast agents, invasive procedures, and cumulative ionizing radiation must be weighed against the risk of disease or delayed diagnosis.48
Multi-detector computed tomography (MDCT) can detect coronary atherosclerosis and stenoses and is reliable for ruling out significant CAD in patients with low-to-moderate probability of CAD.49 The tests for detection of ischaemia are based on either reduction of perfusion or induction of ischaemic wall motion abnormalities during exercise or pharmacological stress. The best-established stress imaging techniques are echocardiography and perfusion scintigraphy. Both may be used in combination with exercise stress or pharmacological stress. Newer stress imaging techniques also include stress magnetic resonance imaging (MRI), positron emission tomography (PET), and combined approaches. The term ‘hybrid imaging’ refers to imaging systems in which two modalities [MDCT and PET; MDCT and single photon emission computed tomography (SPECT)] are combined in the same scanner, allowing both studies to be performed in a single imaging session. Ischaemia imaging has been regarded the most appropriate in patients with intermediate pre-test probability (15-85%) of significant CAD,47 while in asymptomatic patients or in those with low or high pre-test probability, the tests are generally not recommended. More detailed information about the imaging tests in the detection of CAD are available in the ESC Guidelines on the management of SCAD47 and in the Web addenda.
5.2 INVASIVE TESTS
Invasive coronary angiography has been regarded as the reference standard for the detection and the assessment of the severity of CAD but, as an invasive procedure, it is associated with specific procedure-related adverse events. Even experienced interventional cardiologists cannot, without functional information, accurately predict the significance of many intermediate stenoses on the basis of visual assessment or quantitative coronary angiography. When non-invasive stress imaging is contraindicated, non-diagnostic, or unavailable, the measurement of fractional flow reserve (FFR) or coronary flow reserve is helpful during diagnostic coronary angiography.50 Deferral of PCI or CABG in patients with FFR >0.80 appears safe.51-53
Fractional flow reserve measurement is indicated for the assessment of the functional consequences of moderate coronary stenoses. FFR-guided PCI with medical therapy has been shown to decrease the need for urgent revascularization compared with the best available medical therapy alone.54
5.3 DETECTION OF MYOCARDIAL VIABILITY
Non-invasive assessment of myocardial viability has been used to guide the management of patients with chronic ischaemic systolic LV dysfunction. Multiple imaging techniques, including PET, SPECT, and dobutamine stress echocardiography, have been evaluated for assessment of viability and prediction of clinical outcome after myocardial revascularization.55 In general, nuclear imaging techniques have a high sensitivity, whereas techniques evaluating contractile reserve have a somewhat lower sensitivity but higher specificity. MRI has a high diagnostic accuracy for assessing the transmural extent of myocardial scar tissue and can also assess contractile reserve, but its ability to detect viability and predict recovery of wall motion is no better than other imaging techniques. The differences in performance between the various imaging techniques are small, and experience and availability commonly determine which technique is used. The evidence is mostly based on observational studies or meta-analyses. One RCT, relating to PET imaging, showed that patients with a substantial amount of dysfunctional but viable myocardium are likely to benefit from myocardial revascularization.56
6. Revascularization for stable coronary artery disease
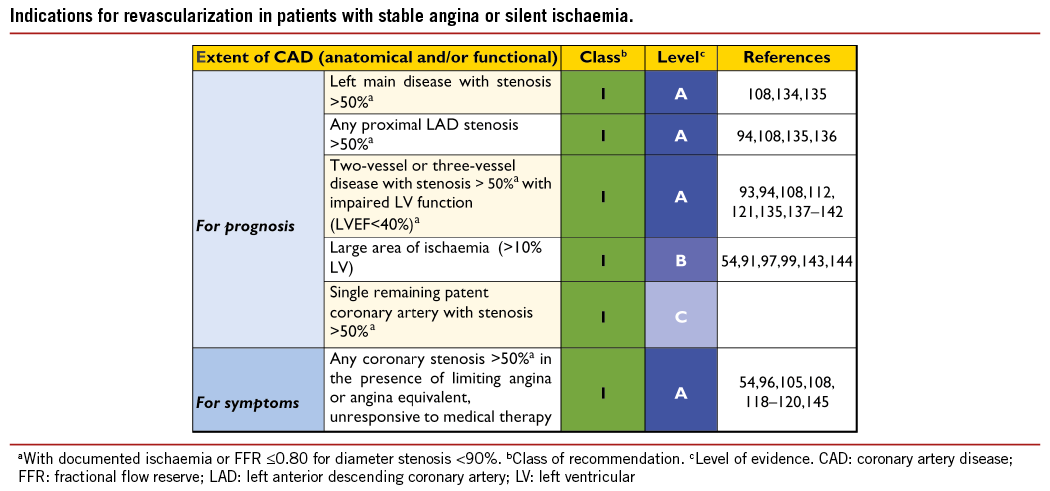
6.1 RATIONALE FOR REVASCULARIZATION
Prior to revascularization, patients with SCAD must receive guideline- recommended medical treatment, due to its established benefits in terms of prognosis and symptom relief.47 Revascularization, by either PCI or CABG, may be indicated in flow-limiting coronary stenoses to reduce myocardial ischaemia and its adverse clinical manifestations.85-87 The indications for revascularization in patients with SCAD are persistence of symptoms despite medical treatment and/or improvement of prognosis.47 Consequently, revascularization and medical therapy should be seen as complementary, rather than competitive treatment strategies. Specific evidence and recommendations for diabetic patients are addressed in section 10.
Angina is associated with impaired quality of life, reduced physical endurance, mental depression, and recurrent hospitalizations and outpatient visits.88 Revascularization by PCI or CABG more effectively relieves angina, reduces the use of anti-angina drugs, and improves exercise capacity and quality of life, compared with a strategy of medical therapy alone (Table 2 Web addenda).54,89-96
Ischaemia is of prognostic importance in patients with SCAD, particularly when occurring at low workload.97,98 Revascularization relieves myocardial ischaemia more effectively than medical treatment alone.92,97,99,100 The extent, location, and severity of coronary artery obstruction as assessed by coronary angiography or coronary computed tomography (CT) angiography are important prognostic factors in addition to ischaemia and left ventricular function.101-103
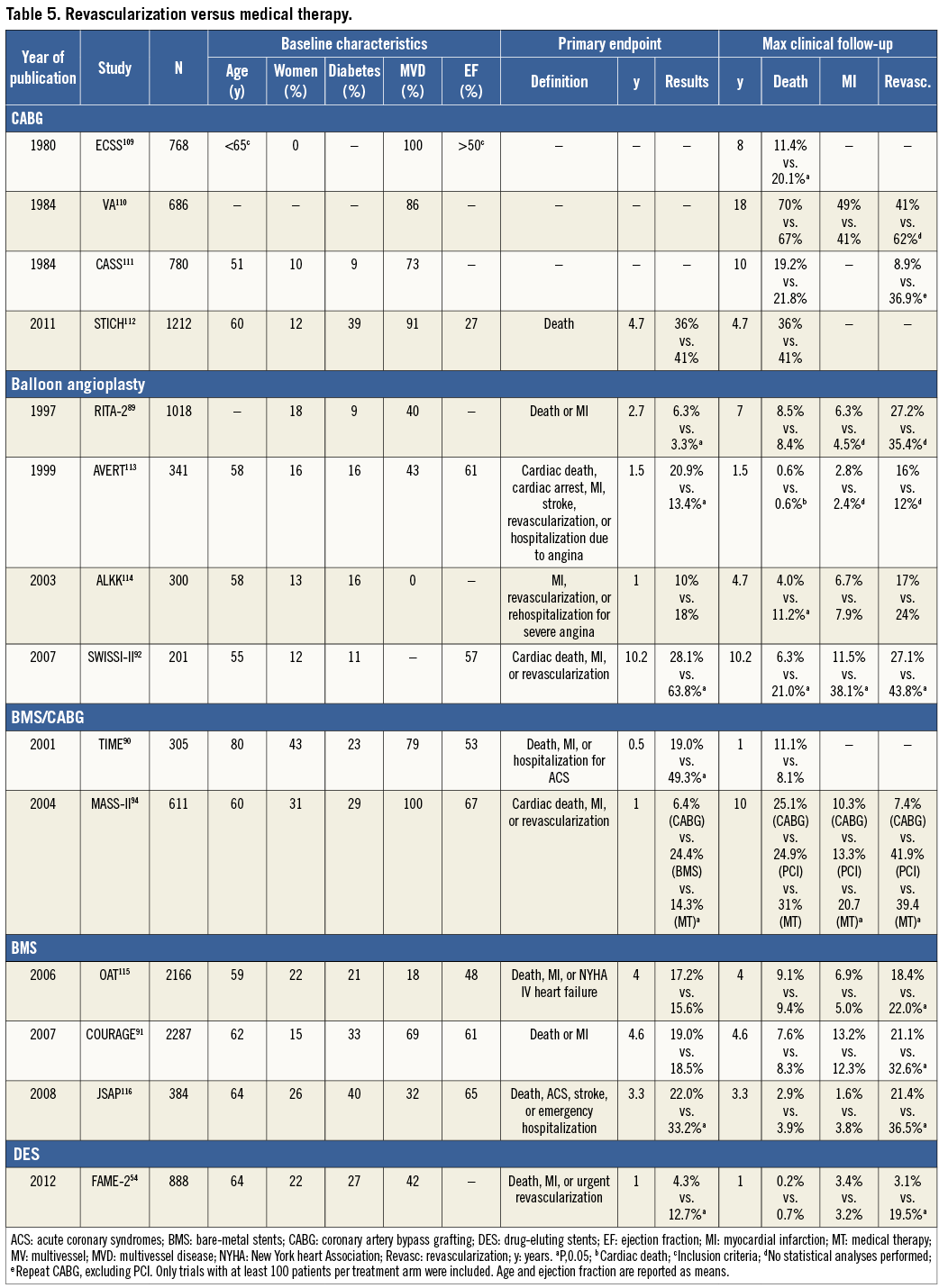
6.2 EVIDENCE BASIS FOR REVASCULARIZATION
The evidence basis for revascularization with PCI and/or CABG, compared with medical treatment, is derived from several RCTs that are summarized in Table 5. It is important to consider that the best current revascularization results achieved with PCI are with new-generation drug-eluting stents (DES) and for CABG with maximal use of arterial grafts. Although revascularization procedures are associated with the risk of biomarker-defined periprocedural myocardial infarction, several studies indicate that pre-PCI –but not post-PCI– biomarker elevations impact adversely on prognosis.104 While spontaneous myocardial infarction has a well established adverse impact on prognosis and notably mortality, recent studies suggest that, compared with medical treatment, PCI is associated with a lower risk of spontaneous myocardial infarction.105
Although individual RCTs and subsequent meta-analyses constitute the highest hierarchical form of evidence-based medicine,106-108 extrapolation of their results to routine clinical practice has its limitations. The majority of RCTs included mainly male patients who were relatively young [with the exception of Trial of Invasive Medical therapy in the Elderly (TIME)], had preserved LV function, and had not previously undergone revascularization. Patients were highly selected, as randomization was usually performed following delineation of coronary anatomy by angiography without routine assessment of ischaemia. By design, all the RCTs compared treatment strategies that allowed subsequent revascularization when patients deteriorated on medical therapy. As a result, the proportion of patients who did not undergo revascularization progressively declined during follow-up, camouflaging differences between the two strategies and making analysis according to the intention-to-treat principle more problematic. Finally, limited duration of follow-up (usually <5 years) incompletely depicts the advantages of CABG related to arterial grafts, which accrue with time but which may also eventually be eroded by progressive vein graft failure.
6.2.1 REVASCULARIZATION WITH THE USE OF PERCUTANEOUS CORONARY INTERVENTION
The efficacy of PCI in addition to medical therapy in patients with SCAD has been addressed in several RCTs,54,91,94 meta-analyses,106,107,117-120 and large-scale registries.121 The most important recent studies and their data are summarized in Table 5.
The Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE)91 trial included 2287 patients with SCAD, who showed objective evidence of ischaemia and significant CAD, randomizing them to medical therapy alone or medical therapy plus PCI with BMS. At a median follow-up of 4.6 years, there were no significant differences between the PCI and medical therapy groups in the composite of death, myocardial infarction and stroke. Freedom from angina was significantly greater in the PCI group at 1 year and 3 years but the advantage was eroded by 5 years, by which time 21% of the PCI group and 33% of the medical therapy group had received additional revascularization (P<0.001). The severity of CAD in COURAGE was moderate and the majority of patients (70%) had no or mild ischaemia at baseline and most patients had normal LV function.122 Patients with LM disease were excluded.
The Medical, Angioplasty or Surgery Study II (MASS II) trial, covering 611 patients with multivessel disease, all recruited at a single institution, is the only RCT comparing medical therapy with PCI (72% with BMS; 28% with balloon angioplasty only) and with CABG. Over 10 years, comparing medical therapy with PCI, the respective rates for all-cause mortality were 31% and 24.1% (P=0.09), for myocardial infarction 20.7% and 13.3% PCI (P=0.01), and for freedom from angina 43% and 59% (P<0.001).94
In the Fractional Flow Reserve versus Angiography for Multivessel Evaluation 2 (FAME-2) trial,54 patients with SCAD and at least one functionally significant stenosis (invasive FFR ≤0.80) were randomly assigned to medical therapy alone or to medical therapy plus FFR-guided PCI. The trial was planned to include 1632 patients but the data safety monitoring board stopped the study prematurely after enrolment of 888 patients, due to a highly significant difference in the incidence of the primary endpoint (a composite of death, myocardial infarction, and urgent revascularization) in favour of FFR-guided PCI that was unlikely to be neutralized with recruitment of more patients. Final analysis showed an incidence of the primary endpoint of 4.3% in the PCI group and 12.7% in the medical therapy group (P<0.001) but without a difference in rates of death or myocardial infarction between the two groups. Interpretation of FAME-2 is complicated, in that the decision for urgent revascularization may have been influenced by the open nature of the trial. The definition of ‘urgent revascularization’ met the criteria for the clinical presentation of an acute coronary syndrome (ACS) and 50% of the patients undergoing urgent revascularization displayed objective evidence of continuing ischaemia.
Most meta-analyses comparing a strategy of PCI against initial medical therapy found no evidence in favour of an invasive strategy, in terms of survival or myocardial infarction.117,118,123,125
Two reported a small survival benefit for PCI over medical therapy, although this might have been influenced by the inclusion of a subset of patients who had had a recent (<4 weeks) myocardial infarction.107,119
One meta-analysis, updated for more recent RCTs, showed that, compared with an initial strategy of medical therapy, PCI was not associated with significant improvement in all-cause mortality [risk ratio (RR) 0.85; 95% confidence interval (CI) 0.71-1.01], cardiac death (RR 0.71; 95% CI 0.47-1.06), myocardial infarction (RR 0.93; 95% CI 0.70-1.24), or repeat revascularization (RR 0.93; 95% CI 0.76-1.14) during short- or long-term follow-up.96 In a meta-analysis of five RCTs covering 5286 patients and site-reported ischaemia at baseline, there were no differences between PCI and medical treatment in terms of death, myocardial infarction, unplanned revascularization or angina during a median follow-up of five years.100
In the New York State’s Cardiac Diagnostic Catheterization Database, 9586 patients were identified between 2003 and 2008, who had either PCI (n=8486; 89%) or medical therapy (n=1100; 11%). A comparison of 933 propensity-score matched patients in each group showed, with PCI over 4 years, a lower incidence of the composite of mortality and myocardial infarction (16.5% vs. 21.2%, respectively; P=0.003) as well as the individual components: death (10.2% vs. 14.5%, respectively; P=0.02) and myocardial infarction (8.0% vs. 11.3%, respectively; P=0.007).121 The authors caution that part of the difference in outcomes might be explained by the differences between the groups in their use of routine medical therapy.
6.2.2 PERCUTANEOUS CORONARY INTERVENTION WITH DRUG-ELUTING STENTS VS. BARE-METAL STENTS
The major limitation of most of the previous comparisons is the lack of use of DES. Several meta-analyses of RCTs comparing early-generation DES with bare-metal stents (BMS) reported similar rates of death, cardiac death, and non-fatal myocardial infarction, but a 50-70% relative risk reduction (RRR) in the need for subsequent or repeat target vessel revascularization (TVR) with DES.124,125
New-generation DES, with thin strut stent platforms, biocompatible durable or biodegradable polymers and limus-based antiproliferative agents, have further advanced efficacy and safety compared with early-generation DES and BMS (see section 17 for more information). Compared with early-generation DES, repeat revascularization was reduced by 10-20%.126 -129 Compared with bare-metal stents and early-generation DES, new-generation DES have also improved safety outcomes including death, myocardial infarction and stent thrombosis. Several studies have reported an approximately 50% lower risk of definite or probable stent thrombosis, than with early-generation DES, particularly during the late phase,128-131 and some studies reported a lower risk of stent thrombosis than with BMS.125,131 A mixed-treatment comparison of DES and BMS, embracing 76 RCTs and 117 762 patient-years of follow-up, did not report a lower risk of death but a lower risk (20-35%) of myocardial infarction with DES (except paclitaxel-eluting stents) than with BMS.132 The randomized Basel Stent Kosten Effektivitäts Trial – Prospective Validation Examination (BASKET-PROVE) trial, comparing DES with BMS among patients with large vessels (>3 mm) showed no significant differences between sirolimus-eluting, everolimus-eluting, and bare-metal stents in terms of the rate of death or myocardial infarction; however, there was a lower risk of cardiac death or myocardial infarction with DES (pooled DES vs. BMS: RR 0.60; 95% CI 0.39-0.93; P=0.02) at 2 years of follow-up.133 An individual patient-data meta-analysis of three RCTs including 4989 patients, which compared new-generation everolimus-eluting stents with early-generation paclitaxel-eluting stents, reported a lower risk of death (3.2% vs. 5.1%; hazard ratio (HR) 0.65; 95% CI 0.49-0.86; P=0.003), cardiac death or myocardial infarction (4.4% vs. 6.3%; HR 0.70; 95% CI 0.54-0.90; P=0.005), and stent thrombosis (0.7% vs. 1.7%; HR 0.45; 95% CI 0.26-0.78; P=0.003) after 3 years of follow-up.126 A patient-level pooled analysis of 26 RCTs in 11 557 women, reported a lower incidence of the composite of death or myocardial infarction in female patients treated with new-generation DES (9.2%) compared with both early-generation DES (10.9%) and BMS (12.8%; P=0.001) at 3 years.129 Similarly, the incidence of definite or probable stent thrombosis was lowest with new-generation DES (1.1%) followed by BMS (1.3%), and early-generation DES (2.1%; P=0.01).
6.2.3 REVASCULARIZATION WITH THE USE OF CORONARY ARTERY BYPASS GRAFTING
The superiority of CABG to a strategy of initial medical therapy for specific subsets of SCAD was established in a meta-analysis of seven RCTs.108 It demonstrated a survival benefit from CABG in patients with LM or three-vessel SCAD, particularly when the proximal LAD coronary artery was involved. Benefits were greater in those with severe symptoms, early positive exercise tests, and impaired LV function. Notably, in these early studies only 10% of CABG patients received an internal mammary artery (IMA), which is an important prognostic component of CABG. Furthermore, 40% of patients in the medical group crossed over to CABG during follow-up. A more recent meta-analysis has reported a reduction in the risk of death with CABG vs. medical therapy (HR 0.62; 95% CI 0.50-0.77).107
The MASS II trial randomly compared medical therapy with PCI and CABG. At ten years, compared with medical therapy, CABG was associated with reduced rates of cardiac mortality, myocardial infarction and angina.94 In the Surgical Treatment IsChemic Heart failure (STICH) trial, 1212 patients with CAD and a left ventricular ejection fraction (LVEF) of ≤35% were randomized to medical therapy or CABG. Patients with LM disease were excluded, and 17% of patients on medical therapy underwent CABG and 6% of patients underwent PCI by the end of the follow-up period. In the intention-to-treat analysis, all-cause mortality was not significantly lower with CABG than with medical therapy (36% vs. 41%; HR 0.86; 95% CI 0.72-1.04; P=0.12); however, all-cause mortality or hospitalization for cardiovascular causes occurred less frequently among patients undergoing CABG (58% vs. 68%; HR 0.74; 95% CI 0.64-0.85; P<0.001). The results with respect to all other secondary clinical outcomes also favoured CABG. In addition, CABG was associated with a reduced risk for the primary outcome, death, in the ‘as treated’ analysis (HR 0.70; 95% CI 0.58-0.84; P<0.001).112
6.3 PERCUTANEOUS CORONARY INTERVENTION VS. CORONARY ARTERY BYPASS GRAFTING
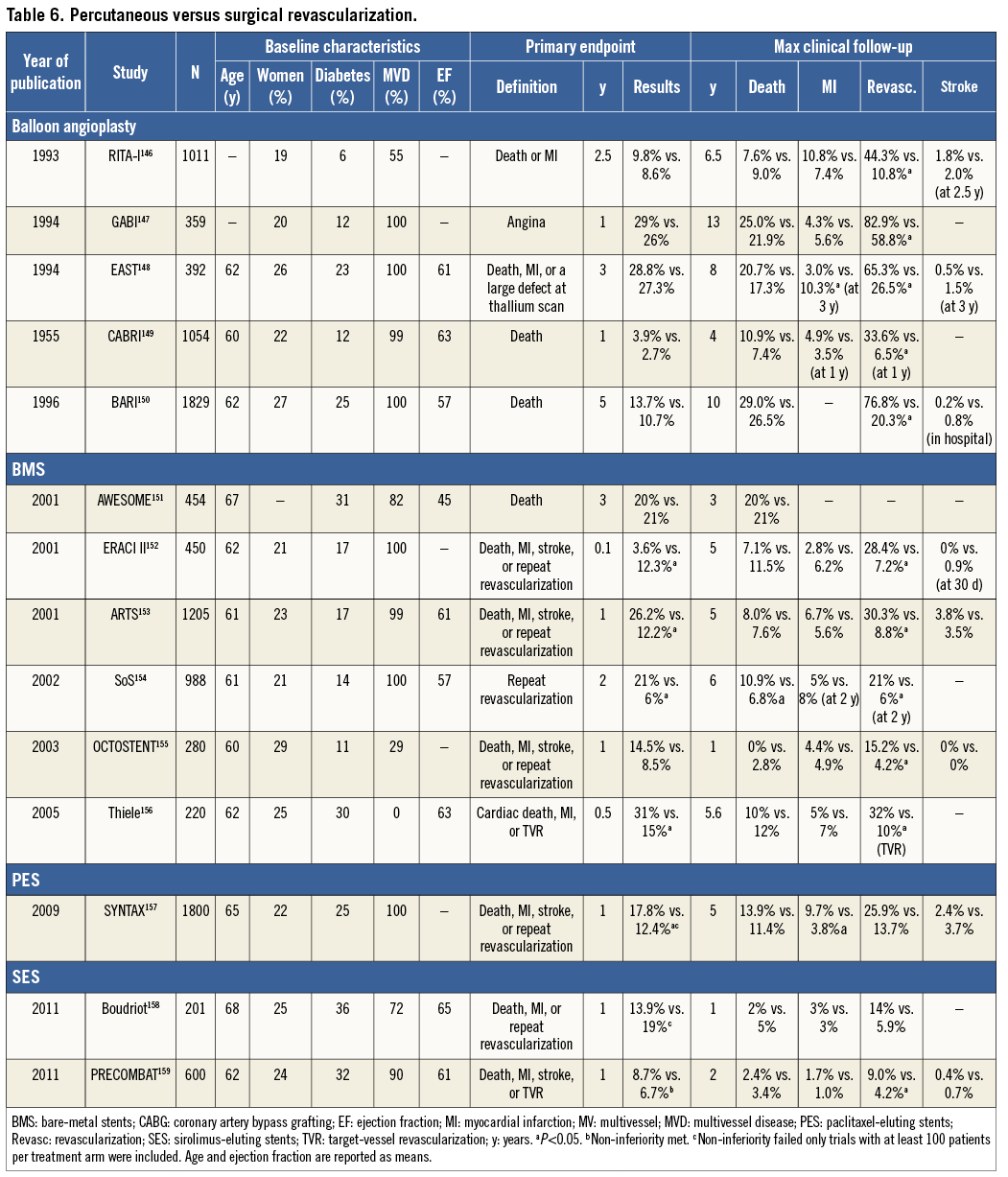
The multitude of studies comparing these two revascularization strategies has shown that neither PCI nor CABG alone can provide a solution for the entire spectrum of SCAD patients who need revascularization; however, CABG results in more complete revascularization than PCI, and the placement of bypass grafts on the mid-coronary vessel makes the complexity of proximal lesions less relevant for the procedure, especially when there are chronic proximal occlusions. The evidence derived from RCTs comparing CABG with PCI is summarized in Table 6.
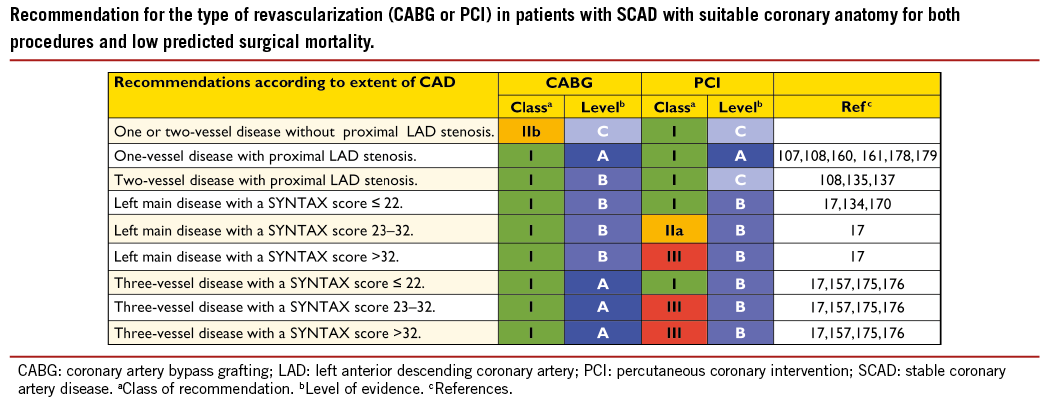
6.3.1 PROXIMAL LEFT ANTERIOR DESCENDING CORONARY ARTERY DISEASE
Two meta-analyses –one including nine RCTs involving 1210 patients with isolated proximal LAD lesions followed for up to 5 years,160 and the other including six RCTs and two non-randomized studies with a total of 1952 patients with isolated proximal LAD lesions, who were followed for up to 4 years161– reported no significant difference in mortality, myocardial infarction, or stroke, but a three-fold increase in recurrent angina and a five-fold increase in repeat revascularization with PCI compared with CABG. Most of the above-mentioned studies have used BMS in the PCI arm, while DES have markedly reduced the risk of repeat revascularization. Similarly, only few trials in patients with isolated proximal LAD lesions have reported long-term outcomes, although the angiographic patency of the IMA has been documented to be >90% at two decades of follow-up. Furthermore, the survival benefit of a single IMA in patients with multivessel CAD, initially reported after a decade of follow-up, has now been extended into the second and third decades, especially with bilateral IMAs.162-165
6.3.2 LEFT MAIN CORONARY ARTERY DISEASE
For several decades, CABG was regarded as the standard of care for significant LM disease in patients eligible for surgery, largely based on the Coronary Artery Surgery Study (CASS) registry.108 It has been suggested that two important pathophysiological features mitigate against the success of PCI in LM lesions (i) up to 80% of LM disease involves the bifurcation, which is known to be at higher risk of restenosis and (ii) up to 80% of LM patients also have multivessel SCAD, where CABG offers a survival advantage independent of the presence of LM disease.159,166,167 More recent evidence suggests, however, that PCI provides at least equivalent results to CABG for lower-severity LM lesions at up to five years of follow-up.
The SYNTAX trial included a pre-specified subgroup analysis of limited power in 705 patients with predominant distal LM disease, who were randomly assigned to CABG or PCI. The primary endpoint of one-year MACCE –the composite of death, myocardial infarction, stroke, and repeat revascularization– was comparable for both revascularization strategies (CABG 13.7% vs. PCI 15.8%; P=0.44).168 At five years’ follow-up, rates of death (CABG=14.6% vs. PCI=12.8%; P=0.53) and myocardial infarction (CABG=4.8% vs. PCI=8.2%; P=0.10) were not significantly different, whereas CABG was associated with a higher rate of stroke (4.3% vs. 1.5%; P=0.03) and a lower risk of repeat revascularization (15.5% vs. 26.7%; P<0.001) with no significant difference in the overall MACCE rates (31.0% vs. 36.9%; P=0.12).17,169 MACCE outcomes were comparable for PCI and CABG in the lower (0-22: 30.4% vs. 31.5%; P=0.74) and intermediate (23-32; 32.7% vs. 32.3%; P=0.88) SYNTAX score tertiles. In patients with SYNTAX scores >32, CABG was associated with numerically lower mortality (14.1% vs. 20.9%; P=0.11) and a significantly reduced need for repeat revascularization (11.6% vs. 34.1%; P<0.001) albeit at a numerically higher risk of stroke (4.9% vs. 1.6%; P=0.13).
The Premier of Randomized Comparison of Bypass Surgery vs. Angioplasty Using Sirolimus-Eluting Stent in Patients with Left Main Coronary Artery Disease (PRECOMBAT) trial randomized 600 patients with LM disease to PCI or CABG.159 The primary end-point –the 1-year composite rate of death, myocardial infarction, stroke, or repeat revascularization– was 6.7% in the CABG group and 8.7% in the PCI group (P=0.37). The 1-year composite rate of death, myocardial infarction or stroke was 4.0% for CABG and 3.3% for PCI (P=0.66). The lack of significant differences between the two groups was maintained over 2 years from randomization and was also valid for mortality (3.4% in the CABG group and 2.4% in the PCI group; P=0.45) and for the composite rate of death, myocardial infarction, or stroke (4.4% in the CABG group and 4.7% in the PCI group; P=0.83). In contrast to the findings in SYNTAX, the incidence of stroke was similar for PCI (0.4%) and CABG (0.7%).
A meta-analysis170 pooled the results of three dedicated RCTs on PCI vs. CABG for LM disease158,159,171 and one pre-specified LM lesion subset from the largest RCT.168 In total, this meta-analysis assessed the 1-year outcomes of 1611 patients. The composite of death, myocardial infarction, stroke, or TVR was observed in 11.8% of the CABG group and 14.5% of the PCI group (P=0.11); the composite of death, myocardial infarction, or stroke was 6.8% in the CABG group and 5.3% in the PCI group (P=0.26). Whilst there was no significant difference in mortality (4.1% in the CABG group and 3.0% in the PCI group; P=0.29) or myocardial infarction (2.8% in the CABG group and 2.9% in the PCI group; P=0.95), the CABG group showed a higher rate of stroke (1.7% vs. 0.1%; P=0.01) but a lower rate of TVR (5.4% vs. 11.4%; P<0.001).
The ASAN Medical Centre-Left Main Revascularization Registry compared the outcomes of patients with LM disease who were treated by either PCI or CABG within the same period. In two analyses –one of 10-year outcomes among 100 patients treated with BMS and 250 patients with CABG, and the other of 5-year outcomes among 176 patients with DES and 219 patients with CABG– neither mortality nor the composite of death, myocardial infarction, or stroke was significantly different between the two treatment approaches. CABG was associated with a decreased risk of revascularization in both comparisons.172 In a registry of 810 patients with LM disease treated by CABG (335 patients) or PCI (475 patients), which ran in parallel with the RCT, no significant difference was observed between the two treatment options in terms of the composite of death, myocardial infarction, or stroke over 2 years, whereas the risk of re-intervention was significantly lower with CABG.159
6.3.3 THREE-VESSEL CORONARY ARTERY DISEASE
A meta-analysis, based on individual patient data from RCTs that were performed before the introduction of DES, reported no difference in mortality between PCI and CABG, although mortality was reduced by CABG in diabetic patients and those aged 65 years or more.106 A meta-analysis of six randomized trials involving 6055 patients, which compared CABG with arterial grafts and PCI (balloon angioplasty, BMS and DES), reported a significant reduction in mortality (RR 0.73; 95% CI 0.62-0.86), myocardial infarction (RR 0.58; 95% CI 0.48-0.72) and repeat revascularization (RR 0.29; 95% CI 0.21-0.41) in favour of CABG.173 There was a trend toward excess strokes with CABG (RR 1.36; 95% CI 0.99-1.86; P=0.06). Several RCTs and meta-analyses indicate that CABG is associated with a greater risk of stroke than PCI, which diminishes during long-term follow-up.174,175
SYNTAX randomly assigned 1800 patients with LM and/or three-vessel CAD to either an early-generation paclitaxel-eluting stent or CABG.157 At 1 year, 12.4% of CABG and 17.8% of PCI patients (P=0.002) reached the primary composite endpoint of MACCE. At 5 years, CABG, as compared with PCI, significantly reduced overall MACCE with respective rates of 26.9% vs. 37.3% (P<0.001), 11.4% vs. 13.9% had died (P=0.10), 3.8% vs. 9.7% (P<0.0001) had a myocardial infarction, 3.7% vs. 2.4% (P=0.09) incurred a cerebrovascular accident, and 13.7% vs. 25.9% (P<0.0001) of the patients required repeat revascularization.17 In the 1095 patients with three-vessel CAD, in comparison with PCI, CABG resulted in lower total death (9.2% vs. 14.6%; P=0.006), cardiac death (5.3% vs. 9.0%; P=0.003), myocardial infarction (3.3% vs. 10.6%; P<0.001) and repeat revascularization (12.6% vs. 25.4%; P<0.001).176 In these patients with low SYNTAX score (0–22), rates of MACCE were similar (26.8% vs. 33.3%; P=0.21) for CABG and PCI, respectively. Conversely, when compared with PCI in patients with intermediate and high SYNTAX scores, CABG showed lower rates of MACCE (22.6% vs. 37.9%; P=0.0008 and 24.1% vs. 41.9%; P=0.0005, respectively), including its mortality, myocardial infarction and repeat revascularization components.176 Notably, patients who were included in the CABG registry of the SYNTAX trial because of ineligibility for PCI had lower MACCE rates than the randomized CABG cohort (23.3% vs. 26.9%, respectively), this being potentially related to more complete revascularization (76% vs. 63%, respectively).17
An observational study based on the New York State registry assessed patients with CAD who had been treated with either isolated bypass surgery (13 212 patients) or DES (20 161 patients) between 2003 and 2005, with focus on 5-year survival.177 The difference in absolute survival in the overall population was small (CABG 78.5% vs. PCI 76%). The main analysis was performed after propensity matching of 8121 pairs of patients, with survival at 5 years of 80.4% for CABG and 73.6% for PCI with DES (HR 0.71; 95% CI 0.67-0.77; P<0.001). A lower risk of death was noted in all subgroups, except for those with two-vessel CAD without proximal LAD lesions. Two main findings can be highlighted from this study: (i) the presence of LAD disease conferred a survival benefit to CABG and (ii) the survival benefit with CABG became evident only during the second half of the 5-year follow-up. In the ASCERT registry of elective patients >65 years of age with two- or three-vessel CAD, 86 244 patients underwent CABG and 103 549 patients underwent PCI (78% with early-generation DES). Using propensity scores and inverse probability adjustment, mortality at 4 years –but not at 1 year– was lower for CABG than for PCI (16.4% vs. 20.8%; RR 0.79; 95% CI 0.76-0.82).26 The observational nature of the studies does not permit assessment of how each patient was selected for each kind of treatment and, despite statistical adjustments, residual confounders cannot be excluded. Early-generation DES were used, which are devoid of the advantages of the newer generation.125-131,133 There is notable consistency in the findings on the survival advantage of CABG over PCI for more severe three-vessel CAD.
7. Revascularization in non-ST-segment elevation acute coronary syndromes
Non-ST-segment elevation acute coronary syndrome (NSTE-ACS) is the most frequent manifestation of ACS, and mortality and morbidity remain high and equivalent to those of patients with ST-segment elevation myocardial infarction (STEMI) during long-term follow-up. The key objectives of coronary angiography and subsequent revascularization are symptom relief and improvement of prognosis. Overall quality of life, length of hospital stay, and potential risks associated with invasive and pharmacological treatments must also be considered when deciding on a treatment strategy.
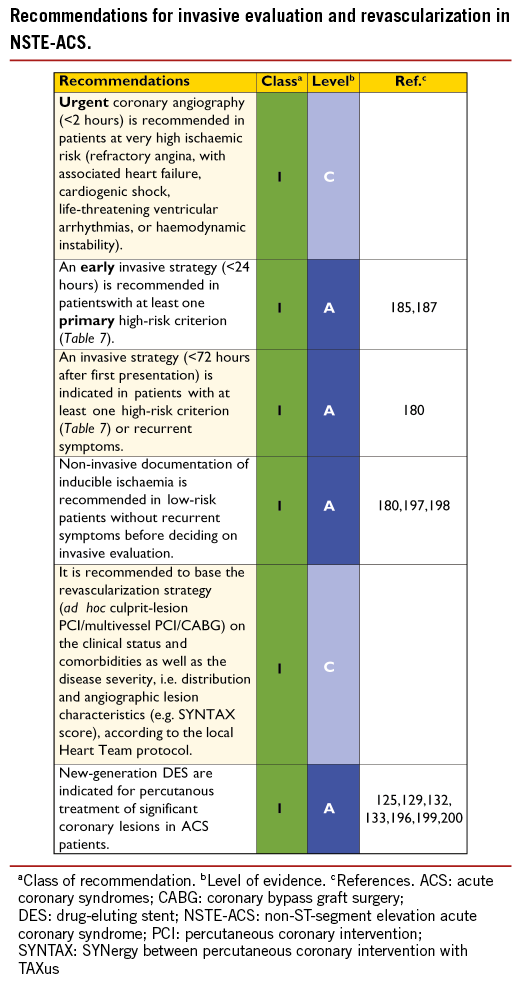
Early risk stratification is important, in order to identify patients at high immediate- and long-term risk for death and cardiovascular events, in whom an early invasive strategy with adjunctive medical therapy may reduce that risk. Patients in cardiogenic shock, or after resuscitation, should undergo immediate angiography (within 2 hours) because of the high likelihood of critical CAD, but it is equally important to identify patients at low risk, in whom invasive and medical treatments provide little benefit or may even cause harm. Details on risk stratification, particularly with respect to the interpretation of troponins, are found in the ESC Guidelines on NSTE-ACS.180
7.1 EARLY INVASIVE VS. CONSERVATIVE STRATEGY
A meta-analysis of seven RCTs that compared routine angiography followed by revascularization against a selective invasive strategy, showed reduced rates of combined death and myocardial infarction [odds ratio (OR) 0.82; 95% CI 0.72-0.93; P=0.001].181 The routine revascularization strategy was associated with a risk of early death and myocardial infarction during the initial hospitalization; however, four of the seven trials included in this meta-analysis were not contemporary, due to marginal use of stents and glycoprotein (GP) IIb/IIIa receptor inhibitors. Another meta-analysis, covering seven trials with more up-to-date adjunctive medication, showed a significant reduction in risk for all-cause mortality (RR=0.75; 95% CI 0.63-0.90; P<0.001) and myocardial infarction (RR=0.83; 95% CI 0.72-0.96; P=0.012), for an early invasive vs. conservative approach at 2 years without excess of death and myocardial infarction at 1 month.182 A further meta-analysis of eight RCTs showed a significant lower incidence of death, myocardial infarction, or rehospitalization for ACS (OR=0.78; 95% CI 0.61-0.98) for the invasive strategy at 1 year.183 The benefit was carried mainly by improved outcomes in biomarker-positive (high-risk) patients. In a gender-specific analysis, a similar benefit was found in biomarker-positive women, compared with biomarker-positive men. Importantly, biomarker negative women tended to have a higher event rate with an early invasive strategy, suggesting that early invasive procedures should be avoided in low-risk, troponin-negative, female patients. A more recent meta-analysis, based on individual patient data from three studies that compared a routine invasive- against a selective invasive strategy, revealed lower rates of death and myocardial infarction at 5-year follow-up (HR=0.81; 95% CI 0.71-0.93; P=0.002), with the most pronounced difference in high-risk patients.184 Age, diabetes, previous myocardial infarction, ST-segment depression, hypertension, body mass index (<25 kg/m2 or >35 kg/m2), and treatment strategy were found to be independent predictors of death and myocardial infarction during follow-up. All results supported a routine invasive strategy but highlight the importance of risk stratification in the decision-making process management.
7.2 TIMING OF ANGIOGRAPHY AND INTERVENTION
Patients at highest risk (i.e. those with refractory angina, severe heart failure or cardiogenic shock, life-threatening ventricular arrhythmias, or haemodynamic instability) were generally not included in RCTs, in order not to withhold potentially life-saving treatments. It has been generally accepted that such patients should be taken for an immediate (<2 hours) invasive evaluation, regardless of electrocardiogram (ECG) or biomarker findings.180
An early invasive strategy (0.5-14 hours of diagnosis), as opposed to a delayed invasive strategy (within 21-86 hours), was tested in several RCTs. In a meta-analysis of three recent trials, early catheterization, followed by coronary intervention on the first day of hospitalization, was shown to be safe and superior in terms of lower risk of recurrent ACS (–41%) and shorter hospital stay (–28%).185 Similar findings were reported in a more recent meta-analysis.186
There is growing evidence to suggest benefit from an invasive strategy within 24 hours in patients with a high-risk profile. The Timing of Intervention in Patients with Acute Coronary Syndromes (TIMACS) trial revealed a significant 38% lower incidence of death, myocardial infarction, or stroke at 6 months in high-risk patients (Global Registry of Acute Coronary Events (GRACE) score >140), with an early (≤24 hours), as compared with a delayed (≥36 hours) strategy.187 No significant difference was observed in patients with a low- to intermediate-risk profile (GRACE score ≤140). Notably, there was no safety issue relating to an early invasive strategy. In the Acute Catheterization and Urgent Intervention Triage strategY (ACUITY) data analysis, a delay of more than 24 hours before PCI was an independent predictor of 30-day and 1-year mortality.188 This increased ischaemic event rate was most evident among moderate- and high-risk patients [according to the Thrombolysis in Myocardial Infarction (TIMI) risk score].
In summary, the timing of angiography and revascularization should be based on patient risk profile. Patients at very high risk (as defined above) should be considered for urgent coronary angiography (in less than 2 hours). In patients at high risk, with at least one primary high-risk criterion, an early invasive strategy within 24 hours appears to be the reasonable timescale. In lower-risk subsets, with a GRACE risk score of <140 but with at least one secondary high-risk criterion (Table 7), the invasive evaluation can be delayed without increased risk but should be performed during the same hospital stay, preferably within 72 hours of admission. In other low-risk patients without recurrent symptoms, a non-invasive assessment of inducible ischaemia should be performed before hospital discharge.
7.3 TYPE OF REVASCULARIZATION
There are no specific RCTs comparing PCI with CABG in patients with NSTE-ACS. In all trials comparing an early invasive with a late strategy, or an invasive with a medical management strategy, the decision on whether to perform CABG or PCI was left to the investigator’s discretion.
In stabilized patients, the choice of revascularization modality can be made in analogy to patients with SCAD. In approximately one-third of patients, angiography will reveal single-vessel disease, allowing ad hoc PCI in most cases. Multivessel disease will be present in another 50%. Here the decision is more complex and the choice has to be made between culprit-lesion PCI, multivessel PCI, CABG, or a combined (hybrid) revascularization. The distribution of PCI vs. CABG in patients with multivessel disease suitable for revascularization is approximately 80% vs. 20%.189 The revascularization strategy in patients with multivessel CAD should be determined early by the Heart Team and based on the patient’s clinical status, as well as the severity and distribution of the CAD and the characteristics of the lesion. The SYNTAX score has proved to be strongly predictive of death, myocardial infarction and TVR.190
Culprit-lesion PCI is usually the first choice in most patients with NSTE-ACS and multivessel disease; however, there are no prospective studies comparing culprit-lesion PCI with early CABG. In stabilized patients with multivessel disease and a high SYNTAX score (>22), particularly when there is no clearly identified culprit lesion, a strategy of urgent CABG should be preferred. The strategy of multivessel PCI for suitable significant stenoses –rather than PCI limited to the culprit lesion– has not been evaluated in an appropriate, randomized fashion. In a large database including 105 866 multivessel CAD patients with NSTE-ACS, multivessel PCI was compared with single-vessel PCI and was associated with lower procedural success but similar in-hospital mortality and morbidity.191 Complete revascularization at the time of the index procedure did not result in lower mortality rates over 3 years, as compared with a staged procedure strategy.192 However, incomplete revascularization appears to be associated with more 1-year adverse event rates.193
CABG was compared with PCI in a propensity-matched analysis among patients with multivessel disease from the ACUITY trial.189 PCI- treated patients had lower rates of stroke, myocardial infarction, bleeding, and renal injury, similar 1-month and 1-year mortality, but significantly higher rates of unplanned revascularization at both 1 month and 1 year. However, only 43% of CABG patients could be matched and there was a strong trend for a higher rate of major adverse cardiac events (MACE) at 1 year with PCI, compared with CABG (25.0% vs. 19.5%, respectively; P=0.05). These results are consistent with the 1-year and 5-year outcomes of the multivessel SYNTAX trial, which included 28.5% of patients with a recent ACS, in both the PCI and the CABG arms.17,157 However, a subanalysis of these patients has not been reported.
Culprit-lesion PCI does not necessarily require a case-by-case review by the Heart Team when, on clinical or angiographic grounds, the procedure needs to be performed ad hoc after angiography. This is the case when there is continuing or recurrent ischaemia, haemodynamic instability, pulmonary oedema, recurrent ventricular arrhythmias, or total occlusion of the culprit coronary artery requiring urgent revascularization. For all other scenarios, revascularization should be discussed in a multidisciplinary setting, with protocols based on the SYNTAX score at each institution, defining specific anatomical criteria and clinical subsets that can be treated ad hoc or transferred to CABG. After culprit-lesion PCI, patients with scores in the two higher terciles of the SYNTAX score should be discussed by the Heart Team, in the context of functional evaluation of the remaining lesions. This also includes the assessment of patients’ comorbidities and individual characteristics.
7.3.1 CORONARY ARTERY BYPASS SURGERY
As there is no randomized study comparing an early- with a delayed CABG strategy, the general consensus is to wait 48-72 hours in patients who had culprit-lesion PCI and have residual severe CAD. In a large database analysis of unselected patients admitted for ACS, performance of early CABG, even in higher-risk patients, was associated with low in-hospital mortality.194 In registries, unadjusted and adjusted analyses showed no difference in outcomes between patients undergoing early (≤48 hours) or in-hospital late (>48 hours) surgery, although CABG was delayed more often in higher-risk patients, suggesting that timing might be appropriately determined by multidisciplinary clinical judgement.195 Therefore, in patients assigned for CABG, timing of the procedure should be decided on an individual basis, according to symptoms, haemodynamic stability, coronary anatomy, and signs of ischaemia. When there is continuing or recurrent ischaemia, ventricular arrhythmias, or haemodynamic instability, CABG should be performed immediately. Patients with LM or three-vessel CAD involving the proximal LAD should undergo surgery during the same hospital stay. In this decision process, it is important to consider the risk of bleeding complications when initially applying aggressive antiplatelet treatment; however, pre-treatment with a dual antiplatelet regimen should be considered only as a relative contraindication to early CABG and does require specific surgical measures to minimize bleeding.
7.3.2 PERCUTANEOUS CORONARY INTERVENTION
The safety and efficacy of DES have not been prospectively tested in a specific population of patients with NSTE-ACS, but this subset comprises up to 50% of patients included in most stent trials particularly those with an all-comer design. There is no particular safety concern in NSTE-ACS as new-generation DES have shown superior safety and efficacy in both SCAD and STEMI patients. Accordingly, new-generation DES are preferred over BMS as the default option.196 Dual Antiplatelet therapy (DAPT) should be maintained for 12 months, irrespective of stent type.
8. Revascularization in ST-segment elevation myocardial infarction
8.1 TIME DELAYS
Delays in the timely implementation of reperfusion therapy are key issues in the management of STEMI, since the greatest benefit gained from reperfusion therapy occurs within the first 2-3 hours of symptom onset.201,202 The total ischaemic time, between symptom onset and provision of reperfusion therapy (either starting fibrinolysis or mechanical reperfusion by primary PCI), is probably the most important factor. The aim is to provide optimal care while minimizing delays, in order to improve clinical outcomes (Figure 2).201 The reduction of first-medical-contact-to-balloon (FMCTB) time –defined as the time from the (first) medical/hospital door to the time of primary PCI– relies on efficient coordination of care between first medical contact or referral hospitals, the emergency medical service (EMS), and the receiving hospitals. It is currently estimated that about 66% of patients achieve a guideline-recommended overall first-hospital-door-to-balloon time of <120 minutes.203 The door-to-balloon (DTB) time refers to patients presenting in PCI-capable centres and should be less than 60 minutes. Door-in to door-out (DI-DO) time is a performance measure that assesses the timeliness and quality of initial reperfusion care. It is defined as the duration from arrival to discharge at the first or STEMI-referral hospital. A DI-DO time ≤30 minutes is associated with shorter reperfusion delays (i.e. a first-hospital DTB time <120 minutes) and lower in-hospital mortality, and should be implemented in non-PCI-capable hospitals as a quality metric.204,205
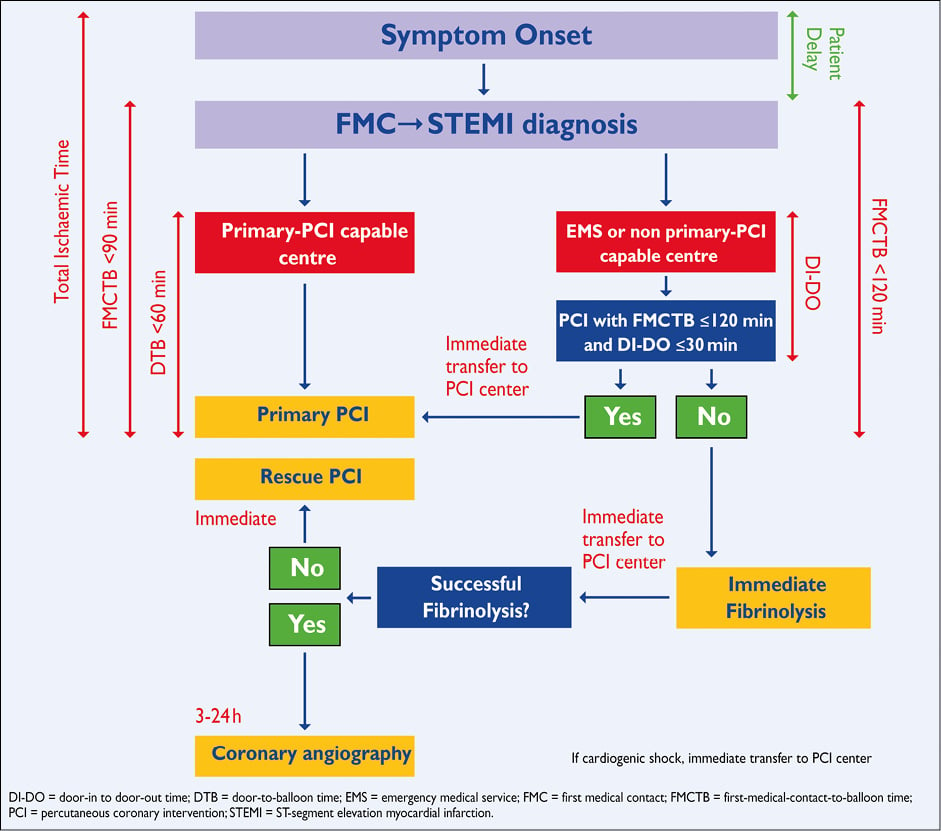
Figure 2. Organization of STEMI patient disposal describing pre- and in-hospital management and reperfusion strategies within 12 hours of first medical contact with ideal time interval for interventions.
8.2 SELECTION OF REPERFUSION STRATEGY
Primary PCI is defined as percutaneous catheter intervention in the setting of STEMI, without previous fibrinolysis. It has replaced fibrinolysis as the preferred reperfusion strategy in patients with STEMI, provided it can be performed in a timely manner in high- volume PCI centres with experienced operators and 24-hour, 7-day catheterization laboratory activation.201,206 -209 In settings where primary PCI cannot be performed in a timely fashion, fibrinolysis should be considered, particularly if it can be administered pre-hospital (e.g. in the ambulance)210-212 and within the first 120 minutes after symptom onset (Figure 2).213-215 It should be followed by transfer to PCI-capable centres for routine coronary angiography in all patients and for rescue PCI in case of unsuccessful fibrinolysis.
During the past decade, primary PCI has become established as the dominant reperfusion therapy in Europe, irrespective of whether patients present early or the journey to the primary PCI- capable hospital is long.202,203,216,217 Four European Union countries have documented full implementation of primary PCI as the preferred reperfusion strategy, including countries in which travelling can be difficult.218 In most other European countries, fibrinolysis for STEMI is becoming a rare therapy; for example 6% of cases in the UK, 7% in Poland, and 8% in France.218 It is interesting to note that, even in countries with a large catchment area, such as Denmark –with one primary PCI centre per 1.4 million inhabitants and correspondingly long transportion distances– the STEMI case-fatality rate is among the lowest recorded in Europe, with an in-hospital mortality of only 3%. The initial diagnosis of STEMI is operational and based on ECG findings with a predictive value of 85%.205 False activation of the catheterization laboratory may therefore occur in 15-30% of cases,216 in which PCI can be deferred but where fibrinolysis is a hazard. In either case, there are costs and some inherent risks associated with the procedure or treatment.
8.3 PRIMARY PERCUTANEOUS CORONARY INTERVENTION
Key points for optimizing and guiding primary PCI are summarized below:
─ The infarct-related artery should be systematically treated during the initial intervention. Evidence supporting immediate (preventive) intervention in non-infarct-related lesions is a matter of debate.233 On the one hand, patients with extensive CAD in vessels remote from the infarct-related artery have reduced success in reperfusion and an adverse prognosis following primary PCI.188 Staged PCI in patients with multivessel disease and no haemodynamic compromise is an independent predictor of survival, and more frequent ischaemic events have been reported in direct vs. staged revascularization of STEMI patients with multivessel disease.234-236 In the recent, randomized Preventive Angioplasty in Acute Myocardial Infarction (PRAMI) trial (n=465), preventive PCI in non-infarct-related coronary arteries with stenosis ≥50%, when compared with PCI limited to the infarct artery, was associated with a reduced risk of the composite of death, myocardial infarction, or refractory angina (HR in the preventive-PCI group 0.35; 95% CI 0.21-0.58; P<0.001). The HR for non-fatal myocardial infarction was 0.32 (95% CI 0.13-0.75). It remains to be determined how clinicians can identify lesions that should be revascularized beyond the culprit lesion and whether complete revascularization should be performed in single- or multi-stage procedures. At present, multivessel PCI during STEMI should be considered in patients with cardiogenic shock in the presence of multiple, critical stenoses or highly unstable lesions (angiographic signs of possible thrombus or lesion disruption), and if there is persistent ischaemia after PCI on the supposed culprit lesion.
─ The radial approach should be the preferred method of access, as it has been shown to reduce the incidence of acute bleeding events –especially in ACS– and was associated with lower mortality in the subset of STEMI patients that were enrolled in the RadIal Vs. femorAL access for coronary intervention (RIVAL) trial.237-239
─ However, the benefit of radial over femoral access depends upon the operators’ expertise in the radial technique.240
─ Stenting should be preferred over balloon angioplasty in the setting of primary PCI,241,242 as it reduces the risk of abrupt closure, re-infarction, and repeat revascularization. Although early-generation DES have not been associated with an increased risk of death, myocardial infarction, or stent thrombosis during long-term follow-up,243 there have been concerns over an increased risk of very late stent thrombosis, owing to delayed arterial healing of stents implanted into lesions with a large necrotic core.244,245
─ More recent evidence has, however, demonstrated the superiority of new-generation everolimus-eluting stents in reducing major acute vascular events in STEMI patients, as compared with early-generation sirolimus-eluting stents.246 Two dedicated trials directly compared new-generation DES with BMS among STEMI patients undergoing primary PCI. The everolimus-eluting stent vs. BMS in ST-segment elevation myocardial infarction (EXAMINATION) trial in 1504 STEMI patients reported no significant differences for the primary endpoint of all-cause death, re-infarction and any revascularization, in patients assigned to everolimus-eluting stents, compared with those assigned to BMS, (11.9% vs. 14.2%, respectively, difference –2.3%; 95% CI –5.8-1.1%; P=0.19) at 1 year.247 However, everolimus-eluting stents were associated with a lower risk of revascularization of the target lesion (2.1% vs. 5.0%; P=0.003) and definite stent thrombosis (0.5% vs. 1.9%; P=0.02). The Comparison of Biolimus Eluted From an Erodible Stent Coating With Bare-Metal Stents in Acute ST-Elevation Myocardial Infarction (COMFORTABLE AMI) trial, examining patients assigned to either BMS or to biolimus-eluting stents with a biodegradable polymer, reported that the latter showed a lower risk of the composite primary endpoint of cardiac death, target-vessel myocardial infarction, and target-lesion revascularization (4.3% vs. 8.7%; HR 0.49; 95% CI 0.30-0.80; P=0.004) as well as a lower risk of target-vessel myocardial infarction (0.5% vs. 2.7%; HR 0.20; 95% CI 0.06-0.69; P=0.01) and a trend towards a lower risk of definite stent thrombosis (0.9% vs. 2.1%; HR 0.42; 95% CI 0.15-1.19; P=0.10).248
─ Results were maintained throughout 2 years of follow-up and a pooled analysis of both trials confirmed a lower risk of stent thrombosis and re-infarction with DES than with BMS.249 Overall, these findings suggest that new-generation DES are more effective and potentially safer than BMS during primary PCI in STEMI.
─ Thrombus aspiration has been proposed as an adjunct during primary PCI, to further improve epicardial and myocardial reperfusion by prevention of distal embolization of thrombotic material and plaque debris. Individual RCTs and meta-analyses have suggested that the use of manual aspiration thrombectomy during primary PCI may be beneficial to improve epicardial and myocardial reperfusion and reduce the rate of MACE including mortality.250-255 In the largest randomized trial to date, the Thrombus Aspiration during PCI in Acute Myocardial Infarction (TASTE) study (7244 patients), the primary endpoint of all-cause mortality occurred in 2.8% of patients in the thrombus aspiration group and in 3.0% in the PCI-only group (HR 0.94; 95% CI 0.72-1.22; P=0.63) at 30 days.256 However, events were evaluated at short-term follow-up, and there was a trend towards a reduction of non-adjudicated events including stent thrombosis (0.2% vs. 0.5%, respectively; HR 0.47; 95% CI 0.20-1.02; P=0.06) and re-infarction (0.5% vs. 0.9%, respectively; HR 0.61; 95% CI 0.34-1.07; P=0.06) in favour of thrombus aspiration. Taken together, these results suggest that routine use of thrombus aspiration is not necessary but selected use may be useful to improve Thrombolysis in Myocardial Infarction (TIMI) 3 flow or prevent stent thrombosis. No clinical benefit of routine rheolytic thrombectomy has been demonstrated in primary PCI.255,257-259
─ Pre- and post-conditioning warrant randomized trials before these procedures can be recommended in routine clinical practice. Remote ischaemic pre-conditioning has engendered little enthusiasm.260 Early administration of metoprolol before PCI in STEMI patients presenting with Killip Class II or less has been shown to reduce infarct size, with a trend toward fewer ischaemic events.261 Trials evaluating the use of antithrombotic and vasodilator agents have been disappointing.
─ Incomplete stent deployment and undersizing should be avoided.262 Massive thrombotic burden and low-pressure delivery, to avoid distal embolization, are the two major contributing factors in stent malapposition in STEMI patients. Self-expanding stents and stents covered with ultra-thin micronets have shown favourable preliminary results in terms of surrogate endpoints.263 However, large-scale clinical outcome studies are required before these devices can be recommended.
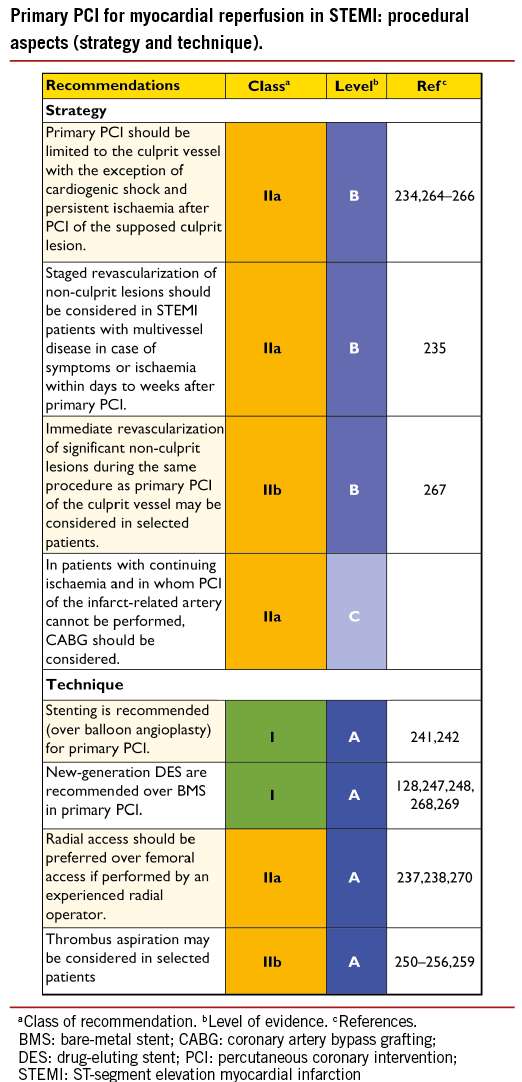
8.4 FIBRINOLYSIS
Despite its frequent contraindications, limited effectiveness in inducing reperfusion, and greater associated risk of bleeding, fibrinolytic therapy –preferably administered as a pre-hospital treatment– remains an adjunct to mechanical revascularization if the latter cannot be performed in time.207,208
The incremental benefit of primary PCI over timely fibrinolysis is diminished when PCI-related delay exceeds 120 minutes, depending on patient age, duration of symptoms, and infarct location. Fibrinolysis is discussed in detail in the ESC Guidelines on STEMI.201
Pre-hospital fibrinolysis has been compared with primary PCI in early-presenting patients in the STrategic Reperfusion Early After Myocardial infarction (STREAM) study. In patients with early STEMI (onset <3 hours previously) who could not undergo primary PCI within 60 minutes after first medical contact, prehospital fibrinolysis (amended to half dose in patients >75 years of age) with timely coronary angiography (6-24 hours in stable patients) and rescue PCI for failed fibrinolysis was as effective as primary PCI in reducing the primary endpoint, a composite of death, shock, congestive heart failure, or re-infarction up to 30 days (12.4% vs. 14.3%, respectively; RR 0.86; 95% CI 0.68-1.09; P=0.21). However, there was a significant increase in intracranial bleeding (1.0% vs. 0.2%; P=0.04) particularly in patients >75 years of age with fibrinolysis. The median times until reperfusion were 100 minutes in the fibrinolysis group and 178 minutes in the primary PCI group, which are an hour shorter on average than the delays in the DANish trial in Acute Myocardial Infarction (DANAMI) trial, which established the superiority of transfer PCI over in-hospital fibrinolysis.219 In view of the lack of superior efficacy and increased rate of intracranial haemorrhage, emphasis should remain on timely PCI within STEMI networks as the preferred treatment for STEMI. Facilitated PCI, defined as routine use of reduced or normal dose fibrinolysis combined with GP IIb/IIIa inhibitors or other antiplatelet agents followed by coronary angiography, has shown no significant advantages over primary PCI alone.271
8.5 SECONDARY PERCUTANEOUS CORONARY INTERVENTION
Several randomized trials and meta-analyses have shown that early, routine, post-thrombolysis angiography with subsequent PCI (if required) reduced the rates of re-infarction and recurrent ischaemia, compared with a strategy of ‘watchful waiting’, in which angiography and revascularization were indicated only in patients with spontaneous or induced severe ischaemia or LV dysfunction.272-281 The benefits of early, routine PCI after thrombolysis were seen in the absence of an increased risk of adverse events (stroke or major bleeding).
Based on data from the four most recent trials, all of which had a median delay between start of thrombolysis and angiography of 2-6 hours, a time-frame of 3-24 hours after successful lysis is recommended.215,272-274 In cases of failed fibrinolysis, or if there is evidence of re-occlusion or re-infarction with recurrence of ST-segment elevation, the patient should undergo immediate coronary angiography and rescue PCI.282
Patients presenting between 12 and 48 hours after onset of symptoms, even if pain-free and with stable haemodynamics, may still benefit from early coronary angiography and possibly PCI.223,224 In patients presenting days after the acute event with a completed myocardial infarction, only those with recurrent angina or documented residual ischaemia –and proven viability on non-invasive imaging in a large myocardial territory– may be considered for revascularization when the infarct artery is occluded. Systematic late PCI of an occluded infarct-related artery after myocardial infarction in stable patients has no incremental benefit over medical therapy.115
8.6 CORONARY ARTERY BYPASS SURGERY
CABG may be indicated in STEMI patients with unsuitable anatomy for PCI, but who have a patent infarct-related artery, since patency of this artery provides time for transfer to the surgical team and a large myocardial area in jeopardy. It should be considered in patients in cardiogenic shock if the coronary anatomy is not amenable to PCI,221 or at the time of repair for patients with mechanical complications.285
CABG is infrequently used and its benefits are uncertain in STEMI patients with failed PCI, coronary occlusion not amenable to PCI, and in the presence of refractory symptoms after PCI since, in most of these cases, time for implementation of surgical reperfusion will be long and the risks associated with surgery are increased in this setting.286
When possible, in the absence of persistent pain or haemodynamic deterioration, a waiting period of 3-7 days appears the best compromise.286 Patients with multivessel disease, who are receiving primary PCI or secondary (post-fibrinolysis) PCI on the culprit artery, will need risk stratification and further, staged revascularization with PCI or surgery following a Heart Team discussion.
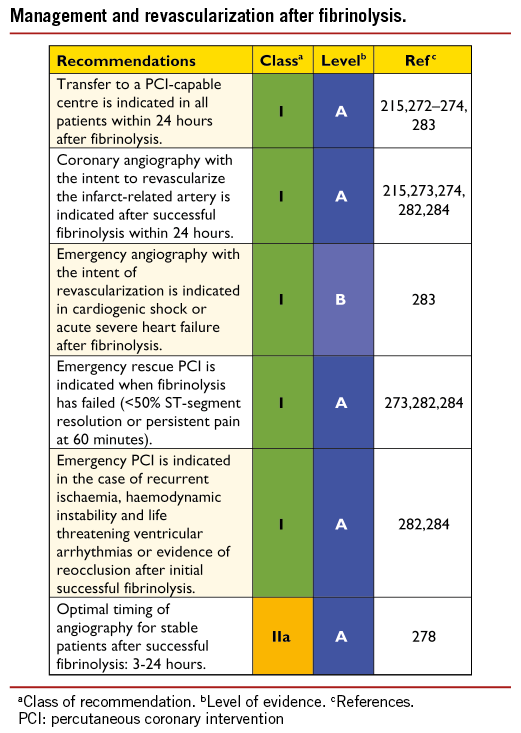
9. Revascularization in patients with heart failure and cardiogenic shock
9.1 CHRONIC HEART FAILURE
Coronary artery disease remains the most common cause of chronic heart failure; patients with depressed LV function remain at risk of sudden cardiac death with or without revascularization, and the indication for prophylactic implantable cardioverter defibrillator (ICD) therapy should always be examined.287
9.1.1 REVASCULARIZATION
Revascularization with CABG or PCI is indicated for symptomatic relief of angina pectoris in patients with heart failure. The prognostic importance of surgical revascularization in patients with chronic heart failure has recently been studied in the STICH trial,112 with the aim of comparing the efficacy of initial medical therapy with that of revascularization by CABG plus medical therapy in a sample of 1212 patients with CAD and LV dysfunction (EF ≤35%). Patients with significant LM disease or CCS Classes III and IV were excluded. Most patients had two-vessel (31%) or three-vessel (60%) CAD, and 68% had a proximal LAD stenosis. Although the primary outcome of all-cause mortality was not significantly reduced by CABG (HR with CABG 0.86; 95% CI 0.72-1.04; P=0.12) in the intention-to-treat analysis, it offered superior pre-specified secondary outcomes, including cardiovascular mortality (HR 0.81; 95% CI 0.66-1.00; P=0.05) and all-cause mortality or hospitalization for heart failure (HR 0.84; 95% CI 0.71-0.98; P=0.03). Among patients allocated to medical therapy, 17% crossed over to CABG and 6% to PCI. The ‘as-treated’ analysis compared the outcomes of 592 patients treated with medical therapy throughout the first year after randomization with those of 620 patients who underwent CABG –either as a consequence of randomization or crossover– and reported significantly lower all-cause mortality in favour of CABG (HR 0.70; 95% CI 0.58-0.84; P<0.001).112 These findings have been confirmed in a recent propensity-matched observational cohort of similar patients during long-term follow-up over 10 years.288 The choice between CABG and PCI should be made by the Heart Team after careful evaluation of the patient’s clinical status and coronary anatomy, including SYNTAX score, comorbidities, and expected completeness of revascularization. A specialist in heart failure should be consulted.
9.1.2 MYOCARDIAL VIABILITY AND REVASCULARIZATION
The risk-benefit balance for revascularization in patients without angina/ischaemia or viable myocardium remains uncertain. In an observational study using cardiac imaging techniques (stress-rest Rb-82/F-18 fluorodeoxyglucose PET) in 648 patients with an LVEF of 31%±12%, hibernating myocardium, ischaemic myocardium, and scarred myocardium were associated with all-cause death (P=0.0015; P=0.0038, and P=0.0010, respectively). An interaction between treatment and hibernating myocardium was present, such that early revascularization in the setting of hibernating myocardium, when compared with medical therapy, was associated with improved survival, especially when the extent of viability exceeded 10% of the myocardium.289,290 The viability sub-study of the STICH trial found viable myocardium in 487 of 601 patients (81%) and no viable myocardium in 114 (19%).289 Among patients without viability, 60 were allocated to CABG and 54 to medical therapy and, among the 487 patients with myocardial viability, 244 were assigned to CABG and 243 to medical therapy. The differences in baseline characteristics, between patients who underwent myocardial viability testing and those who did not, indicate some selection bias driven by clinical factors. Viability was arbitrarily defined using different cut-off values for the different tests used. By univariate analysis, there was a significant association between myocardial viability and outcome; however, this association was not significant on multivariable analysis that included other prognostic variables. It is likely that other variables, such as LV volumes and ejection fraction, are causally determined by the extent of viable myocardium. The lack of correlation between myocardial viability status and benefit from CABG in this study indicates that assessment of myocardial viability should not be the sole factor in selecting the best therapy for these patients.
9.1.3 VENTRICULAR RECONSTRUCTION
The aim of surgical ventricular reconstruction (SVR) is to remove scar tissue from the LV wall by an endoventricular patch plasty, thereby restoring physiological volume, and to restore an elliptical rather than spherical shape. The decision to add SVR to CABG should be based on a careful evaluation of symptoms (heart failure symptoms should take priority over angina), measurement of LV volumes, and assessment of the transmural extent of myocardial scar tissue, and should be performed only in centres with a high level of surgical expertise. The STICH trial failed to show a difference in the primary outcome (death from any cause or hospitalization for cardiac causes) between CABG and the combined procedure (CABG and SVR). The reduction in end-systolic volume index in STICH –smaller than in previously reported observational studies treating larger aneurysms– might explain the inconsistent finding and, thus, the value of reasonable SVR might be underestimated.291,292
Subgroup analyses of the STICH trial suggest that patients with less-dilated LV and better LVEF may benefit from SVR, while those with larger LV and poorer LVEF may do worse.293 In the STICH trial, a post-operative left ventricular end-systolic volume index (LVESVI) of 70 mL/m2 or lower, after CABG plus SVR, resulted in improved survival compared with CABG alone. In another study, in patients treated with CABG and SVR, a post-operative LVESVI of less than 60 ml/m2 was associated with improved survival compared with a post-operative LVESVI of 60 ml/m2 or more.294 In some patients with large aneurysms, who would have been excluded from STICH (due to acute heart failure, inotropic support or violation of other inclusion criteria), surgical ventricular restoration has shown favourable outcomes although in the absence of a comparator.295
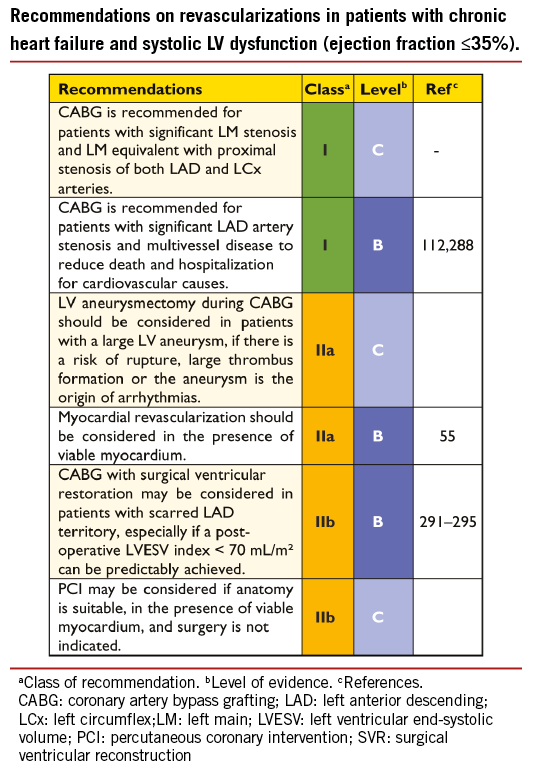
9.2 CARDIOGENIC SHOCK
Acute myocardial infarction accounts for approximately 75% of all patients with cardiogenic shock, and the incidence has remained somewhat constant for many years at 6-8%.296-298 Cardiogenic shock complicating acute myocardial infarction is caused by LV failure in about 80% of cases. Mechanical complications, such as papillary muscle rupture with severe mitral valve incompetence (6.9%), ventricular septal defect (3.9%), or free wall rupture (1.4%), are other precipitating causes. Because revascularization is the corner-stone of the treatment in patients with cardiogenic shock complicating ACS, emergency coronary angiography is indicated. The general triage and treatment of these complex patients is presented in Figure 3.
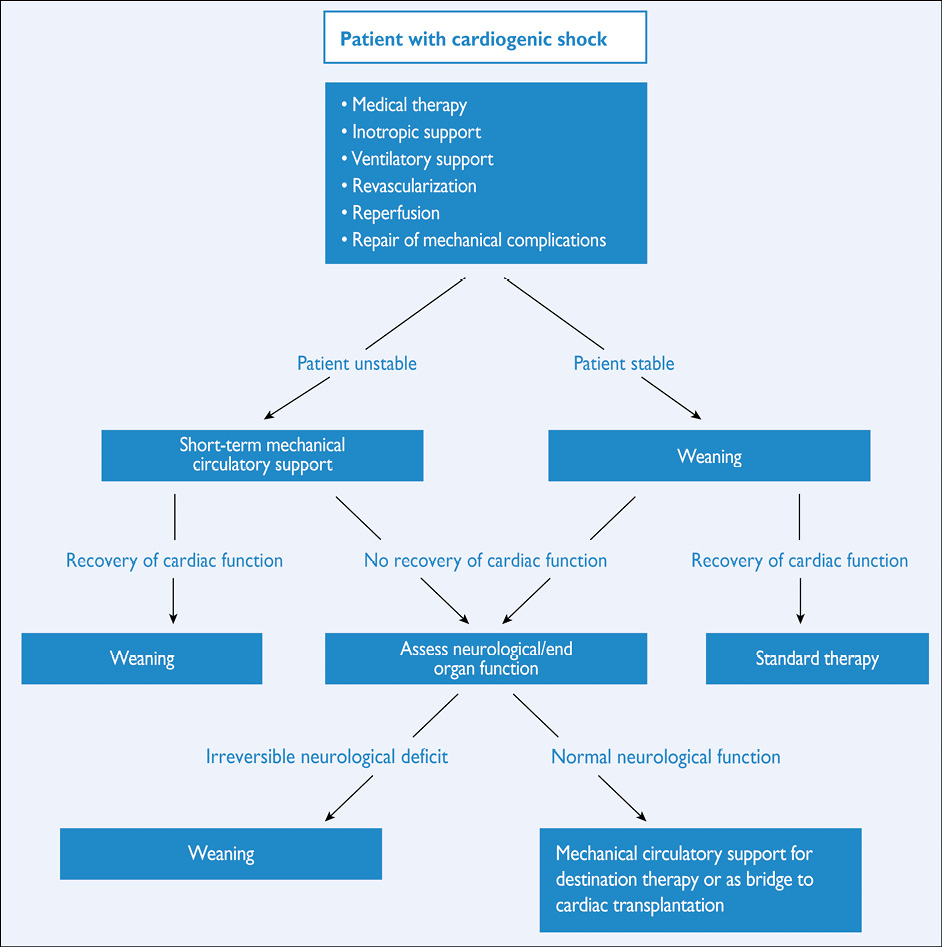
Figure 3. Treatment of patients with cardiogenic shock.
9.2.1 REVASCULARIZATION
The Should We Emergently Revascularize Occluded Coronaries for Cardiogenic Shock (SHOCK) trial demonstrated that, in patients with cardiogenic shock due to acute myocardial infarction, emergency revascularization with PCI or CABG improved long-term survival when compared with initial intensive medical therapy. Allcause mortality at 6 months was lower in the group assigned to revascularization than in the group assigned to medical therapy (50.3% vs. 63.1%, respectively; RR 0.80; 95% CI 0.65-0.98; P=0.03).221 Subgroup analysis revealed that the only variable that correlated significantly with treatment both at 30 days and at 6 months was age, with little or no effect of invasive treatment on mortality in elderly patients (>75 years); however, these findings were not corroborated in the SHOCK trial registry, in which a covariate-adjusted model also suggested a lower mortality among elderly patients (>75 years) undergoing revascularization, as compared with initial intensive medical therapy (RR 0.46; 95% CI 0.28-0.75; P=0.002).299
9.2.2 MECHANICAL CIRCULATORY SUPPORT
Intra-aortic balloon pump (IABP) counterpulsation has been widely used as mechanical support in cardiogenic shock.300 The efficacy of IABP in cardiogenic shock has recently been challenged in the large, randomized Intraaortic Balloon Pump in Cardiogenic Shock IABP-SHOCK II trial, which included 600 patients with cardiogenic shock complicating acute myocardial infarction, who were assigned to IABP or no IABP. The primary endpoint of 30-day mortality was not reduced with the use of IABP (39.7% IABP vs. 41.3% control; RR 0.96; 95% CI 0.79-1.17; P=0.69) and there was no long-term benefit.301,302 As a result, the use of IABP for this indication is not routinely recommended but remains an adjunct for patients with mechanical complications as a bridge to surgery.
Three randomized trials and a large registry have demonstrated superior haemodynamic support with percutaneous mechanical circulatory assist systems than with IABP, without differences in mortality but with an increased risk of adverse events.303-306 A meta-analysis, comparing the safety and efficacy of percutaneous left ventricular assist devices (LVAD) in IABP in patients with cardiogenic shock, found LVAD-treated patients to have a similar mortality and incidence of lower extremity ischaemia, but more bleeding than those treated with IABP.307
In younger patients with no contraindication for cardiac transplantation, LVAD therapy can be implemented as a bridge to transplantation. In patients not eligible for transplant, LVADs may be inserted as a bridge to recovery or with the goal of destination therapy.308-310
9.2.3 RIGHT VENTRICULAR FAILURE
Almost 50% of patients with inferior acute myocardial infarction show echocardiographic evidence of right ventricular dysfunction, with haemodynamic compromise developing in <25% of cases.311-315 Isolated right ventricular failure accounts for 2.8% of cases of cardiogenic shock complicating acute myocardial infarction.316,317 Successful primary PCI leads to a haemodynamic improvement, recovery of right ventricular free wall and global function and, hence, improved survival compared with unsuccessful reperfusion.317-319
9.2.4 MECHANICAL COMPLICATIONS
Mechanical complications of acute myocardial infarction comprise myocardial rupture, resulting in either mitral regurgitation due to papillary muscle rupture, ventricular septal defect (VSD), or free wall rupture with tamponade.320-322
Ventricular septal defect, characterized by haemodynamic compromise, is treated by IABP followed by early surgical repair.323 Percutaneous closure devices for patients’ post-infarct VSDs have been reported in case series and, in centres with appropriate experience, may be considered in selected cases as alternatives to surgery.324-326
Rupture of the free wall, resulting in tamponade, should be salvaged by prompt pericardial drainage and surgical intervention. Left ventricular free wall rupture accounts for approximately 15% of in-hospital mortality from myocardial infarction.327 Data from the SHOCK trial registry, on patients with and without LV free wall rupture who underwent surgery, showed similar mortality rates.327,328
Acute mitral regurgitation due to rupture of the papillary muscle should be treated by immediate surgery and revascularization.317,329,330
10. Revascularization in patients with diabetes
10.1 EVIDENCE FOR MYOCARDIAL REVASCULARIZATION
Data from randomized trials on revascularization in diabetic patients are summarized in Table 8. For additional information on diabetes, we refer to the ESC Guidelines on diabetes.84 Diabetic patients undergoing revascularisation, either with CABG or PCI, are at greater risk for kidney injury than patients without diabetes.
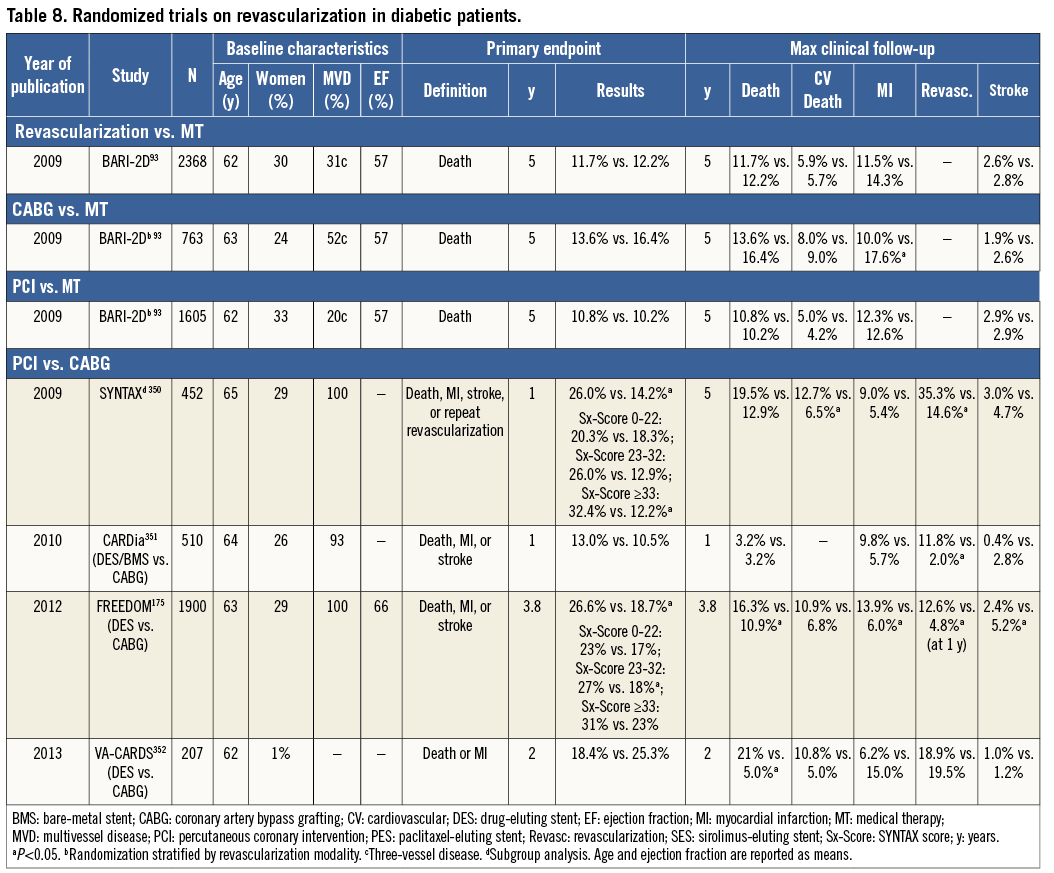
10.1.1 STABLE CORONARY ARTERY DISEASE
The Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI-2D) trial specifically addressed the question of myocardial revascularization in diabetic patients with SCAD.334 A total of 2368 patients with diabetes and evidence of ischaemia, or symptoms of angina in the presence of angiographically defined SCAD, were randomized to medical therapy or to myocardial revascularization in addition to medical therapy. Before randomization, patients were placed in either the PCI or CABG stratum of revascularization as deemed appropriate by the responsible physician. The enrolment target of 2800 patients was not met and follow-up had to be extended by 1.5 years to 5.3 years. Patients with LM disease, those who were unstable, requiring immediate revascularization, and patients with creatinine values >2.0 mg/dl or moderate-to-severe heart failure were excluded. The primary endpoint was all-cause mortality and the principal secondary endpoint was a composite of death, myocardial infarction, or stroke (MACCE). The use of DES (35%) was low and restricted to early-generation devices. A total of 42% of patients in the medical therapy group underwent clinically indicated revascularization during follow-up.
At 5 years, survival did not differ between the medical therapy and revascularization groups, and there were no differences in MACCE (Table 8). In the PCI group, there was no outcome difference between PCI and medical therapy. In the CABG stratum, where patients had more extensive CAD, freedom from MACCE was significantly higher with revascularization than with medical treatment.334 Survival, however, was not significantly different, which may reflect a power issue or the fact that patients with more extensive myocardial perfusion abnormalities or LV function impairment were more likely to receive revascularization over time in the medical therapy group.335 Compared with medical therapy, the revascularization strategy at the 3-year follow-up had a lower rate of worsening angina (8% vs. 13%, respectively; P<0.001), new angina (37% vs. 51%, respectively; P<0.001), and subsequent coronary revascularizations (18% vs. 33%, respectively; P<0.001), and a higher rate of angina-free status (66% vs. 58%, respectively; P<0.003).
The investigators speculated that the benefit of CABG over medical therapy emerged due to a preference for CABG rather than PCI among patients with more advanced CAD. This was further substantiated in a study of the impact of angiographic (BARI-2D score) risk stratification on outcomes. Among the CABG stratum patients with high-risk angiographic scores, the 5-year risk of death, myocardial infarction or stroke was significantly lower and amplified for those assigned to revascularization, when compared with medical therapy (24.8% vs. 36.8%, respectively; P=0.005).336
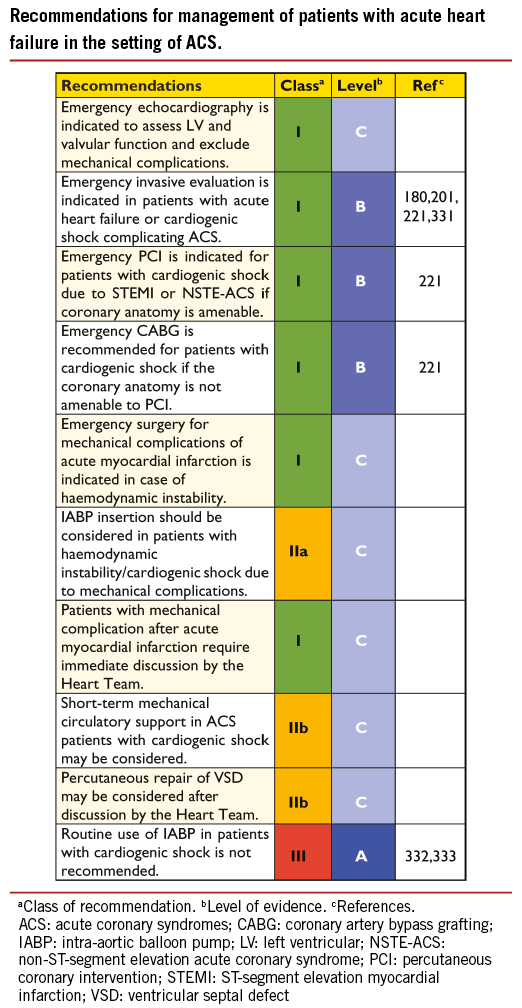
10.1.2 ACUTE CORONARY SYNDROMES
Approximately 20-30% of patients with NSTE-ACS have known diabetes, and at least as many have undiagnosed diabetes or impaired glucose tolerance.337 Mortality in patients with ACS is two- to three-time increased in diabetic patients, compared with non-diabetic.338 Despite the higher risk, revascularization and thienopyridines are less frequently prescribed among diabetics than non-diabetics, with an impact on in-hospital and long-term mortality.339-341
In NSTE-ACS patients, there is no clear correlation between the treatment effect of myocardial revascularization and diabetic status.342,343,364 In both the Fragmin during Instability in Coronary Artery Disease-2 (FRISC-2) and Treat angina with Aggrastat and determine Cost of Therapy with an Invasive or Conservative Strategy-Thrombolysis in Myocardial Infarction 18 (TACTICS-TIMI 18) trials,342,343,364 an early invasive strategy in ACS patients was associated with better outcomes than with a conservative strategy; in TACTICS-TIMI 18,364 the magnitude of the benefit to diabetic patients was greater than that to non-diabetic patients. In a recent meta-analysis of nine RCTs with 9904 ACS patients, diabetic patients (n=1789) had a higher rate of death (9.3% vs. 3.2%; P<0.001), non-fatal myocardial infarction (11.3% vs. 7.1%; P<0.001), and rehospitalization with ACS (18.1% vs. 13.0%; P<0.001) than non-diabetic patients at 1 year post-procedure. An early invasive strategy was associated with a similar risk reduction in death, myocardial infarction, or rehospitalization for ACS in diabetic and non-diabetic patients (RR 0.87; 95% CI 0.70-1.03 vs. 0.86; 95% CI 0.70-1.06; P for interaction 0.83).338 Accordingly, diabetes presents a secondary indication for high risk and for invasive management, and further efforts need to be made to give diabetic patients with ACS better access to revascularization therapy.180
Compared with non-diabetic patients, diabetics with STEMI present later, are more likely to experience haemodynamic instability and end-organ damage, and have delayed revascularization. In STEMI patients, the Primary Coronary Angioplasty vs. Thrombolysis (PCAT)-2 collaborative analysis of 19 RCTs with individual patient data from 6315 patients (14% with diabetes mellitus) showed a similar benefit of primary PCI over fibrinolytic treatment in diabetic and non-diabetic patients.363 The OR for mortality in favour of primary PCI was 0.49 for diabetic patients (95% CI 0.31-0.79). Recurrent myocardial infarction and stroke were also significantly lower in favour of primary PCI. Patients with diabetes had significantly delayed initiation of reperfusion treatments and longer ischaemic times, probably related to atypical symptoms causing significant delays in initiating reperfusion therapy. Owing to a higher absolute risk, the number needed to treat to save one life at 30 days was significantly lower for diabetic patients (number needed to treat=17; 95% CI 11-28) than for non-diabetic patients (number needed to treat=48; 95% CI 37-60).
10.2 TYPE OF MYOCARDIAL REVASCULARIZATION
The presence of diabetes mellitus defines the treatment strategy for an important subset of patients with multivessel CAD.
10.2.1. RANDOMIZED CLINICAL TRIALS
The Future Revascularization Evaluation in Patients with Diabetes Mellitus (FREEDOM) trial is the only adequately powered, randomized study comparing CABG against PCI with use of early-generation DES (94%) in diabetic patients undergoing elective revascularization for multivessel disease without LM coronary stenosis.175 Between 2005 and 2010, 33 966 patients were screened, of whom 3309 were considered eligible and 1900 (6%) enrolled. Their mean SYNTAX score was 26 ± 9. The primary outcome of death from any cause, non-fatal myocardial infarction, or stroke was lower in the CABG than the PCI group, with divergence of the curves starting at 2 years. This difference was driven by a borderline reduction of all-cause mortality (P=0.049) and by a markedly lower rate of myocardial infarction favouring the CABG group (P<0.001). Conversely, rates of stroke were doubled in the CABG group (P=0.03). The superiority of CABG over PCI was consistent across all pre-specified subgroups, including SYNTAX score, the only exception being that patients recruited outside the USA (n=1130) had a less-pronounced relative benefit from CABG than those enrolled in the USA (n=770) (P=0.05 for interaction).175 Detailed assessment of quality of life revealed substantial and durable improvements in cardiovascular-specific health status with both PCI and CABG groups. During the first month after treatment, PCI resulted in more rapid improvement in health status and quality of life, this changing between 6 months and 2 years in favour of CABG and differences disappearing beyond 2 years.344
It is unclear, however, whether the SYNTAX score was analysed by a blinded ‘core’ laboratory, which is essential for reproducibility. It should be noted that the SYNTAX score became operational during the FREEDOM trial and is not mentioned in the FREEDOM study protocol.345 Therefore, the validity of the observation that CABG led to better outcomes than PCI, irrespective of the SYNTAX score, remains unclear, and it is not consistent with the findings related to the diabetic subgroup of the SYNTAX trial. The increased risk of stroke raises the question of treatment selection, particularly among elderly patients. In addition, the median follow-up was 3.8 years but only 23% of patients were at risk at 5 years.
In the subset of 452 diabetic patients with multivessel CAD who were enrolled in the SYNTAX trial, there were no significant differences at 5 years in the composite of all-cause death, myocardial infarction, or stroke (CABG 19.1% vs. PCI 23.9%; P=0.26) or in the individual components such as all-cause death (P=0.07), stroke (P=0.34), or myocardial infarction (P=0.20).346 However, repeat revascularization was less frequently required in the CABG group (P<0.001). Among patients with low SYNTAX score ( ≤ 22), rates of MACCE were similar for CABG and PCI (33.7% vs. 42.5%, respectively; P=0.38) but repeat revascularization remained more frequent in the PCI group (18.5% vs. 38.5%, respectively; P=0.01). Interestingly, in the SYNTAX trial, diabetes was not an independent predictor of outcomes once the SYNTAX score was entered into the multivariable model.25
In the Coronary Artery Revascularization in Diabetes (CARDia) trial, 510 diabetic patients with multivessel or complex single-vessel CAD, enrolled at 24 sites, were randomly assigned to either CABG or PCI with use of either BMS or DES and routine use of abciximab. There were no differences between CABG and PCI for the primary endpoint, the 1-year composite of death, myocardial infarction, or stroke.347 Comparing the subset of patients treated with DES, the primary outcome rates were 12.4% in the CABG and 11.6% in the PCI group (HR 0.93; 95% CI 0.51-1.71; P=0.82). Repeat revascularization was more common among patients assigned to PCI (P<0.001), whereas stroke tended to be less common among patients assigned to PCI (P=0.07).
Hence, taking currently available evidence into consideration, CABG is the revascularization modality of choice among diabetic patients with multivessel CAD; however, PCI can be considered as a treatment alternative among diabetic patients with multivessel disease and low SYNTAX score (≤22).
10.2.2 META-ANALYSES
A meta-analysis of individual data from 10 RCTs of elective myocardial revascularization106 confirms a survival advantage for CABG over PCI in diabetic patients, whereas no difference was found for non-diabetic patients; the interaction between diabetic status and type of revascularization was significant. In this pooled analysis, PCI patients were treated with either balloon angioplasty or BMS. A more recent meta-analysis –dedicated to diabetic patients treated with either CABG or PCI, with at least 80% of arterial conduit(s) or stents (BMS and early-generation DES)– showed significantly lower mortality with CABG at 5 years or the longest follow-up (RR 0.67; 95% CI 0.52-0.86; P=0.002).349 On the other hand, this pooled analysis showed increased rates of stroke using CABG vs. PCI at 5-year follow-up (RR 1.72; 95% CI 1.18-2.53; P=0.005). Similarly, a meta-analysis –restricted to four RCTs covering 3052 patients, which compared PCI with use of early-generation DES vs. CABG in diabetic patients with multivessel CAD– reported a higher risk of death and myocardial infarction with revascularization by early-generation DES (RR 1.51; 95% CI 1.09-2.10; P=0.01) but a lower risk of stroke (2.3% vs. 3.8%; RR 0.59; 95% CI 0.39-0.90; P=0.01).350 A sensitivity analysis revealed that the superiority of CABG over early-generation DES for the endpoint MACCE were most pronounced among patients with high SYNTAX score, but not significant in those with low SYNTAX score. All RCTs have shown higher rates of repeat revascularization procedures after PCI compared with CABG, in diabetic patients.106,346
10.3 REVASCULARIZATION WITH THE USE OF PERCUTANEOUS CORONARY INTERVENTION
A collaborative network meta-analysis has compared DES with BMS in 3852 diabetic patients.351 The need for target-lesion revascularization was considerably lower with DES than with BMS [OR 0.29 for sirolimus-eluting stent; 0.38 for paclitaxel-eluting stent]. A more recent mixed-treatment comparison of 42 trials with 22 844 patient-years of follow-up assessed the efficacy and safety of several early and new-generation DES and BMS in patients with diabetes. Compared with BMS, all DES showed a rate of TVR that was lower by 37-69%. Compared with BMS, there were no differences in rates of death, myocardial infarction, or stent thrombosis for any DES in diabetic patients.352 There are no robust data to support the use of any one DES over another in patients with diabetes.
10.4 REVASCULARIZATION WITH THE USE OF CORONARY ARTERY BYPASS GRAFTING
There is no direct, randomized evidence for or against the use of one vs. two IMA conduits in diabetic patients. Whether use of bilateral IMA increases the risk of deep sternal wound complications is still a matter of debate, although diabetic patients are particularly prone to sternal infections in bilateral IMA operations. However, observational evidence, with follow-up periods up to 30 years, suggests that bilateral IMA use improves long-term outcomes.23,24 Pending the long-term results of the randomized Arterial Revascularisation Trial (ART) trial,353 it is still not clear whether bilateral IMA grafting produces better outcomes, but the superior survival observed with bilateral IMA grafting has been seen not to depend on diabetic status.354 In a recent analysis, there was no significant correlation with diabetic status over 15-year follow-up when using multiple arterial grafts.355 Indeed, alternative strategies –including use of the radial artery in patients with an excessively high risk for sternal complications (e.g. obese patients)– have been shown to be safe during follow-up, and to prolong survival compared with using vein grafts.356
10.5 ANTITHROMBOTIC PHARMACOTHERAPY
There is no indication that antithrombotic pharmacotherapy should differ between diabetic and non-diabetic patients undergoing revascularization. Although a correlation between diabetic status and efficacy of GP IIb/IIIa inhibitors was noted in earlier trials without concomitant use of thienopyridines, this was not confirmed in the more recent Early glycoprotein IIb/IIIa inhibition in non-ST-segment elevation acute coronary syndrome (EARLY-ACS) trial.357 In the current context of use of oral P2Y12 inhibitors, diabetic patients do not specifically benefit from the addition of GP IIb/IIIa inhibitors.
10.6 ANTI-DIABETIC MEDICATIONS
Only a few specific trials of anti-diabetic medications have been conducted in patients undergoing myocardial revascularization.
Metformin
Because of the risk of lactic acidosis in patients receiving iodinated contrast media, it is generally stated that administration of metformin should be suspended before angiography or PCI, and resumed 48 hours later, subject to adequate renal function. The plasma half-life of metformin is 6.2 hours; however, there is no convincing evidence for such a recommendation. Checking renal function after angiography in patients on metformin and witholding the drug when renal function deteriorates might be an acceptable alternative to automatic suspension of metformin. In patients with renal failure, metformin should preferably be stopped before the procedure. Accepted indicators for metformin-induced lactic acidosis are arterial pH <7.35, blood lactate >5 mmol/l (45 mg/dl), and detectable plasma metformin concentration. Accurate recognition of metformin-associated lactic acidosis and prompt initiation of haemodialysis are important steps towards rapid recovery.
Other drugs
Observational data have raised concern over the use of sulphonylureas in patients treated with primary PCI for acute myocardial infarction. Such concern has not been backed up by a post hoc analysis of the Diabetes, Insulin Glucose Infusion in Acute Myocardial Infarction (DIGAMI)-2 trial, although the number of patients undergoing primary PCI in this trial was low.358 Arrhythmias and ischaemic complications were also less frequent in patients receiving gliclazide or glimepiride.359 Thiazolidinediones may be associated with lower rates of restenosis after PCI with BMS,360 but carry an increased risk of heart failure resulting from water retention in the kidney.
No trial has demonstrated that the administration of insulin or glucose-insulin-potassium improves PCI outcome after STEMI. Observational data in patients undergoing CABG suggest that use of a continuous intravenous (i.v.) insulin infusion to achieve moderately tight glycaemic control (6.6-9.9 mmol/l or 120-180 mg/dl) is independently associated with lower rates of mortality and major complications than those observed after tighter (6.6 mmol/l or 120 mg/dl) or more lenient (9.9 mmol/l or 180 mg/dl) glycaemic control.361 In the BARI-2D trial, outcomes were similar in patients receiving insulin sensitization vs. insulin provision to control blood glucose. In the CABG group, administration of insulin was associated with more cardiovascular events than the insulin-sensitization medications.139
In the Saxagliptin and Cardiovascular Outcomes in Patients with Type 2 Diabetes Mellitus SAVOR-TIMI 53 trial, dipeptidyl peptidase 4 (DPP-4) inhibition with saxagliptin neither increased nor decreased the incidence of ischaemic events, although the rate of hospitalization for heart failure was increased.362
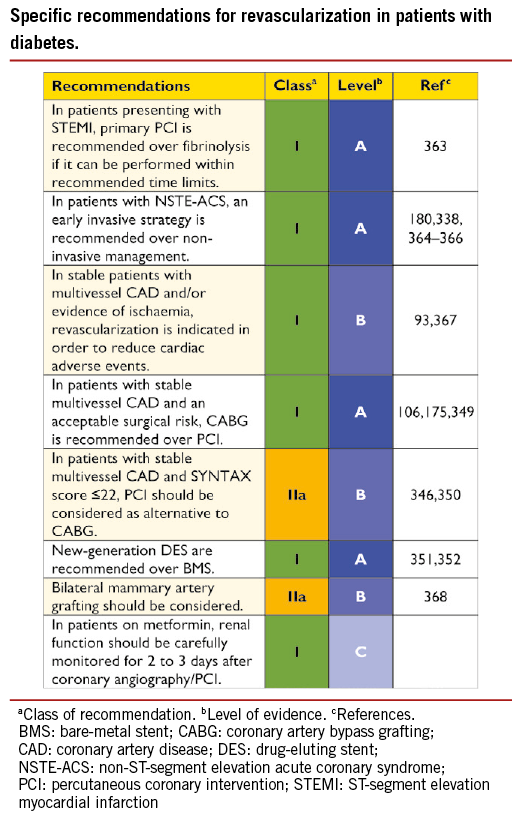
11. Revascularization in patients with chronic kidney disease
11.1 EVIDENCE-BASE FOR REVASCULARIZATION
Myocardial revascularization is underused in patients with chronic kidney disease (CKD).369-371 In all categories of kidney function (defined in the web addenda), observational studies suggest that CKD patients with multivessel disease who undergo revascularization have better survival than those who receive medical therapy.372,373 Particularly among patients with ACS, large-scale registries indicate better short- and long-term survival with early revascularization than with medical therapy across all CKD stages.371,374 When there is an indication for PCI, DES should be preferred over BMS, because of its lower risk of revascularization and the absence of safety concerns.375,376 Notwithstanding, the use of contrast media during diagnostic and interventional vascular procedures represents the most common cause of acute kidney injury in hospitalized patients. In addition, patients with CKD have frequent comorbidities that increase the risk of periprocedural ischaemic and bleeding events. Notably, there is little evidence from RCTs, as most therapeutic RCTs on revascularization have excluded CKD patients. Current treatment strategies are therefore based on retrospective analyses of RCTs and data from large registries.
11.1.1 PATIENTS WITH MODERATE CHRONIC KIDNEY DISEASE
Observational studies suggest an increased risk of perioperative and short-term (~12 months) fatal events but lower medium-to-long-term mortality after CABG compared with PCI.377,378 The absolute risk for end-stage renal disease is smaller than that for fatal events in this patient population and the combined incidence of death or end- stage renal disease may remain lower after CABG at long-term follow-up. In the post hoc analysis of patients with CKD (25% of 1205 patients) in the randomized Arterial Revascularization Therapies Study (ARTS) trial, which compared CABG against multivessel PCI with the use of BMS, no difference was observed in the primary endpoint of death, myocardial infarction, or stroke (19% vs. 17%; HR 0.93; 95% CI 0.54-1.61; P=0.80) as well as mortality after 3 years of follow-up; however, the risk of repeat revascularization was reduced in favour of CABG (25% vs. 8%; HR 0.28; 95% CI 0.14-0.54; P=0.01).379 There is some evidence that suggests that the off-pump approach may reduce the risk of perioperative acute renal failure and/or progression to end-stage renal disease in these patients.380 Predictive tools have been proposed, which hold promise as a means of identifying CKD patients who are likely to derive the most benefit from one particular revascularization strategy, but these have not been systematically validated externally.381
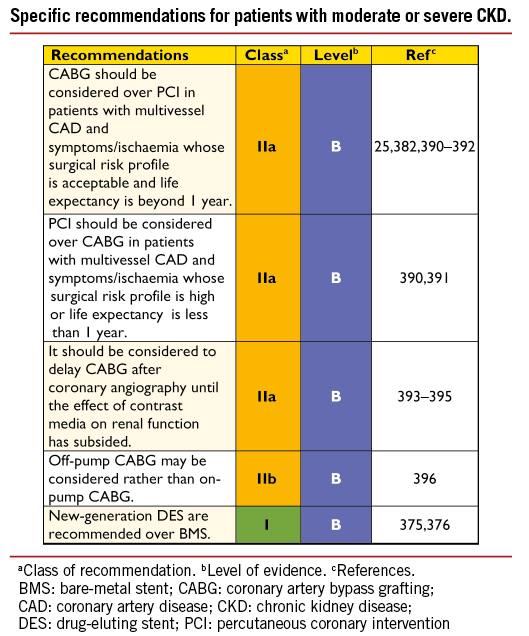
11.1.2 PATIENTS WITH SEVERE CHRONIC KIDNEY DISEASE AND END-STAGE RENAL DISEASE OR IN HAEMODIALYSIS
In the absence of data from RCTs, results from a large cohort of 21 981 patients with end-stage renal disease (data from US Renal Data System) with poor 5-year survival (22-25%) suggest that CABG should be preferred over PCI for multivessel coronary revascularization in appropriately selected patients on maintenance dialysis.382 Compared with PCI, CABG was associated with significantly lower risks for both death and the composite of death or myocardial infarction.382 Selection of the most appropriate revascularization strategy must therefore account for the general condition and life expectancy of the patient, the least invasive approach being more appropriate in the most fragile and compromised patients.
Candidates for renal transplantation must be screened for myocardial ischaemia, and those with significant CAD should not be denied the potential benefit of myocardial revascularization. Renal transplant recipients have been reported to have similar long-term survival after CABG and PCI.383
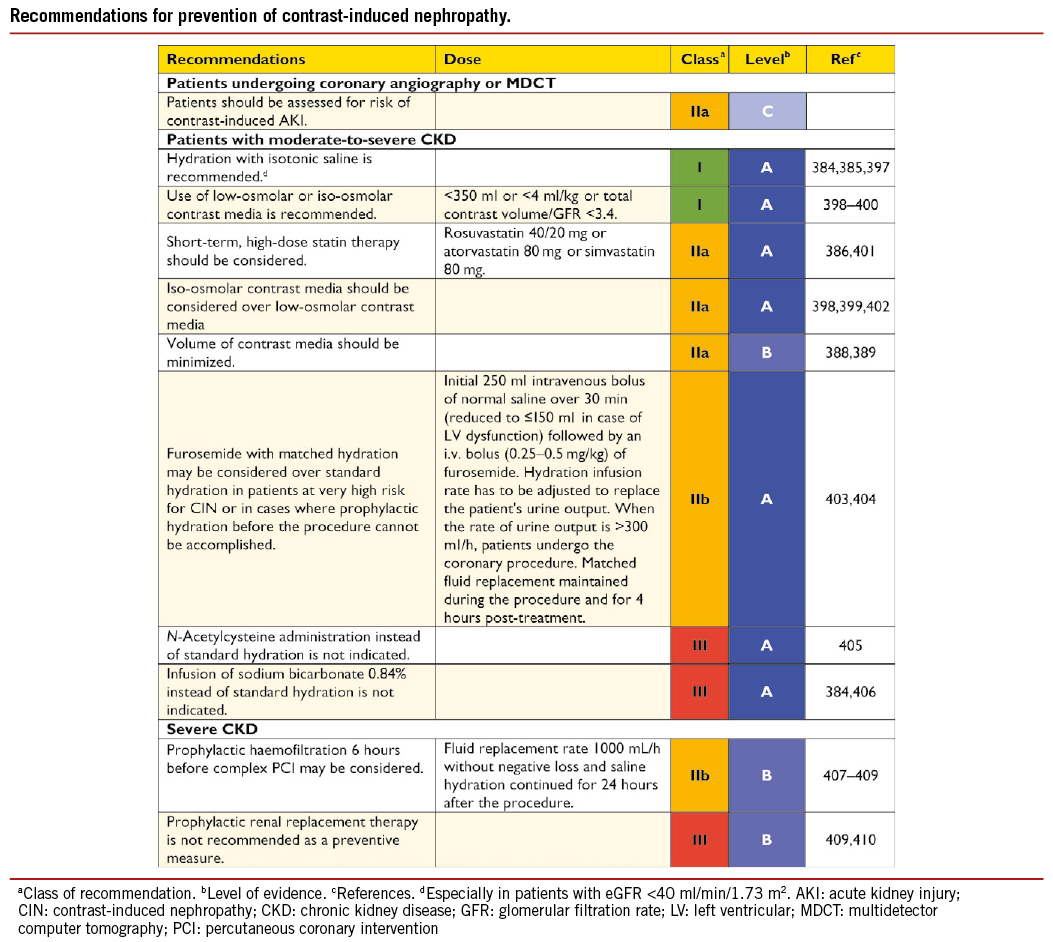
11.2 PREVENTION OF CONTRAST-INDUCED NEPHROPATHY
Especially if glomerular filtration rate (GFR) is <40 ml/min/1.73 m2, all patients with CKD who undergo diagnostic catheterization should receive preventive hydration with isotonic saline, to be started approximately 12 hours before angiography and continued for at least 24 hours afterwards to reduce the risk of contrast-induced nephropathy (CIN).384,385 The implementation of high-dose statin before diagnostic catheterization has been shown to reduce the incidence of CIN and should be considered as an additional preventive measure in patients without contraindications.386 The antioxidant ascorbic acid has been explored in oral and intravenous preparations, for protection against CIN. A recent meta-analysis of nine RCTs in 1536 patients suggested a somewhat lower risk of CIN among pre-existing CKD patients who received ascorbic acid, than in patients who received placebo or an alternate treatment (9.6% vs.16.8%, respectively; RR=0.67; 95% CI 0.47 to 0.97; P=0.034)387 but more evidence is required to make definite recommendations. Although performing diagnostic and interventional procedures separately reduces the total volume exposure to contrast media, the risk of renal atheroembolic disease increases with multiple catheterizations. Therefore, in CKD patients with diffuse atherosclerosis, a single invasive approach (diagnostic angiography followed by ad hoc PCI) may be considered, but only if the contrast volume can be maintained <4 ml/kg. The risk of CIN increases significantly when the ratio of total contrast volume to GFR exceeds 3.7:1.388,389
For patients undergoing CABG, the effectiveness of the implementation of pharmacological preventive measures –such as clonidine, fenoldopam, natriuretic peptides, N-acetylcysteine or elective pre-operative haemodialysis– remains unproven.
12. Revascularization in patients requiring valve interventions
12.1 PRIMARY INDICATION FOR VALVE INTERVENTIONS
Overall, 40% of patients with valvular heart disease will have concomitant CAD. Coronary angiography is recommended in all patients with valvular heart disease requiring valve surgery, apart from young patients (men <40 years and pre-menopausal women) without risk factors for CAD or when the risks of angiography outweigh the benefits (e.g. in cases of aortic dissection, a large aortic vegetation in front of the coronary ostia, or occlusive prosthetic thrombosis leading to an unstable haemodynamic condition).411
In patients undergoing aortic valve replacement (AVR) who also have significant CAD, the combination of CABG and aortic valve surgery reduces the rates of perioperative myocardial infarction, perioperative mortality, late mortality, and morbidity, when compared with patients not undergoing simultaneous CABG.412-415
This combined operation, however, carries an increased risk of mortality over isolated AVR.11,416-418 In a contemporary analysis of a large cohort, the greater risk of the combined operation than with isolated AVR was associated with effects of pre-existing ischaemic myocardial damage and comorbidities.419
In patients with severe comorbidities, the Heart Team may opt for transcatheter valve interventions. Although a systematic review of observational studies has shown no significant impact of CAD on mortality in patients undergoing transcatheter aortic valve implantation (TAVI),420 a recent single-centre investigation found an increased risk of cardiovascular adverse events among patients with advanced CAD (SYNTAX score >22).421 PCI, among patients with CAD undergoing TAVI, does not appear to increase the short-term risks of death, myocardial infarction, or stroke, compared with patients undergoing isolated TAVI; however, its impact on long-term prognosis is not well established.422-425 The selection of lesions treated by PCI is usually based on clinical presentation and angiography, as functional methods of detecting ischaemia have not been validated among patients with severe aortic stenosis.422,423,426-428 Currently, there is no conclusive evidence as to whether PCI should be performed as a staged intervention or during the same procedure, and the decision may be made on an individual basis according to the leading clinical problem, renal failure, and complexity of the underlying CAD.422,424,425,428,429 Published experience with PCI and percutaneous mitral valve repair is currently limited to case reports.
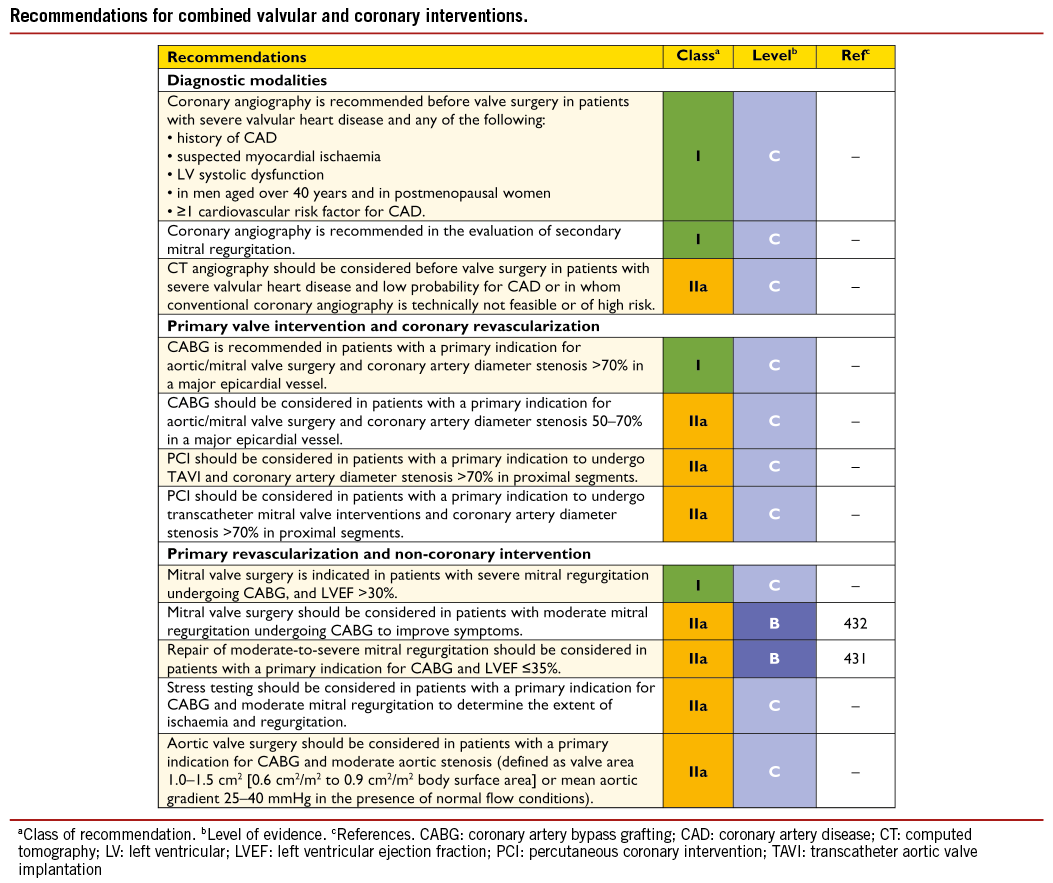
Alternative treatments for high-risk patients also include ‘hybrid’ procedures, which involve a combination of scheduled surgery for valve replacement and planned PCI for myocardial revascularization.
At present, however, the data on hybrid valve/PCI procedures are very limited, being confined to case reports and small case series.430 Individual treatment decisions in these complex patients are best formulated by the Heart Team.
12.2 PRIMARY INDICATION FOR CORONARY REVASCULARIZATION
Many patients with CAD and reduced LV function have concomitant secondary mitral regurgitation. Observational data from the STICH trial suggest that adding mitral valve repair to CABG in patients with LV dysfunction (LVEF ≤35%) and moderate-to-severe mitral regurgitation offers better survival than CABG alone.431 Likewise, in patients undergoing CABG for the clinically leading problem of CAD, aortic valves with moderate stenosis should be replaced.411 Case-by-case decisions by the Heart Team are needed for patients with an indication for PCI and moderate-to-severe valve disease.
13. Associated carotid/peripheral artery disease
13.1 ASSOCIATED CORONARY AND CAROTID ARTERY DISEASE
The prevalence of severe carotid artery stenosis increases with the severity of CAD and is an indicator of impaired prognosis.433 Although the association between carotid artery stenosis and CAD is evident, the prevalence of significant carotid artery stenosis in the entire cohort remains relatively low. Conversely, up to 40% of patients undergoing carotid endarterectomy (CEA) have significant CAD and may benefit from pre-operative cardiac risk assessment.
13.1.1 RISK FACTORS FOR STROKE ASSOCIATED WITH MYOCARDIAL REVASCULARIZATION
The incidence of stroke after CABG varies depending on age, comorbidities and surgical technique. The FREEDOM trial, which compared PCI with CABG in diabetic patients with multivessel CAD, showed a 30-day rate of stroke of 1.8% after CABG and 0.3% after PCI (P=0.002).175 Similarly, a greater risk of stroke was reported in the SYNTAX trial, which diminished during long-term follow-up and was no longer significant at 5 years (CABG 3.7% vs. PCI 2.4%; P=0.09).17 In a meta-analysis of 19 randomized trials with 10 944 patients, the risk of stroke was lower among patients assigned to PCI than in those assigned to CABG after 30 days and at 1 year.131 These findings indicate that CABG carries a greater periprocedural risk of stroke but that the long-term risk of cerebrovascular events persists with both treatments.17 The most common cause of CABG-related stroke is embolization of atherothrombotic debris from the ascending aorta, particularly during aortic cannulation. The risk of periprocedural stroke after CABG in patients with carotid artery stenosis is associated with the severity of stenosis but even more with a history of stroke or transient ischaemic attack (TIA) (within 6 months).434 There is a lack of strong evidence that CAD is a significant cause of perioperative stroke.435 The extension of atherosclerotic disease to intracerebral and extracerebral territories, radiographic demonstration of previous stroke and aortic atheromatous disease, are the most important factors for predicting an increased risk of perioperative stroke.435
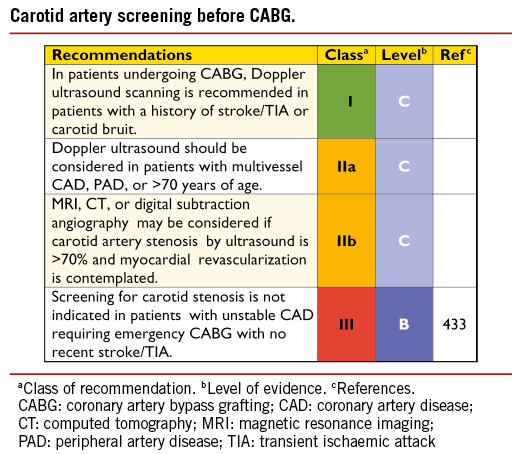
Although symptomatic carotid artery stenosis is associated with a greater risk of stroke, 50% of patients suffering strokes after CABG do not have significant carotid artery disease and 60% of territorial infarctions on CT scan/autopsy cannot be attributed to carotid disease alone. Furthermore, only around 40% of strokes following CABG are identified within the first day after surgery, while 60% of strokes occur after uneventful recovery from anaesthesia. In a recent study including 45 432 patients undergoing CABG, 1.6% experienced a stroke and risk factors for all strokes were age, smaller body surface area, emergency surgery, previous stroke, pre-operative atrial fibrillation (AF), and on-pump CABG with hypothermic circulatory arrest. For intraoperative strokes, additional risk factors were peripheral and carotid artery disease, previous cardiac surgery, worse clinical condition, LV dysfunction, left circumflex (LCx) coronary artery stenosis >70%, and on-pump CABG with arrested heart or hypothermic circulatory arrest.436
Although the risk of stroke is low among patients with carotid artery disease undergoing PCI, ACS, heart failure, and extensive atherosclerosis are independent risk factors for this adverse event. In a large registry of 348 092 PCI patients, the rates of stroke and TIA amounted to only 0.11% and did not differ between transfemoral and radial access.437
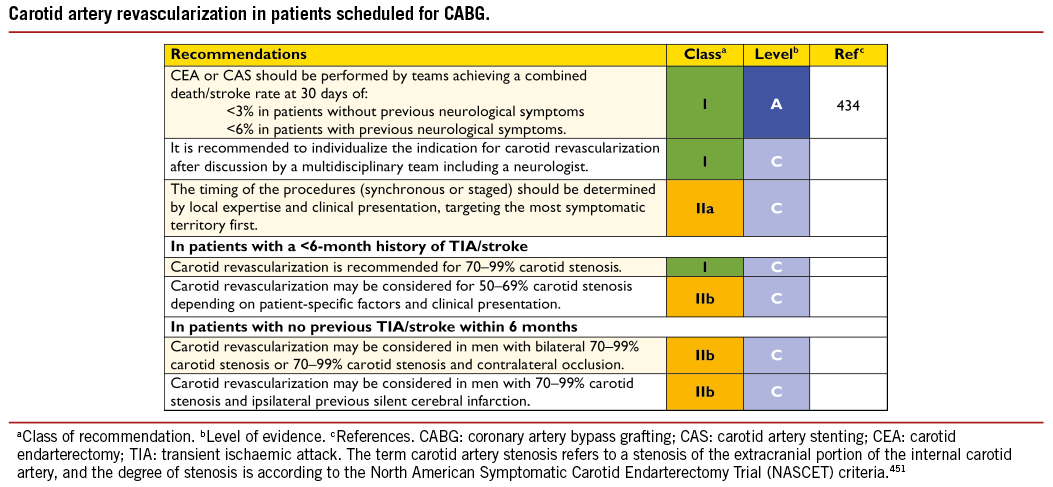
13.1.2 PREVENTIVE MEASURES TO REDUCE THE RISK OF STROKE AFTER CORONARY ARTERY BYPASS GRAFTING
Detection of severe carotid artery bifurcation disease may lead to concomitant carotid revascularization in selected cases. Identification of an atherosclerotic aorta is believed to be an important step in reducing the risk of stroke after CABG. Pre-operative CT scan or intraoperative ultrasound epiaortic scanning –better than aortic palpation– can lead to modifications in the operative management that may reduce the risk of stroke associated with CABG.438,439 There is conflicting evidence regarding the influence of off-pump CABG on the incidence of stroke.440 A recent randomized trial showed no difference in the incidence of stroke between off-pump CABG and on-pump CABG at 30 days.441 However, studies employing a ’minimal touch’ technique for the aorta reported a lower risk of stroke and MACCE with off-pump CABG.442,443
Perioperative medical therapy plays a fundamental role in the prevention of neurological complications following CABG. Statins in combination with beta-blockers have shown a protective effect on the risk of stroke after CABG.444
13.1.3 CAROTID REVASCULARIZATION IN PATIENTS SCHEDULED FOR MYOCARDIAL REVASCULARIZATION
In patients with previous TIA or stroke and the presence of carotid artery stenosis (50-99% in men; 70-99% in women), CEA performed by experienced teams may reduce the risk of perioperative stroke or death.434 Conversely, isolated myocardial revascularization should be performed among patients with asymptomatic unilateral carotid artery stenosis because of the small risk reduction in stroke and death achieved by concomitant carotid revascularization (1% per year).434 Carotid revascularization may be considered in asymptomatic men with bilateral severe carotid artery stenosis or contralateral occlusion, provided that the risk of stroke or death within 30 days can be reliably documented to be <3% in the presence of a life expectancy >5 years. In women with asymptomatic carotid disease or patients with a life expectancy of <5 years, the benefit of carotid revascularization remains unclear.434 In the absence of clear proof that staged or synchronous CEA or carotid artery stenting (CAS) is beneficial in patients undergoing CABG, patients should be assessed on an individual basis by a multidisciplinary team including a neurologist. This strategy is also valid for patients scheduled for PCI. The strategy of combining PCI with CAS in the same procedure in elective patients is not routinely recommended, except in the infrequent circumstance of concomitant acute severe carotid and coronary syndromes.
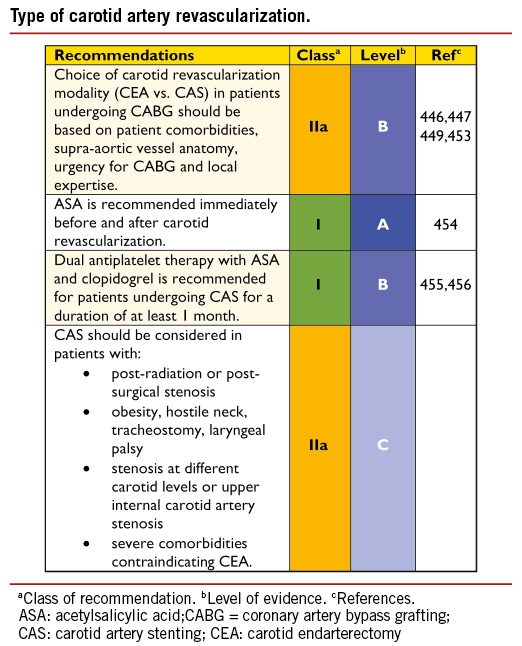
13.1.4 TYPE OF REVASCULARIZATION IN PATIENTS WITH ASSOCIATED CAROTID AND CORONARY ARTERY DISEASE
Few patients scheduled for CABG require synchronous or staged carotid revascularization.445-448 In the absence of randomized trials comparing management strategies in patients with concomitant CAD and carotid disease, the choice of carotid revascularization modality (CEA vs. CAS) should be based on patient comorbidities, supra-aortic vessel anatomy, degree of urgency for CABG and local expertise.449 Operator proficiency impacts on results of both carotid revascularization methods but even more in CAS, with higher mortality rates in patients treated by low-volume operators or early in their experience.450 If CAS is performed before elective CABG, the need for dual antiplatelet therapy (DAPT) usually delays cardiac surgery for 4-5 weeks.451,452
13.2 ASSOCIATED CORONARY AND PERIPHERAL ARTERIAL DISEASE
Peripheral artery disease (PAD) is an important predictor of adverse outcome after myocardial revascularization, and portends a poor long-term outcome.457,458 Patients with clinical evidence of PAD are at increased risk for procedural complications after either PCI or CABG. When comparing the outcomes of CABG vs. PCI in patients with PAD and multivessel disease, CABG is associated with a trend for better survival. Risk-adjusted registry data have shown that patients with multivessel disease and PAD undergoing
CABG have better survival at 3 years than similar patients undergoing PCI, in spite of higher in-hospital mortality. In the case of CABG, surgeons should avoid harvesting veins from legs that are affected by significant clinical symptoms of PAD; however, with no solid data available in this population, the two myocardial revascularization approaches are probably as complementary in patients with PAD as they are in other CAD patients.
Non-cardiac vascular surgery in patients with associated coronary artery disease
Patients scheduled for non-cardiac vascular surgery are at greater risk of cardiovascular morbidity and mortality due to a high incidence of underlying symptomatic or asymptomatic CAD.451,459 Results of the largest RCT have demonstrated that, among 510 patients randomized to prophylactic myocardial revascularization (by either PCI or CABG) or to medical therapy alone, there is no advantage in terms of incidence of perioperative myocardial infarction, early or long-term mortality before major vascular surgery.460 Patients included in this study had preserved LV function and SCAD. A RCT with 208 patients at moderate or high cardiac risk, who were scheduled for major vascular surgery, reported similar results: patients undergoing systematic pre-operative coronary angiography and revascularization had similar in-hospital outcomes but greater freedom from cardiovascular events at 4 years than with a selective strategy.461 In summary, selected high-risk patients may benefit from staged or concomitant myocardial revascularization, with options varying from a one-stage surgical approach to combined PCI and peripheral endovascular repair or hybrid procedures.
RCTs involving high-risk patients, cohort studies, and meta-analyses provide consistent evidence, in patients undergoing high-risk non-cardiac vascular surgery or endovascular procedures, of lower incidences of cardiac mortality and myocardial infarction related to medical therapy including statins.458 In summary, perioperative cardiovascular complications are common in PAD patients with associated CAD and result in significant morbidity following non-cardiac vascular surgery. All patients require pre-operative screening to identify and minimize immediate and future risk, with a careful focus on known CAD, risk factors for CAD, and functional capacity.451,462
14. Repeat revascularization and hybrid procedures
14.1 EARLY GRAFT FAILURE
Early graft failure after CABG is reported in up to 12% of grafts (left IMA 7%; saphenous vein graft 8%) as evaluated by intraoperative angiographic control,463 but only a minority, around 3%, are clinically apparent.464 Graft failure can be due to conduit defects, anastomotic technical errors, poor native vessel run-off, or competitive flow with the native vessel. When clinically relevant, acute graft failure may result in myocardial infarction with consequently increased mortality and major cardiac events. The suspicion of graft failure should arise in the presence of ECG signs of ischaemia, ventricular arrhythmias, important biomarker modifications, new wall motion abnormalities, or haemodynamic instability.465 Owing to the low specificity of ECG modifications and echocardiographic wall motion abnormalities during the post-operative course and the delay in appearance of biomarker changes, a careful assessment of all variables will influence the decision-making for angiographic evaluation.
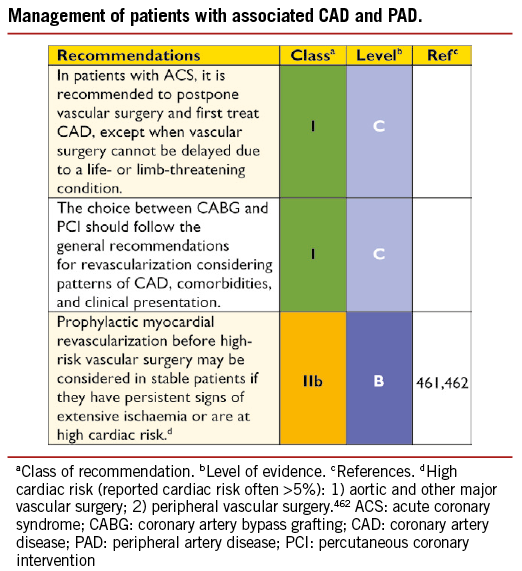
Perioperative angiography is recommended in cases of suspected myocardial ischaemia to detect its cause and help decide on appropriate treatment.463,465,466 In symptomatic patients, early graft failure can be identified as the cause of ischaemia in about 82% of cases.467 In early post-operative graft failure, emergency PCI may limit the extent of myocardial infarction compared with re-do surgery.467 The target for PCI is the body of the native vessel or the IMA graft, while the acutely occluded saphenous vein graft (SVG) and the anastomosis should be avoided due to concerns over embolization or perforation. Re-do surgery should be favoured if anatomy is unsuitable for PCI, or if several important grafts are occluded. Early mortality in the range of 9-15% has been reported in this group of patients, without any difference between the two revascularization strategies.467 In asymptomatic patients, repeat revascularization should be considered if the artery is of appropriate size and supplies a large territory of myocardium. The optimal treatment strategy in patients with acute graft failure should be decided by ad hoc consultation between cardiovascular surgeon and interventional cardiologist, on the basis of the patient’s clinical condition and extent of myocardium at risk.
14.2 DISEASE PROGRESSION AND LATE GRAFT FAILURE
Ischaemia after CABG may be due to progression of disease in native vessels or disease of bypass grafts (Table 9). Repeat revascularization in these patients is indicated in the presence of significant symptoms despite medical treatment, and in asymptomatic patients with objective evidence of myocardial ischaemia (>10% LV).54,143 The survival of patients with patent left IMA to LAD and ischaemia in the territories of the right- and circumflex arteries does not appear to be influenced by mechanical revascularization when compared with medical therapy alone.468
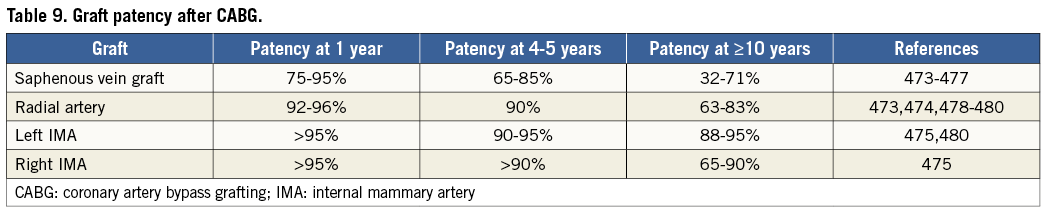
Re-do coronary artery bypass grafting or percutaneous coronary intervention
Percutaneous coronary intervention in patients with previous CABG has worse acute and long-term outcomes than in patients without prior CABG. Re-do CABG has a two- to four-fold increased mortality compared with first-time CABG.477,478 There are limited data comparing the efficacy of PCI vs. re-do CABG in patients with previous CABG. In the Angina With Extremely Serious Operative Mortality Evaluation (AWESOME) RCT and registry, overall in-hospital mortality was higher with re-do CABG than with PCI.151,479 More recent observational data have shown similar long-term results in patients treated by re-do CABG and PCI, with a higher revascularization rate for the PCI group.479,480 In view of the higher risk of procedural mortality with re-do CABG and the similar long-term outcome, PCI is the preferred revascularization strategy in patients with patent left internal mammary artery (LIMA) and amenable anatomy. CABG is preferred for patients with extensively diseased or occluded bypass grafts, reduced systolic LV function, several total occlusions of native arteries and absence of patent arterial grafts. The IMA is the conduit of choice for revascularization during re-do CABG.481
Percutaneous coronary intervention via the by-passed native artery should be the preferred approach provided the native vessel is not chronically occluded. Percutaneous coronary intervention for a chronic total occlusion (CTO) may be indicated when ischaemic symptoms are present with evidence of significant ischaemia and viable myocardium in the territory supplied. If PCI in the native vessel fails, PCI in the diseased SVG remains an option.
Percutaneous coronary intervention for saphenous vein graft lesions
Percutaneous coronary intervention for SVGs is associated with an increased risk of distal coronary embolization, resulting in periprocedural myocardial infarction. Percutaneous coronary intervention of de-novo SVG stenosis is considered a high-risk intervention because SVG atheroma is friable and more prone to distal embolization. A pooled analysis of five RCTs reported that GP IIb/IIIa inhibitors are less effective for interventions in SVGs than in native vessels.483 Several different approaches have been evaluated to prevent distal embolization of particulate debris, including distal occlusion/aspiration, proximal occlusion, suction, filter devices or mesh-covered stents.484 Unlike occlusive devices, distal protection using filters offers the inherent advantage of maintaining antegrade perfusion and the opportunity for contrast injections. Combined data, mostly from comparative studies between devices and surrogate endpoints, support the use of distal embolic protection during SVG PCI.485,486
In an RCT comparing different distal-protection devices in SVG PCI, the only independent predictor of 30-day MACE was plaque volume, and not the type of protection device used.487 Experience with other devices used for SVG PCI, such as mesh-based stents, is limited.488
Implantation of DES in SVG lesions is associated with a lower risk of repeat revascularization than with BMS.489-497 In the Swedish Coronary Angiography and Angioplasty Registry (SCAAR) of 3063 procedures with 4576 stents –including BMS and DES in SVG lesions– the incidence of death was lower among patients who received DES.489 However, no differences in terms of death, myocardial infarction, or stent thrombosis were observed in the randomized Is Drug-Eluting-Stenting Associated with Improved Results in Coronary Artery Bypass Grafts (ISAR-CABG) trial.495
Long-term results (up to 7 years post-procedure) of early- generation DES in SVG lesions are satisfactory, with no excess risk of stent thrombosis and maintained lower rate of restenosis than with BMS.494,496 Compared with PCI of native coronary vessels, patients undergoing PCI of SVGs have impaired long-term clinical outcomes.498
14.3 ACUTE PERCUTANEOUS CORONARY INTERVENTION FAILURE
Most PCI-related complications (including dissections, vessel occlusion, intracoronary thrombosis, and coronary perforation) are successfully treated in the catheterization laboratory;499,500 on-site or stand-by surgery is therefore not required during these procedures. The need for urgent surgery to manage PCI-related complications is uncommon and only required in patients with major complications that cannot be adequately resolved by percutaneous techniques.499,500 This is mainly confined to patients with a large, evolving myocardial infarction due to iatrogenic vessel occlusion that cannot be salvaged percutaneously, and to those with iatrogenic cardiac tamponade with failed pericardiocentesis or recurrent tamponade.499,500 When severe haemodynamic instability is present, IABP or mechanical circulatory assistance may be desirable before emergency surgery.
14.4 REPEAT PERCUTANEOUS CORONARY INTERVENTION
Recurrence of symptoms or ischaemia after PCI is the result of restenosis, incomplete initial revascularization, or disease progression. Infrequently, patients may require repeat PCI due to late and very late stent thrombosis.
Restenosis
Restenosis associated with angina or ischaemia should be treated by repeat revascularization and repeat PCI remains the strategy of choice for these patients if technically feasible. Originally, balloon angioplasty was frequently used in this setting, with good initial results but high rates of recurrence.501,502 Bare-metal stents provided superior early results in patients with in-stent restenosis but produced unfavourable late outcomes and were therefore reserved for patients with suboptimal initial results after balloon angioplasty or for those with large vessels.501,502 Ablative techniques (including rotational atherectomy and laser) have failed to improve results in such patients. Although brachytherapy was effective for in-stent restenosis, it never achieved widespread use, mainly due to logistical issues. Currently DES implantation is recommended in patients with BMS or DES in-stent restenosis. In this setting, the results from DES are superior to those obtained with balloon angioplasty, BMS implantation or brachytherapy.501-505 Drug-coated balloons are also effective in these patients and are particularly attractive when more than two stent layers are already present in the vessel. Drug-coated balloons are superior to balloon angioplasty and give results similar to early-generation DES in patients with BMS or DES in-stent restenosis.506-512 The use of intracoronary imaging may provide insights into the underlying mechanisms of in-stent restenosis. The presence of an underexpanded stent should, if possible, be corrected during the repeat procedure. In patients with recurrent episodes of diffuse in-stent restenosis –and in those with associated multivessel disease, especially in the presence of other complex lesions such as chronic total occlusions– CABG should be considered before a new PCI attempt.
Disease progression
Patients with symptomatic disease progression after PCI account for up to 50% of re-interventions.513,514 They should be managed using criteria similar to patients without previous revascularization if angiographic and functional results of previous interventions remain satisfactory. Percutaneous coronary intervention is an excellent therapy for these patients but care should be taken to identify the sites of prior patent stents as, occasionally, these may complicate re-interventions in the same vessel. Preventive pharmacological strategies should be maximized in this population.
Stent thrombosis
Although stent thrombosis is very rare it may have devastating clinical consequences. Stent thrombosis usually presents as a large myocardial infarction and patients should undergo emergency primary PCI.515 Owing to the rarity of this complication, the interventional strategy of choice remains unsettled but the use of thromboaspiration and intracoronary IIb/IIIa platelet inhibitors is frequently advocated. Aggressive, high-pressure balloon dilation should be used to correct underlying, stent-related, predisposing, mechanical problems.516 In this challenging setting, it has been suggested that intracoronary diagnostic techniques be used to correct mechanical problems and optimize final results.516,517 While optical coherence tomography (OCT) provides superior near-field resolution to intravascular ultrasound imaging (IVUS) and is able to identify red thrombus, thrombus shadowing may interfere with imaging of the underlying structures.516 Some patients with very late stent thrombosis actually have neoatherosclerosis as the underlying pathological substrate, and this can be recognized with intracoronary imaging.516 Although the value of repeat stenting in patients with stent thrombosis is under debate and should be avoided when satisfactory results are obtained with balloon dilation, a new stent may be required to overcome edge related dissections and adjacent lesions or to optimize final results. Detection and correction of any predisposing thrombogenic milieu remains important during these interventions.516
Adequate inhibition of platelet aggregation is of great importance in minimizing the risk of stent thrombosis, as well as its recurrence. Hence, in patients presenting with stent thrombosis, particular care should be taken to select the most appropriate P2Y12 inhibitor and ensure the importance of compliance by adequate patient information. There is no evidence to suggest that platelet function testing is effective in guiding the decision-making process with respect to type of P2Y12 inhibitor in this specific setting. Since prasugrel and ticagrelor lower the risk of primary ST,341,518 these agents should be preferred over clopidogrel, if clinically indicated. Duration of treatment should be at least 12 months after the acute event and potentially longer if well tolerated. In cases where these new agents are not available or contra-indicated, doubling the dose of clopidogrel may be reasonable.519
14.5 HYBRID PROCEDURES
Hybrid myocardial revascularization is a planned intervention combining cardiac surgery with a catheter-based intervention performed within a predefined time.520-523 Procedures can be performed consecutively in a hybrid operating room, or sequentially on separate occasions in the conventional surgical and PCI environments. The Heart Team discussion and the design of a joint strategy are critical for these patients. Hybrid procedures consisting of IMA to LAD and PCI of other territories appear reasonable when PCI of the LAD is not an option or is unlikely to portend good long-term results or when achieving a complete revascularization during CABG might be associated with an increased surgical risk.520,521 Although in most centres the number of hybrid procedures is relatively small, it remains important to consider when they may be clinically indicated.
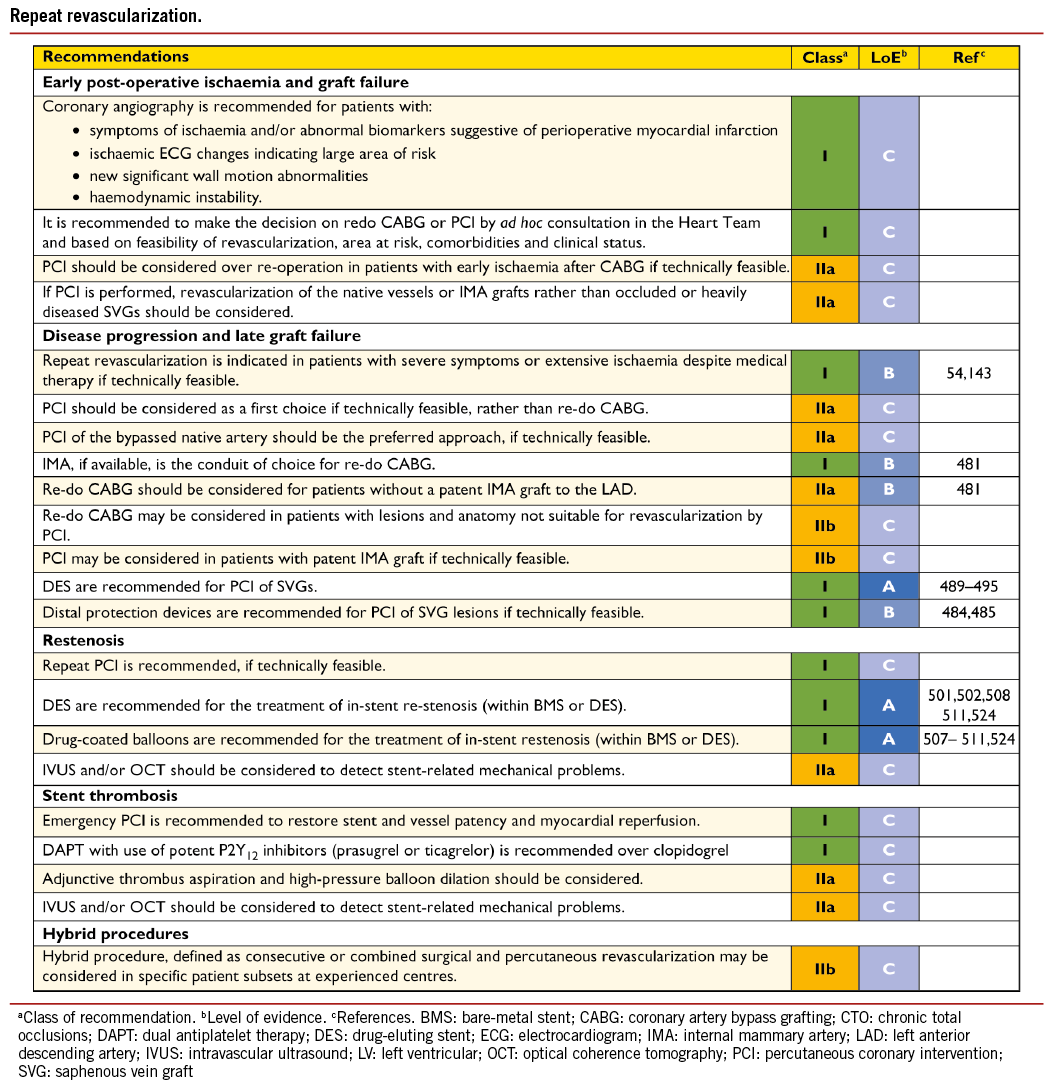
Options include:
(1) Selected patients with single-vessel disease of the LAD, or in multivessel disease but with poor surgical targets except for the LAD territory, in whom minimally invasive direct coronary artery bypass grafting (MIDCAB) can be performed to graft the LAD using the LIMA. The remaining lesions in other vessels are subsequently treated by PCI.
(2) Patients who had previous CABG and now require valve surgery, and who have at least one important patent graft (e.g. IMA to LAD) and one or two occluded grafts witha native vessel suitable for PCI.
(3) Combination of revascularization with non-sternotomy valve intervention (e.g. PCI and minimally invasive mitral valve repair, or PCI and transapical aortic valve implantation).
In addition, some patients with complex multivessel disease presenting with STEMI initially require primary PCI of the culprit vessel, but subsequently may require complete surgical revascularization. A similar situation occurs when patients with combined valvular and CAD require urgent revascularization with PCI. Finally, when a heavily calcified aorta is found in the operating room the surgeon may elect not to attempt complete revascularization and to offer delayed PCI.
15. Arrhythmias
15.1 VENTRICULAR ARRHYTHMIAS
15.1.1 REVASCULARIZATION FOR PREVENTION OF SUDDEN CARDIAC DEATH IN PATIENTS WITH STABLE CORONARY ARTERY DISEASE AND REDUCED LEFT VENTRICULAR FUNCTION
Revascularization plays an important role in reducing the frequency of ventricular arrhythmias in normal and mildly reduced LV function (CASS study,525 European Coronary Surgery Study).109 Thus, revascularization significantly decreased the risk for sudden cardiac death in patients with CAD and LVEF <35% [Studies of Left Ventricular Dysfunction (SOLVD)].526 Likewise, simultaneous ICD implantation during CABG did not improve survival in patients with reduced LV function (CABG Patch).527 Conversely, an adjusted increased risk of ventricular tachycardia (VT) or ventricular fibrillation (VF) of 5% or 8%, respectively, was observed with every 1-year increment of time elapsed from revascularization, irrespective of the mode of revascularization, potentially related to a gradual progression of CAD (Multicenter Automatic Defibrillator Implantation Trial – Cardiac Resynchronization Therapy (MADIT-CRT).528 Indirect evidence for a protective effect of coronary revascularization in terms of sudden cardiac death is provided by retrospective analysis of data from the Multicentre Automatic Defibrillator Implantation Trial II (MADIT II) and Sudden Cardiac Death in Heart Failure Trial (SCD-HEFT) studies, in which ICD implantation was performed for primary prophylaxis of sudden cardiac death in patients with CAD and an ejection fraction <30-35%, respectively. In these studies, ICD implantation did not reduce sudden death if revascularization had been performed within 6 months (MADIT II)608 or 2 years (SCD-HEFT)529 prior to ICD implantation. Finally, the STICH trial, which investigated the effect of revascularization (CABG) in patients with reduced LV function (<35%) revealed a non-significant trend towards lower overall mortality in the CABG group but a significant benefit in cardiovascular endpoints (e.g. death from cardiac causes including sudden death).112 Because of the protective effect of revascularization of ventricular arrhythmias, patients with ischaemic LV dysfunction (LVEF <35%) who are considered for primary preventive ICD implantation should be evaluated for residual ischaemia and for potential revascularization targets.
Since revascularization by CABG led to a 46% risk reduction of sudden cardiac death in the SOLVD study, and in view of the low risk for sudden cardiac death within 2 years after revascularization in MADIT-II, reassessment of LV function up to 6 months after revascularization may be considered before primary preventive ICD implantation in patients with CAD and LVEF <35%. This is based on the observation that reverse LV remodelling and improvement of LV function may occur up to 6 months after revascularization procedures.530,531
15.1.2 REVASCULARIZATION FOR TREATMENT OF ELECTRICAL STORM
Electrical storm is a life-threatening syndrome related to incessant ventricular arrhythmias, which is most frequently observed in patients with ischaemic heart disease, advanced systolic heart failure, valve disease, corrected congenital heart disease, and genetic disorders such as Brugada syndrome, early repolarisation and long-QT syndromes. In the MADIT-II study, the occurrence of interim post-enrolment ischaemic events (angina or myocardial infarction) was independently predictive of the electrical storm, although there was no close correlation between the timing of the two.532 Urgent coronary angiography and revascularization should be part of the management of patients with electrical storm, as well as antiarrhythmic drug therapy and/or ablation of ventricular tachycardia.
15.1.3 REVASCULARIZATION AFTER OUT-OF-HOSPITAL CARDIAC ARREST
Approximately 70% of survivors of out-of-hospital cardiac arrest have CAD, with acute vessel occlusion observed in 50%.533 Multiple non-randomized studies suggest that emergency coronary angiography and PCI after out-of-hospital cardiac arrest yields a favourable survival rate of up to 60% at 1 year, which is considerably higher than the 25% overall survival rate in patients with aborted cardiac arrest.534,535 More recent data suggest that almost one-quarter of patients, resuscitated from cardiac arrest but without ST-segment elevation, show a culprit lesion (either vessel occlusion or irregular lesion).536,537 Notably, in the prospective Parisian Region Out of Hospital Cardiac Arrest (PROCAT) registry, 96% of patients with STEMI and 58% without STEMI after out-of-hospital cardiac arrest revealed at least one significant coronary artery lesion, and hospital survival rates were significantly higher if immediate PCI was performed successfully.538,539 Thus, in survivors of out-of-hospital cardiac arrest, early coronary angiography and PCI –if appropriate– should be performed irrespective of the ECG pattern if no obvious non-cardiac cause of the arrhythmia is present.540
15.2 ATRIAL ARRHYTHMIAS
15.2.1 ATRIAL FIBRILLATION COMPLICATING PERCUTANEOUS CORONARY INTERVENTION
New-onset AF in patients undergoing PCI occurs in 2-6% of procedures and increases with age, pre-existing heart failure, acute myocardial infarction and arterial hypertension.541-544 Notably, new-onset AF (defined as change from sinus rhythm at admission to AF during/ after PCI) typically occurs during the first 4 days after acute myocardial infarction and is associated with impaired prognosis, more than doubling the risk of death, congestive heart failure and stroke.
The use of oral anticoagulation in addition to antiplatelet therapy appears to decrease the risk of stroke after PCI as found in observational studies.543,545,546 Information on the duration of new-onset AF after PCI is scarce but most of these episodes are probably of paroxysmal nature or are terminated by cardioversion during the hospital stay. It is not clear whether AF represents an independent risk factor for cardiovascular events after PCI, or merely mirrors the severity of underlying heart disease. Antithrombotic treatment for stroke prevention, in patients with AF occurring during or after PCI, should follow the guidelines for antithrombotic treatment of AF that occurs outside the setting of PCI, although prospective studies are scarce (see section 18). A potentially higher bleeding risk in this patient population should be assessed as outlined in the ESC Guidelines for AF.547
15.2.2 ATRIAL FIBRILLATION COMPLICATING CORONARY ARTERY BYPASS GRAFTING
Continuous telemetry during the entire hospital stay revealed that new-onset post-operative AF may occur in one-third of patients undergoing isolated CABG.548 The presence of post-operative AF after CABG is independently associated with increased cardiac morbidity and mortality, prolonged hospitalization, increased healthcare expenditure, and poor long-term prognosis.549,550 Several attempts to prevent and manage post-operative AF have been evaluated, including magnesium, statins, steroids and antioxidative drugs.547
Pre-operative anti-arrhythmic drug treatment may be initiated but will have to be weighed against side-effects. Beta-blockers significantly decrease the risk of AF after CABG.551-557 Because beta-blockers are effective for prevention of post-operative AF and can be applied with low risk, they are recommended for decreasing the incidence of AF after CABG; however, beta blockers may be discontinued after CABG if AF was not present and other reasons for beta-blockade do not apply (e.g. reduced LV systolic function). The optimum treatment period before discontinuing beta blockade is unknown but a three-month period seems reasonable, given the fact that the occurrence of post-operative AF declines rapidly after CABG.631
Amiodarone is effective in preventing post-operative AF,552,558,559 but may cause bradycardia.
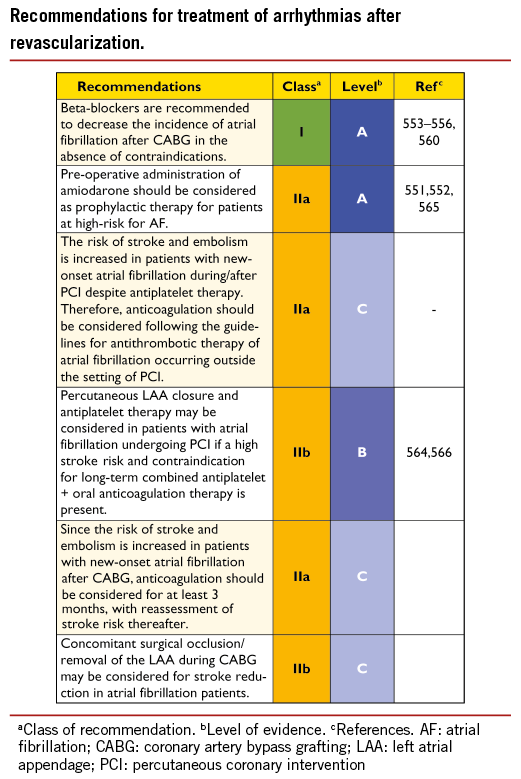
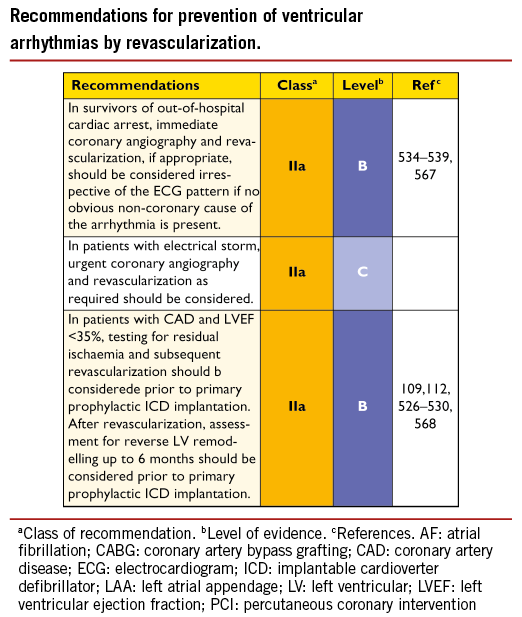
15.2.3 POST-OPERATIVE ATRIAL FIBRILLATION AND STROKE RISK
Post-operative AF carries a two- to fourfold increased risk for embolic events. A recent analysis of >16 000 patients undergoing CABG revealed that oral anticoagulation, initiated at discharge in 20% of patients with post-operative AF, led to a 22% relative risk reduction for death.560 In patients with post-operative AF, the cumulative risk for embolic death increases during the first year after CABG and continues to increase until 2 years after surgery before plateauing, thus indicating that stroke risk in CABG patients with post-operative AF is not just a perioperative issue. Antithrombotic treatment for stroke prevention in patients with post-operative AF should follow the Guidelines for antithrombotic treatment of AF occurring outside the setting of CABG.547 Anticoagulation with heparin or non-vitamin K antagonist oral anticoagulants (NOAC) should be initiated if post-operative AF persists for more than 48 hours and should be maintained for at least 4 weeks after restoration of sinus rhythm; longer in the case of stroke risk factors.547 The absence of documented AF during follow-up –even on subsequent intensified monitoring for AF and stroke risk– should not necessarily result in withholding anticoagulation therapy in light of the high incidence of asymptomatic ‘silent’ AF episodes.561 There are no data on whether prophylactic intraoperative ablation of AF has an impact on the occurrence of post-operative AF.
15.3 CONCOMITANT SURGICAL PROCEDURES FOR ATRIAL FIBRILLATION OR STROKE TREATMENT
The original cut-and-sew ‘maze’ procedure for AF, described by Cox et al.,562 included removal or ligation of the left atrial appendage (LAA). In addition, a retrospective analysis demonstrated that surgical LAA occlusion independent of intraoperative AF surgery reduces the risk of stroke.563 Likewise, transcatheter LAA occlusion in the Watchman Left Atrial Appendage System for Embolic Protection in Patients with Atrial Fibrillation (PROTECT AF) trial was non-inferior to oral anticoagulation with vitamin K antagonists in patients with AF.564 Whether surgical LAA obliteration (which does not employ a prosthesis in direct contact with the blood, thus potentially obviating the need for prolonged antiplatelet/ anticoagulation therapy) reduces stroke risk has not yet been investigated in randomized, prospective studies. Currently, concomitant surgical LAA obliteration may be considered to reduce stroke risk in CABG patients with a history of AF, but randomized studies are needed to further clarify this issue. Removal or closure of the LAA should be considered as an adjunct to anticoagulation and not as an alternative for anticoagulant therapy until more and longer-term data are available.
16. Procedural aspects of coronary artery bypass grafting
16.1 PRE-OPERATIVE MANAGEMENT
Most patients admitted for surgical revascularization are already medically treated with angiotensin-converting enzyme (ACE) inhibitors, statins, antiplatelet drugs, beta-blockers, and/or other antianginal drugs. Beta-blockers should not be stopped to avoid acute ischaemia and statins should be continued until surgery –or initiated if not previously introduced. Angiotensin-converting enzyme inhibitors might be discontinued 1-2 days before CABG, to avoid the potential deleterious consequences of perioperative hypotension.
The section on antithrombotic and antiplatelet therapy (section 18) will cover perioperative care around CABG relating to this particular aspect.
16.2 BLOOD MANAGEMENT
16.2.1 BLOOD SALVAGE INTERVENTIONS
There is strong evidence that use of cell-savers reduces allogenic blood product exposure (OR 0.63; 95% CI 0.43-0.94; P<0.02) but also reduces red blood cells and the mean volume of total allogenic blood products transfused per patient (P<0.002).569
16.2.2 PHARMACOLOGICAL STRATEGIES
Antifibrinolytic drugs are effective in reducing blood loss, the need for allogenic red blood cell transfusion, and the need for re-operation due to continued post-operative bleeding in cardiac surgery.570 Lysine analogues (e.g. tranexamic acid) are effective and relatively free from serious adverse events.
16.2.3 BLOOD TRANSFUSION
There is ample evidence that the number of transfused red blood cell units is an independent risk factor for worse outcomes after cardiac surgery.571,572 Transfusion trigger to a target haematocrit of around 24% is as safe as a liberal strategy of 30% with respect to 30-day mortality and complications.573 Platelet transfusion should be considered in patients recently exposed to P2Y12 inhibitors if there are clinical signs of poor haemostasis.
16.3 SURGICAL PROCEDURES
16.3.1 CONDUIT HARVEST SAPHENOUS VEIN HARVEST
Saphenous vein harvest can be accomplished using open and endoscopic techniques. Endoscopic vein graft harvesting, as well as radial artery harvesting, have been introduced into clinical practice in the past decade. While a reduced rate of leg wound infection and impaired wound healing are well documented in almost all studies, short- and long-term patency of endoscopically harvested vein grafts, compared with openly harvested grafts, has been challenged.574,575 Although there is no unequivocal evidence concerning patency rates, most recent data from meta-analyses and randomized and non-randomized trials do not demonstrate inferior clinical outcomes with endoscopic vein harvest.576-579 Endoscopic vein graft harvest should be undertaken by experienced surgeons or physician assistants with appropriate training and reasonable caseload.580-582 Endoscopic radial harvesting is likewise possible but robust clinical-scientific evidence concerning its safety and efficacy is scarce.583 If performed ‘open’, the ‘no-touch’ SVG harvesting technique may reduce graft injury and improve patency.584,585
Mammary artery harvesting
Internal mammary arteries are dissected from the chest wall, either as a pedicle or as an isolated (skeletonized) vessel. While the skeletonized technique has a higher theoretical potential for injury during harvest, the benefits include a longer conduit, more versatility (sequential anastomosis), higher blood flow and, most importantly, fewer wound healing problems.586-590
16.3.2 CORONARY VESSEL
Coronary artery bypass grafting aims at revascularizing coronary arteries with a flow-reducing luminal stenosis, supplying a viable and sizable area that is otherwise at risk.
The patency of a bypass graft is influenced by the characteristics of the anastomosed vessel, the run-off area, the graft material, its manipulation, and its construction.1 Important coronary characteristics are the internal lumen size, the severity of proximal stenosis, the quality of the wall at the site of anastomosis, and the distal vascular bed.
16.3.3 COMPLETENESS OF REVASCULARIZATION
Ideally, a generally accepted definition of completeness of myocardial revascularization would comprise (i) the size of the vessel, (ii) the severity of the lesion, (iii) the ischaemic burden caused by the lesion and (iv) the viability of the depending myocardial territory.591-593 Current surgical practice is based on an anatomical definition of complete revascularization, defined as bypass grafting to all epicardial vessels ≥1.5 mm with a diameter reduction ≥50% in at least one angiographic view.594 However, in other clinical trials, several different definitions of completeness of revascularization have been used. Coronary artery bypass graft patients with incomplete revascularization had an outcome that was either similar595-599 or inferior594,598,600,601 to that of patients with complete revascularization. A pivotal interventional study has shown superior results from FFR-guided functionally complete revascularization than those obtained by anatomically complete revascularization by PCI.50 Currently, however, these results cannot be extrapolated to this group of CABG patients.53
16.3.4 CONSTRUCTION OF CENTRAL ANASTOMOSIS
Use of in situ grafts, still connected to their native take-off (LIMA, right IMA, right gastroepiploic artery) avoids the need for a proximal anastomosis. If free conduits (vein grafts, radial artery) are used, additional central anastomosis for arterial inflow into the bypass vessels is utilized in the majority of cases. Partial or total aortic cross-clamping allows the construction of central anastomoses to the ascending aorta. With a higher atherosclerotic risk profile, the likelihood of atherosclerotic changes in the ascending aorta increases and requires strategies that reduce or avoid manipulation. A single cross-clamp technique may be preferred to multiple manipulations, with the aim of reducing atheroembolic events, but a strict no-touch technique most effectively reduces embolization of atherosclerotic material.442 In this situation, grafts are anastomosed end-to-side in a Y- or T-shaped configuration to the IMAs, to facilitate arterial inflow. Devices for clampless aortic anastomoses are also available.
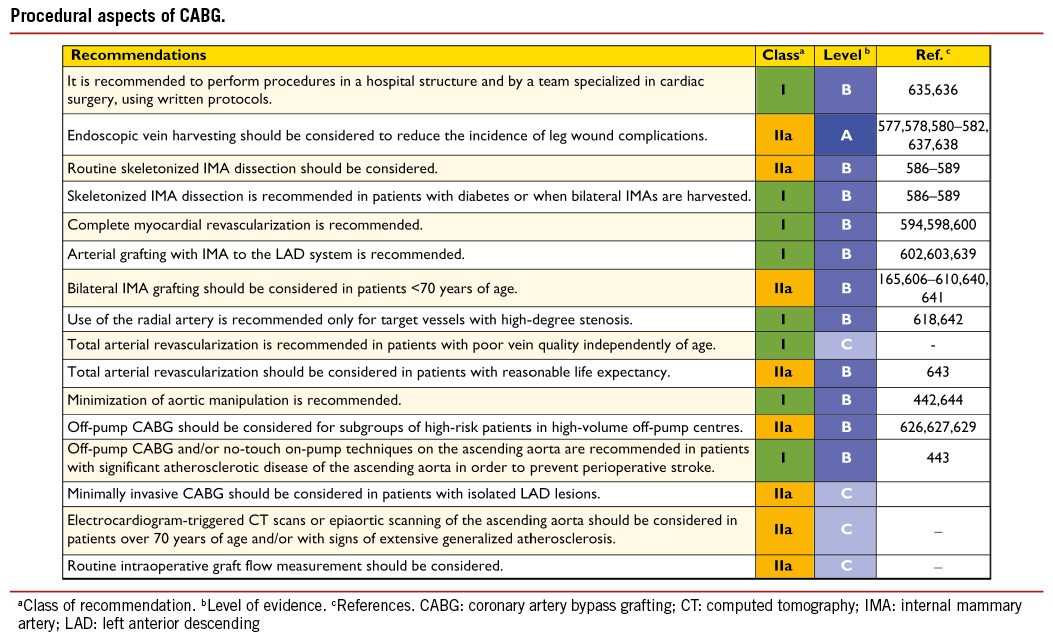
16.3.5 BYPASS GRAFTS
The long-term benefit of CABG is maximized with the use of arterial grafts, specifically the IMA.602,603 Available grafts include the IMA, radial, and gastroepiploic arteries, although the latter is seldomly used in current practice.17,18 Except in rare circumstances, almost all patients should receive at least one arterial graft –the LIMA– preferentially to the LAD.602,604
Data from non-randomized studies reveal unequivocally that the use of bilateral IMA is associated with improved long-term survival, as well as fewer non-fatal events such as myocardial infarction, recurrent angina, and need for re-operation.165,368,605-610 These advantages have also been demonstrated for diabetic patients. Conversely, BIMA grafting is associated with a small increase in sternal dehiscence and increased rate of mediastinitis; obese and diabetic patients being at particular risk.368,586,605,611-614 Thus BIMA grafting is recommended if life expectancy exceeds 5 years and to avoid aortic manipulation.
The radial artery constitutes a reasonable alternative as the second arterial graft, in patients in whom BIMA grafting is contraindicated (e.g. obese, diabetic, old women). Available evidence indicates its superiority (in terms of survival and non-fatal events) over the saphenous vein,615-617 but inferiority to use of the IMA.606 This patency is strongly related to target vessel size and severity of stenosis. Numerous studies have demonstrated a strong, adverse influence on radial artery patency when the native coronary artery stenosis is <70%.618 Furthermore, using radial artery grafts increases the number of arterial anastomoses beyond the use of both IMA and helps to achieve total arterial revascularization.
Graft flow measurement may be useful in confirming or excluding a technical graft problem indicated by haemodynamic instability or inability to wean the patient from cardiopulmonary bypass, new regional wall motion abnormalities on transoesophageal echocardiography, or ventricular arrhythmias.619 It has also been shown to reduce the rate of adverse events and graft failure, although interpretation can be challenging in sequential grafts and T-grafts.619,620
16.3.6 ON-PUMP AND OFF-PUMP PROCEDURES
Despite improved techniques and experience, part of the morbidity related to CABG is caused by the extracorporeal circulation (cardio-pulmonary bypass) and access for cardiopulmonary bypass, prompting the off-pump approach. Two recent large, international, randomized trials have shown no difference in 30-day or 1-year clinical outcomes between on- and off-pump surgery, when performed by experienced surgeons.441,621,622 There is also enough evidence to conclude that, for most patients and surgeons, on-pump CABG provides the best –or equal– short- and long-term outcomes.621-625 For some surgeons, off-pump CABG is associated with inferior early and late graft patency rates and possibly compromised long-term survival; however, complete off-pump procedures in the hands of highly trained teams appear to be associated with a reduced risk of early morbidity, such as stroke, wound and respiratory infections, as well as fewer transfusions and shorter hospital stay.626-629 In the subgroup of patients with end-stage CKD, there is some evidence that off-pump CABG is associated with lower in-hospital mortality and need for new renal replacement therapy.380 In the subgroup of patients with atherosclerotic changes of the ascending aorta, a no-touch technique –avoiding any manipulations of the ascending aorta either on- or off-pump– is essential to reduce the risk of stroke.443 The consistent cross-over rate of around 5% from on-pump CABG to off-pump CABG in high-quality RCTs suggests the necessity of routine ECG-gated CT scans of the thoracic aorta before bypass surgery in patients over 70 years of age or those with other risk factors for extensive atherosclerosis.
16.3.7 MINIMALLY INVASIVE PROCEDURES
Minimally invasive direct coronary artery bypass may represent an attractive alternative to a sternotomy.630 It has a similar safety and efficacy profile to conventional on- and off-pump CABG, with markedly reduced post-operative length of stay and an early quality-of-life benefit, although spreading of the ribs is associated with increased post-operative pain.631-633
16.4 REPORTING PERIOPERATIVE OUTCOME
Perioperative reporting of outcome after CABG procedures should be done on a risk-adjusted basis. Early clinical outcome at 3 months after CABG is characterized by a 1-2% mortality rate and a 1-2% morbidity rate for each of the following events: stroke, renal, pulmonary and cardiac failure, bleeding, and wound infections. The early risk period after CABG extends up to 3 months, is multifactorial, and depends on the interface between technical variability and patient comorbidity.634
17. Procedural aspects of percutaneous coronary intervention
17.1 PERCUTANEOUS CORONARY INTERVENTION DEVICES
17.1.1 BALLOON ANGIOPLASTY
Plain balloon angioplasty has been displaced in the treatment of de novo coronary lesions after demonstration of the superiority of BMS and, more recently, DES in terms of repeat revascularization.645 Its contribution to the treatment of in-stent restenosis has also diminished after recent studies demonstrated the advantages of DES and drug-coated balloons for this indication.505,511 However, balloon angioplasty might be a valuable PCI option in all patients in whom implantation of stents is technically not achievable, or in a vessel that is too small to be stented (<2.0 mm), and in patients with critical stenoses who require urgent surgery.
17.1.2 CORONARY STENTS
Bare-metal stents
Coronary stents are very effective in repairing dissections and have eliminated the need for urgent CABG due to abrupt vessel closure. Fully covered stents can be life-saving in the rare event of coronary perforation. The contribution of BMS is its approximately 30% lower rate of restenosis than with plain balloon angioplasty.645 Although many efforts have been made to further reduce restenosis by modification of stent design and materials, thinning of stent struts has been the only proven modification capable of reducing restenosis of BMS.646,647 Bare-metal stents have been associated with favourable outcomes in terms of mortality, myocardial infarction, and stent thrombosis.124 However, owing to a 20-30% rate of recurrence of angiographic stenosis within 6-9 months after implantation, restenosis with BMS has often been referred to as the ‘Achilles’ heel’ of PCI.645 There is no indication for BMS over new-generation DES, irrespective of patient and lesion subset. Similarly, there is no clear evidence of a difference between DES and BMS in the risk of stent thrombosis following unplanned disruption of DAPT.648
Early-generation drug-eluting stents
The risk of restenosis with BMS led to the development of DES, which consist of a metallic stent platform with controlled release of antiproliferative drugs, mostly controlled by surface polymers. Early-generation DES released sirolimus (e.g. Cypher®)649 or paclitaxel (e.g. Taxus®).650 Both in native vessels and saphenous vein bypass grafts, DES potently reduced angiographic and ischaemia-driven TVR.124,495 Thus, the risk of clinical restenosis with the use of early-generation DES was 50-70% lower than with BMS, corresponding to a number-needed-to-treat of approximately 7-8.124 In RCTs, no significant differences were observed in the long-term rates of death or myocardial infarction after use of DES or BMS.124,199 Despite the superior anti-restenotic efficacy of early-generation DES over BMS, concerns have been generated by studies showing an increased propensity for very late stent thrombosis.244,651,652 Although early-generation DES represented an important advance in the field of PCI,653 they currently play an irrelevant role in the treatment of CAD and are largely supplanted by new-generation DES.3
New-generation drug-eluting stents
New-generation DES are characterized by thin-strut, metallic platforms that release limus-based antiproliferative drugs from durable polymers with improved biocompatibility and lower polymer mass,654,655 biodegradable polymers,654,656-658 or polymer-free surfaces.659,660 Recent studies have shown the superiority of several new-generation DES over early-generation DES, not only with respect to efficacy but also safety.128,129,661,662 New-generation DES have addressed previous concerns of very late stent thrombosis and are at least as safe as bare-metal stents during long-term follow-up. Table 10 displays a list of Conformité Européenne (CE)-approved new-generation DES, supported by RCT evidence with clinical endpoints. Table 11 shows a list of CE-approved new-generation DES, the proven efficacy of which was based on angiographic findings from studies with or without a control group. These tables only provide a temporary ‘snapshot’ of available products, as new devices will be introduced or new evidence of established devices will become available.
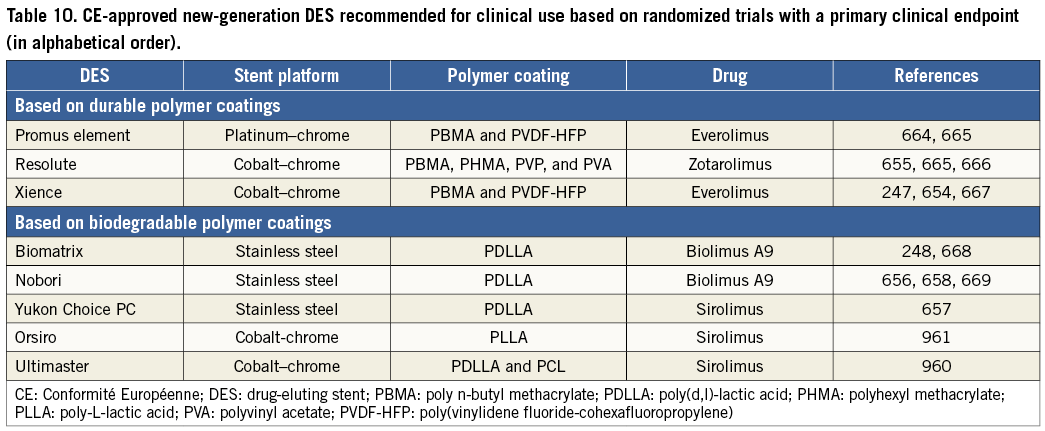
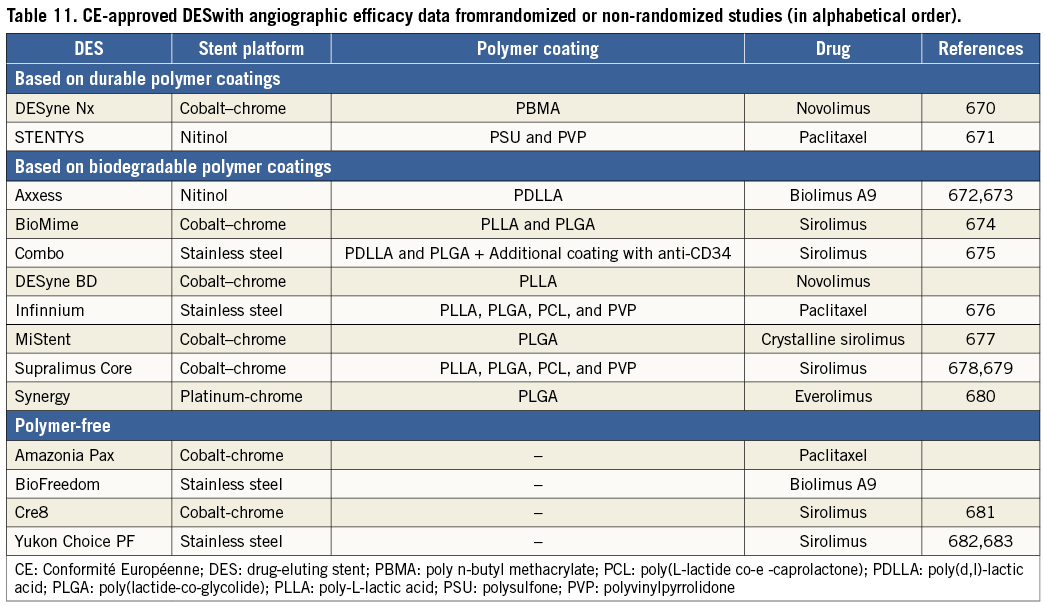
Indications for new-generation DES
Increased efficacy and safety of new-generation DES have enabled their unrestricted use in patients with CAD and an indication for PCI, including patients with diabetes, multivessel and LM disease, acute myocardial infarction, SVG and restenotic lesions, and chronic total occlusions.3 New-generation DES should therefore be considered by default in all clinical conditions and lesion subsets. Among patients who require anticoagulation with NOACs, undergo non-cardiac surgery, experience bleeding complications, or are non-compliant with medication intake, previous concerns relating to differences in the duration of DAPT and risks associated with DAPT cessation are not substantiated in recent data sets.648,663
17.1.3 BIORESORBABLE STENTS
Completely bioresorbable stents, which dissolve after fulfilling their support function in the lesion site of the coronary vessel, have been a perennial aim since the introduction of the metallic stents. The combination of resorbable stent platforms with drug-eluting properties has enhanced the efficacy of these devices. Current stent platforms are based on two technologies: the manufacturing of drug-eluting, bioresorbable, polymer-based stents and drug-eluting, resorbable, metallic (magnesium) stents.684 The resorption process of the stent platforms takes from several months to 2 years, depending on polymer composition. To date, bioresorbable stents have been shown to dissolve completely over time, to restore the vasomotion of treated segments, and to result in positive remodelling with late lumen enlargement. In small series of patients with relatively simple lesions, early results are promising and appear to be similar to newgeneration DES.685-687
However, confirmation in large-scale RCTs is required to establish the indications for these devices.
Table 12 includes the list of devices approved for use in Europe.
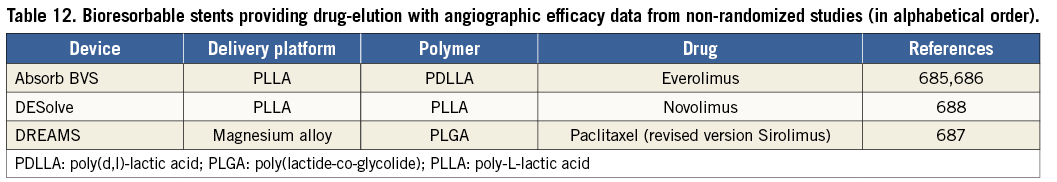
17.1.4 DRUG-COATED BALLOONS
The rationale of using drug-coated balloons is based on the concept that, with highly lipophilic drugs, even short contact times between the balloon surface and the vessel wall are sufficient for effective drug delivery. Using a paclitaxel-coated balloon, three RCTs, Paclitaxel-Coated Balloon Catheter I (PACCOCATH-I) and PAC- COCATH-II,507,508 and Paclitaxel-Eluting PTCA – Catheter In Coronary Disease (PEPCAD)-II,689 have targeted in-stent restenosis following BMS implantation, while three others have targeted in-stent restenosis in patients predominantly treated with DES eluting limus-analogues.509-511 By virtue of the positive results achieved without additional stent implantation, drug-coated balloons may represent an attractive option for patients with restenosis after implantation of DES, although it is not known whether they are as safe and effective for this indication as new-generation DES that elute limus analogues.
In the randomized PEPCAD III study, the combination of a drug-coated balloon with cobalt chromium stent implantation was inferior to a sirolimus-eluting stent for de novo indications.690 Also, the Drug Eluting Balloon in Acute Myocardial Infarction (DEB-AMI) trial showed that drug-coated balloons followed by BMS implantation were inferior to paclitaxel-eluting stents in patients with STEMI.691 A recent angiographic study suggested that drug-coated balloons may serve as an alternative to paclitaxel-eluting stents for the treatment of lesions in small coronary vessels;692 however, the role of drug-coated balloons in this setting has not been evaluated against more effective, new-generation DES with limus analogues. There are various types of drug-coated balloons approved for use in Europe and their main characteristics are listed in Table 13. Most of the differences are related to the drug carrier, whereas paclitaxel is currently the sole active drug used. Although specifically designed comparative studies are lacking, one cannot assume a class effect for all drug-coated balloons.693
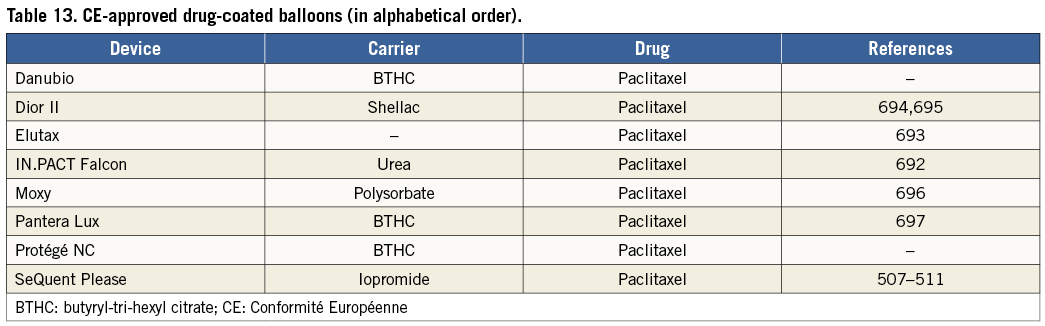
17.1.5 OTHER DEVICES
Although routine use of rotational atherectomy did not improve outcomes after DES,698 such a device might technically be required in cases of tight and calcified lesions, to allow subsequent passage of balloons and stents. There is a resurgence in the use of rotational atherectomy for the purpose of optimal lesion preparation among patients undergoing implantation of bioresorbable stents.
17.2 ADJUNCTIVE INVASIVE DIAGNOSTIC TOOLS
17.2.1 INTRAVASCULAR ULTRASOUND
Coronary angiography is unable to visualize the atherosclerotic involvement of the arterial wall. Intravascular ultrasound imaging allows a real-time, tomographic assessment of lumen area and plaque composition, size, and distribution. As a result of diffuse disease and remodelling, coronary angiography underestimates the extent and severity of the disease compared with IVUS.699 Although invasive by nature, IVUS is the established standard for accurate measurement of plaque burden, and the technique has been systematically used to determine the influence of different drugs on coronary plaque progression or regression.700,701
Several RCTs addressed the potential of IVUS in reducing restenosis and adverse events after BMS implantation –with conflicting results. Most of these RCTs focussed on optimizing stent expansion using IVUS. Findings from meta-analyses subsequently suggested that better clinical and angiographic results may be obtained under IVUS guidance.702-704 In the DES era, a threshold of stent expansion (5.0-5.5 mm2) was proposed to predict the occurrence of late events. In the subset of patients with LM disease, observational studies suggest that IVUS-guided stent implantation is associated with improved survival during long-term clinical follow-up.705 The use of intracoronary imaging has also been advocated in patients with stent failure, including restenosis and stent thrombosis, in order to explicate and correct underlying mechanical factors. In a multicentre all-comers study to establish the frequency, predictors, and timing of stent thrombosis, a pre-specified substudy compared outcomes of IVUS against angiographic guidance of DES implantation.706 IVUS-guided DES implantation (pre- and post-PCI in 63% of included cases) was performed in 3349 of 8583 patients (39%). In propensity- adjusted multivariable analysis, IVUS guidance was associated with reduced rates of definite or probable stent thrombosis (adjusted HR 0.40; 95% CI 0.21-0.73; P=0.003), myocardial infarction (adjusted HR 0.66; 95% CI 0.49-0.88; P=0.004), and MACE (adjusted HR 0.70; 95% CI 0.55-0.88; P=0.003) at 1 year. Notable limitations of this study were the lack of randomization and lack of pre-specified guidelines for performing and acting on IVUS findings.
In addition to conventional grey-scale IVUS, other ultrasound-based techniques have been used to provide additional diagnostic insights. Assessment of plaque composition may be further improved by analysis of the complete radiofrequency signal using different diagnostic algorithms, including those used in ‘virtual histology’.
17.2.2 OPTICAL COHERENCE TOMOGRAPHY
Optical coherence tomography is a light-based modality of intravascular imaging with higher spatial resolution than IVUS (15 vs. 150 mm) and is ideally suited to accurate detection of intraluminal structures. Plaque composition, including the presence of lipid pools and intraluminal thrombi, can also be determined.707 Notably, this is the only technique capable of providing accurate measurements of the thickness of the fibrous cap and to detect even minor cap disruptions.707,708 Early stages of cardiac allograft vasculopathy are frequently angiographically silent, yet can be visualized with OCT or IVUS and are associated with important prognostic implications.708 Optical coherence tomography requires complete blood clearance from the lumen for imaging, has a limited penetration on the vessel wall and is therefore unable to assess the complete plaque burden. After stent implantation, OCT is more accurate than IVUS in detecting subtle morphological details including malapposition, residual thrombus, plaque prolapse, and residual dissections, although the clinical significance of these findings remains to be determined.709,710 During longitudinal follow-up investigations, OCT is more accurate than IVUS for assessing even neointimal thickness, strut apposition, and coverage. These findings are important surrogate markers of the efficacy and safety of DES and are frequently used to compare new DES. A recent retrospective and observational study suggested that OCT-guided stenting might improve clinical outcomes.711 Owing to its very high resolution, OCT is used to reveal the underlying mechanisms in patients with stent failure, including in-stent restenosis and stent thrombosis.516 Likewise, intrastent neointimal tissue may be characterized, including the detection of neoatherosclerosis, which represents a potential link between in-stent restenosis and stent thrombosis.516,712 Further studies are needed to define the clinical value of OCT.
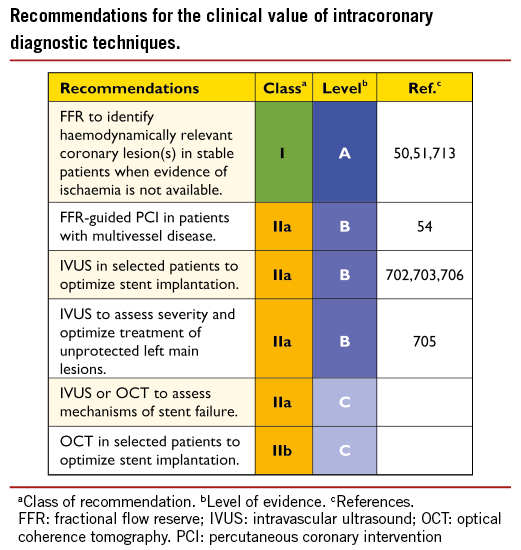
17.2.3 PRESSURE-DERIVED FRACTIONAL FLOW RESERVE
Fractional flow reserve is the current standard of care for the functional assessment of lesion severity.713 Imaging techniques provide useful information (i.e. minimal lumen area) but FFR is able to provide a physiological assessment. Initial studies suggested that the cut-off figure of 0.75 was reliable for identifying ischaemia-producing lesions, but subsequently the 0.80 criterion has gained widespread acceptance and its clinical role has been validated in outcome studies. Fractional flow reserve evaluation is valuable in patients undergoing diagnostic coronary angiography without prior non-invasive functional testing in the presence of borderline lesions and in patients with multivessel disease. The concept of avoiding unnecessary treatment of lesions that are not haemodynamically relevant was demonstrated in the DEFER and Fractional Flow Reserve Vs. Angiography for Multivessel Evaluation (FAME) trials.50,51 More recently, the FAME II trial demonstrated that, in patients with SCAD, FFR-guided PCI using DES resulted in less need for urgent revascularization than with medical treatment.54 While FFR requires maximal and stable hyperaemia –usually obtained by intravenous adenosine– new methods and indices [including instantaneous wave-free ratio (iFR)] that do not rely on the concept of maximal hyperaemia have been proposed, in order to simplify studies and facilitate a wider adoption of physiological assessment. Further studies will need to confirm the value of these new indices in clinical decision-making.714 Fractional flow reserve can also be ascertained along the entire coronary tree using the anatomical information obtained by multislice CT.715,716
Although appealing, owing to its non-invasive nature, CT-derived FFR requires further clinical validation before its clinical use may be justified.
17.3 SPECIFIC LESION SUBSETS
17.3.1 BIFURCATION STENOSIS
Bifurcation lesions are common and represent 10-15% of all coronary interventions.717 Coronary bifurcation lesions are defined as stenosis of a main branch at the origin of a side branch, with or without lesions extending into the ostium of the side branch. They are best described according to the Medina classification, which uses the three components of a bifurcation: the main branch proximal, the main branch distal, and the side branch, giving a binary value (1 or 0) according to whether or not each of the segments previously defined is compromised.29
PCI of bifurcation lesions is technically challenging, owing to multiple factors that include anatomical variability related to bifurcation site, plaque burden and morphology, bifurcation angle, and branch diameter.718-724 Also, bifurcation anatomy may have dynamic variability during PCI, with plaque shift or dissection causing side-branch occlusion and requiring adjustments in the interventional approach.720
Despite many attempts with a variety of different stenting techniques (T-stenting, V-stenting, crush and its modifications, culotte, etc.), the optimal strategy for every anatomical subset has not yet been established. Variables to be considered are plaque distribution, size, and downstream territory of each vessel (main and side branch), and the bifurcation angle. Stent implantation in the main vessel only, followed by provisional balloon angioplasty with or without stenting of the side branch, seems preferable to routine stenting of both vessels,725,726 although some studies have reported similar or improved results with specific strategies of complex stenting.727-732 Fractional flow reserve data from side branches suggest that angiography overestimates the functional severity of side-branch stenosis. Final ‘kissing’ balloon dilation is recommended when two stents are eventually required, with no advantage from final kissing with the one-stent technique.733,734 Several stents, designed specifically for treatment of bifurcation lesions, have undergone extensive evaluation with good angiographic and clinical results, especially with side branch size >2.5 mm.
Percutaneous coronary intervention for left main bifurcations
Significant unprotected LM disease is observed in 5-7% of patients undergoing coronary angiography. For bifurcation and LM lesions, DES are preferred, with special attention to adequate sizing and deployment. Unprotected distal LM bifurcation PCI is a challenging percutaneous procedure and has worse long-term clinical outcome than the favourable results obtained with ostial- or shaft-LM lesions.735,736 There are few systematic data supporting a specific stenting technique for LM bifurcation lesions.737
17.3.2 CHRONIC TOTAL CORONARY OCCLUSION
Chronic total occlusion is defined as complete vessel occlusion with TIMI 0 flow within the occluded segment and an estimated occlusion duration of ≥3 months.738 In a consecutive series of patients without previous CABG surgery or recent myocardial infarction, who underwent angiography, totally occluded vessels were observed in 25% of cases.739 Patients with CTO underwent PCI less frequently than those without CTO (11% vs. 36%, respectively; P<0.0001) but were more frequently assigned to CABG or medical therapy.739
Treatment of CTOs should be considered in the presence of symptoms or objective evidence of viability/ischaemia in the territory of the occluded artery. Given the usually high procedural contrast volume, the potential long-term risk of radiation exposure and contrast-induced nephropathy should be considered. Ad hoc PCI is not recommended for CTOs. Observational studies suggest that successfully revascularized CTOs confer a long-term survival advantage over failed revascularization.740-742,743,744 In addition, better relief of angina and functional status was observed after successful CTO recanalization.745 In the post hoc analysis of 4-year results of the SYNTAX trial, the presence of CTO was the strongest independent predictor of incomplete revascularization (46.6% in the PCI arm), and had an adverse effect on clinical outcomes, including mortality.594
The procedural success rate is lower for PCI of CTO than for non-CTO lesions, with a similar rate of complications.746,747 In a meta-analysis of 13 studies encompassing 7288 patients, recanalization was successful in 69% of cases (ranging from 51-74%).743 Success rates are strongly dependent on operator skills, experience with specific procedural techniques, and the availability of dedicated equipment (specialized guide wires and catheters or very low profile CTO balloons). Bilateral angiography and IVUS can be very helpful, as can special techniques such as guide-anchoring, various retrograde approaches, and specific wiring manipulation techniques, including parallel or anchoring wire.748 A retrograde approach via collateral pathways offers an additional possibility of success after failure of antegrade crossing, especially for right coronary artery and LAD occlusions.749 In general, this technique is not regarded as a first-line approach and is generally reserved for previous failed attempts. The overall success rate with the retrograde approach in a multicentre registry of 175 patients was 83.4%.750
In recently published systematic reviews and one RCT with long-term follow-up, DES provided superior clinical outcome to BMS, mainly due to a lower risk of revascularization.751-754
17.3.3. OSTIAL LESIONS
Ostial disease is defined as a lesion arising within 3 mm of the vessel origin. It may be classified by location as aorto-ostial, non-aorto-ostial, or branch-ostial.755 Coronary ostial lesions are frequently not a manifestation of coronary atherosclerosis, but rather related to aortitis or radiation exposure.756-758
Ostial lesions are usually recognized as fibrotic, calcified, and relatively rigid.759,760 Aorto-ostial disease is resistant to dilation and prone to recoil, due to the greater thickness of muscular and elastic tissue in the aortic wall.755 Coronary stents–particularly DES– have improved procedural efficacy and safety.
In ostial coronary lesions, additional judgement and caution is essential before proceeding to PCI:755
(1) In aorto-ostial lesions coronary spasm has to be absent;
(2) In ostial LAD or LCx stenoses, a decision must be made on whether to attempt precise positioning of the stent at the ostium of the artery or whether stenting across the LCx/ LAD ostium into the LM artery is preferable.
Lesion assessment with IVUS may be helpful, particularly in LM ostial stenosis, including assessment of the degree of calcification, need for adjunctive devices and assessment of stent expansion. Fractional flow reserve measurement may also be valuable in the assessment of angiographically borderline aorto-ostial and side-branch ostial lesions,761 taking special care to avoid a wedge position of the guiding catheter and using intravenous, rather than intracoronary, adenosine.
Proper selection of the guiding catheter is important in aorto-ostial lesions, to avoid deep intubation and compromise of coronary flow. Preparation and debulking of the lesion with rotational atherectomy and special balloons, cutting or scoring, may be useful in highly calcified, rigid ostial lesions.762-765
Drug eluting stents are the default devices for ostial lesions.
The accurate positioning of the stent, precisely in the coronary ostium, may be technically challenging and some specialized techniques have been described that achieve the optimal stent placement.766-768 Treatment of restenotic and saphenous vein graft lesions are discussed in section 14.
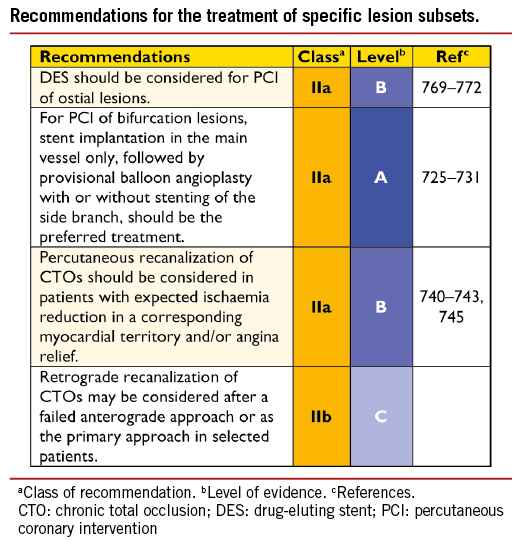
18. Antithrombotic treatments
The choice, initiation, combination, and duration of antithrombotic strategies for myocardial revascularization depend on the clinical setting [SCAD, NSTE-ACS, STEMI], and the urgency and mode (PCI vs. CABG) of the intervention. To maximize the effectiveness of therapy and reduce the hazard of bleeding, ischaemic and bleeding risks should be evaluated on an individual basis.
18.1 PERCUTANEOUS CORONARY INTERVENTION IN STABLE CORONARY ARTERY DISEASE
18.1.1 ORAL ANTIPLATELET THERAPY
Dual antiplatelet therapy includes a 150-300 mg oral loading dose of acetylsalicylic acid (ASA) (or 80-150 mg i.v.) followed by 75-100 mg per os (p.o.) daily plus a clopidogrel 300-600 mg loading dose followed by 75 mg daily.773-775 Acetylsalicylic acid acts via irreversible inhibition of platelet cyclo-oxygenase-1 (COX-1), which is normally complete with chronic dosing ≥75 mg/day. Contrary to the antiplatelet effects, the gastrointestinal side-effects of ASA increase at higher doses. The optimal risk-benefit ratio appears to be achieved with an ASA dosage of 75-150 mg/day.774,776
There is no evidence of benefit for systematic clopidogrel preloading before diagnostic coronary angiography in SCAD.777 A loading dose of 600 mg or more is recommended in patients scheduled for elective PCI if coronary anatomy is known. The use of a higher maintenance dose (150 mg) has been proposed in patients with high thrombotic risk (e.g. in diabetes, after recurrent myocardial infarction, after early and late stent thrombosis, for complex lesions, or in life-threatening situations should occlusion occur); however, no studies have established a short- or long-term benefit of a 150 mg daily maintenance dose. Specifically, the Gauging Responsiveness with A VerifyNow assay: Impact on Thrombosis And Safety (GRAVITAS) trial failed to show a benefit of doubling the clopidogrel maintenance dose in subjects deemed to be non-responders.778
Lifelong single antiplatelet therapy is recommended. Patients should be instructed not to prematurely discontinue oral antiplatelet therapy after stenting, due to the risks of stent thrombosis and myocardial infarction.774,779 Data from the Patterns of Non-Adherence to Anti-Platelet Regimens In Stented Patients (PARIS) registry indicate that cardiac events after cessation of DAPT depend on the clinical circumstances and reason for cessation and that they attenuate over time.648 Half of the cases in which treatment was discontinued within 2 years of stent implantation were due to a physician’s guidance, and did not result in any adverse effect. Disruptions due to bleeding or non-compliance represented 14% of the cessations and were associated with a substantially increased risk of MACE, although this association largely attenuated after 30 days. Although the overall contribution of DAPT cessation to cardiac risk was small –thereby challenging existing paradigms for extension of antiplatelet treatment in otherwise stable patients after PCI– these findings highlights the need for patient education.
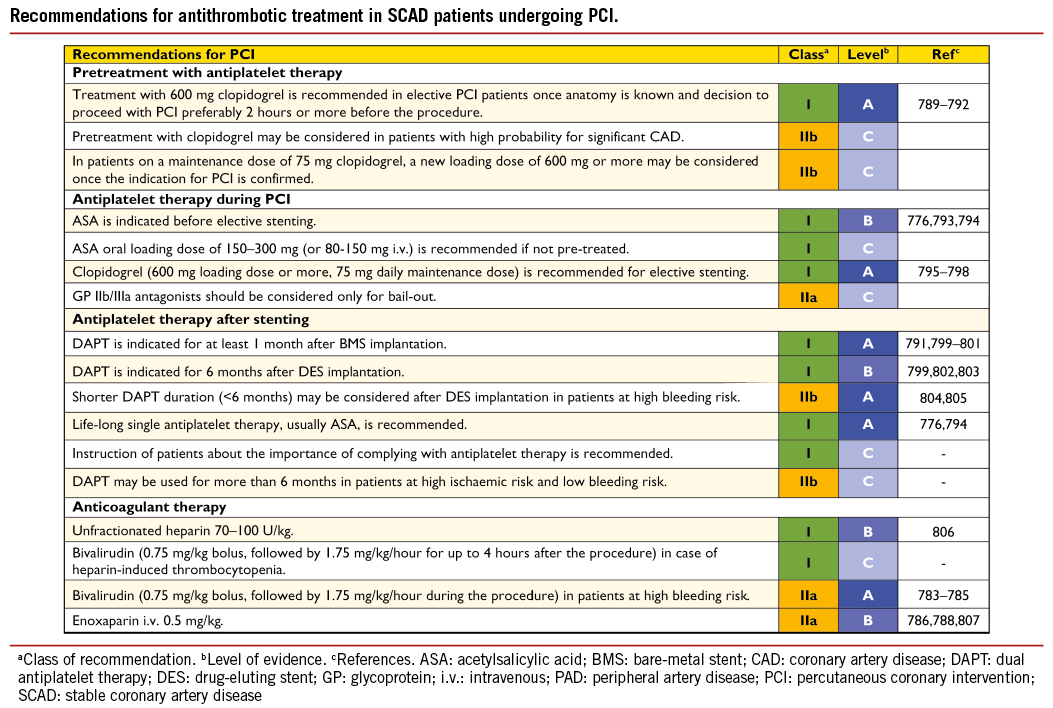
18.1.2 INTRAVENOUS ANTIPLATELET THERAPY
Recent trials did not demonstrate additional benefit from GP IIb/IIIa inhibitors after a clopidogrel loading dose of 600 mg.780-782 Anecdotal experience, however, suggests that GP IIb/IIIa inhibitors may be beneficial in ‘bail-out’ situations (intraprocedure thrombus formation, slow flow, threatened vessel closure).86 The use of cangrelor is reviewed in section 18.4.2.
18.1.3 ANTICOAGULATION
The Randomized Evaluation in PCI Linking Angiomax to Reduced Clinical Events (REPLACE)-2 trial demonstrated that outcome with bivalirudin and provisional GP IIb/IIIa blockade is similar to that of unfractionated heparin (UFH) plus planned GP IIb/IIIa inhibition during PCI for SCAD.783 Subsequently, Intracoronary Stenting and Antithrombotic Regimen – Rapid Early Action for Coronary Treatment (ISAR-REACT) 3, performed in patients pre-treated with clopidogrel, showed similar net clinical outcomes to bivalirudin and UFH,784 but UFH dosage was higher (140 U/kg) than recommended, leading to an excess in major bleeding in patients preferentially undergoing procedures via femoral access. In view of the primary endpoint results and a trend towards a lower risk of myocardial infarction, anticoagulation with UFH with an i.v. bolus of 70-100 U/kg remains the standard anticoagulant treatment for elective PCI. Among PCI patients with negative biomarkers, bivalirudin reduced bleeding without affecting mortality and might therefore be considered for use in patients at high risk for bleeding.785
The Safety and Efficacy of Intravenous Enoxaparin in Elective Percutaneous Coronary Intervention Randomized Evaluation (STEEPLE) trial has demonstrated lower bleeding with intravenous enoxaparin (0.5 mg/kg; P=0.01; 0.75 mg/kg; P=0.05) and 57% less major bleeding with both doses (P<0.01 for both), when compared with UFH with similar efficacy.786 Yet a significant benefit with respect to the primary endpoint was found only in the low-dose arm, which was stopped prematurely because of a non-significant trend towards excess mortality not related to ischaemic events and not confirmed at 1 year of follow-up.787 A recent meta-analysis confirmed the favourable safety profile.788
18.2 NON-ST-SEGMENT ELEVATION ACUTE CORONARY SYNDROME
High ischaemic risk is associated with dynamic ST-segment and troponin changes (primary indications), diabetes status, a GRACE score >140, LV function <40%, creatinine clearance <60 ml/min, recent PCI, and post-myocardial infarction angina (secondary indicators).180 Bleeding risk can be assessed using risk scores, which may remain valid despite the increased use of the radial route to perform PCI.808,809
18.2.1 ORAL ANTIPLATELET THERAPY
Dual antiplatelet therapy includes ASA with an oral loading dose of 150-300 mg (or 80-150 mg i.v.), followed by 75-100 mg p.o. daily, and a P2Y12-receptor antagonist, as discussed below.774
Prasugrel and ticagrelor
Prasugrel (60 mg loading and 10 mg daily maintenance dose), a prodrug that irreversibly blocks the P2Y12 platelet receptor with a faster onset and a more potent antiplatelet inhibition, has been tested in the TRial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet InhibitioN with Prasugrel – Thrombolysis In Myocardial Infarction 38 (TRITON-TIMI-38) trial against the 300 mg loading dose of clopidogrel –both started in the catheterization laboratory after diagnostic angiography in thienopyridine-naïve patients– and proved beneficial with respect to a composite ischaemic outcome.518 Patients with NSTE-ACS treated conservatively were not included in this study. Recurrent cardiovascular events were fewer in prasugrel-treated patients (from 11.2 to 9.3%; RRR 0.82; 95% CI 0.73-0.93; P=0.002), mostly driven by a significantly lower risk for myocardial infarction (from 9.2 to 7.1%; RRR 23.9%; 95% CI 12.7-33.7; P<0.001). Severe bleeding complications were more common with prasugrel than with clopidogrel (TIMI non-CABG major bleeding 2.4% vs. 1.8%, respectively; HR 1.32; 95% CI 1.03-1.68; P=0.03), driven mostly by an increase in spontaneous bleeds (1.6% vs. 1.1%, respectively; HR 1.51; 95% CI 1.09-2.08; P=0.01), but also in fatal bleeding (0.4% vs. 0.1%, respectively; HR 4.19; 95% CI 1.58-11.11; P=0.002). Bleeding was also increased in prasugrel-treated patients referred for early CABG. Excluding patients with a higher bleeding risk, prasugrel offers significant benefit over clopidogrel with respect to cardiovascular events (HR 0.74; 95% CI 0.66-0.84; P<0.001) without significantly increasing major bleeding (HR 1.24; 95% CI 0.91-1.69; P=0.17).518 In diabetic patients presenting with ACS, prasugrel confers a particularly greater treatment effect than clopidogrel, without significantly increased bleeding.337 Prasugrel should be considered in patients who present with stent thrombosis despite adherence to clopidogrel therapy.810 Prasugrel is contraindicated in patients with prior stroke or TIA. Treatment with prasugrel is generally not recommended for patients of ≥75 years of age. If, after a careful individual risk–benefit evaluation by the prescribing physician, treatment is deemed necessary in the ≥75 years age or low body weight (<60 kg) groups then, following a loading dose of 60 mg, a reduced maintenance dose of 5 mg should be prescribed.
Alternatively, ticagrelor can be administered.811 Ticagrelor [180 mg loading dose; 90 mg b.i.d. (twice daily) daily maintenance dose] a cyclo-pentyltriazolopyrimidine, is an oral, reversibly binding P2Y12 inhibitor with a plasma half-life of approximately 6-12 hours. The Study of Platelet Inhibition and Patient Outcomes (PLATO) study randomly assigned ACS patients –with or without prior loading with clopidogrel and irrespective of strategy (invasive vs. non-invasive)– to treatment with ticagrelor or clopidogrel and showed significantly superior results in favour of ticagrelor in the composite ischaemic endpoint (11.7% in the clopidogrel group and 9.8% in the ticagrelor group; HR 0.84; 95% CI 0.77-0.92; P<0.001) and mortality (from 5.1 to 4.0%, respectively; HR 0.79; 95% CI 0.69–0.91; P=0.001).341 Patients undergoing PCI, with moderate- to high-risk NSTE-ACS, were allowed to receive an additional blinded 300 mg loading dose of clopidogrel (total loading dose 600 mg) or its placebo after the initial loading dose. Those patients with a final diagnosis of non-ST-segment elevation myocardial infarction (NSTEMI) had a significantly lower primary endpoint result with ticagrelor than with clopidogrel (11.4% vs. 13.9%, respectively; HR 0.83, CI 0.73-0.94) in contrast to patients with a final diagnosis of unstable angina (8.6% vs. 9.1%, respectively; HR 0.96, CI 0.75-1.22). The rate of TIMI major non-CABG-related bleeding was similar to that with prasugrel in the TRITON-TIMI 38 trial and was higher, at 2.8%, in the ticagrelor group, than the 2.2% of the clopidogrel group (HR 1.25; 95% CI 1.03-1.53; P=0.03). TIMI major CABG-related bleeding occurred in 5.3% of the patients in the ticagrelor group and in 5.8% in the clopidogrel group. There was no difference in the overall rates of fatal haemorrhage (0.3% in both groups) despite a higher rate of fatal intracranial haemorrhage in the ticagrelor group (0.1% vs. 0.001%; P=0.02). Ticagrelor was associated with an increased rate of adverse effects including dyspnoea, increased frequency of ventricular pauses, and asymptomatic increases in uric acid.
Clopidogrel
Clopidogrel is a prodrug that is converted in active metabolites through a two-step reaction involving cytochrome P450 (CYP450) enzymes, leading to an irreversible blockade of the P2Y12 receptor. Compared with prasugrel and ticagrelor, this conversion results in a slower onset of action and a larger variability in oral bioavailability. The Clopidogrel and Aspirin Optimal Dose Usage to Reduce Recurrent Events - Seventh Organization to Assess Strategies in Ischemic Syndromes 7 (CURRENT-OASIS) 7 trial tested whether a double-dose regimen of clopidogrel (600 mg loading dose followed by 150 mg maintenance dose from day 2 to day 7, then 75 mg maintenance dose) was superior to a standard-dose regimen of clopidogrel (300 mg loading dose followed by 75 mg maintenance dose) in ACS patients (treated conservatively and invasively). Overall, the higher dose regimen was no more effective than the conventional dosage, with a similar 30-day rate of the composite endpoint of cardiovascular death, myocardial infarction, or stroke (4.2% vs. 4.4%, respectively; HR 0.94; 95% CI 0.83-1.06; P=0.30), but was associated with increased 30-day rates of TIMI major bleeding (1.7% vs. 1.3%; HR 1.26; 95% CI 1.03-1.54; P=0.03) and the need for blood transfusion (2.2% vs. 1.7%; HR 1.28, 1.07-1.54; P=0.01).519 The primary efficacy endpoint did not differ according to ASA dose (high vs. low) nor did the safety endpoint, major bleeding. When analysing the results from the pre-specified subgroup of 17 263 patients with ACS who underwent PCI, the double-dose regimen of clopidogrel led to 14% fewer cardiovascular events (3.9% vs. 4.5%; HR 0.86; 95% CI 0.74-0.99; P=0.039); however, the P-value for interaction was 0.03 and did not meet the pre-specified criterion (P<0.01) that rendered these results statistically significant. Therefore, the benefit was formally restricted to the 31% lower risk of stent thrombosis (1.6% vs. 2.3%, HR 0.69, 95 CI 0.56-0.87, P=0.001).812 Major bleeding was more common with double-dose than with standard-dose clopidogrel (1.6% vs. 1.1%; HR 1.41; 95% CI, 1.09-1.83; P=0.009). It is difficult to disentangle the impact of the chosen strategy of a short (1 week) treatment period with 150 mg. High-dose and low-dose ASA did not differ for the primary efficacy outcome (4.1% vs. 4.2%, respectively; HR 0.98; 95% CI 0.84-1.13; P=0.76) and the safety outcome major bleeding (1.5% vs. 1.3%; HR 1.18; 95% CI, 0.92-1.53; P=0.20). Based on these findings, the high-dose clopidogrel regimen of 600 mg loading dose and 150 mg maintenance dose in the first week may be considered only when prasugrel and ticagrelor are not available or if they are contraindicated.
18.2.2 INTRAVENOUS ANTIPLATELET THERAPY
In the era before DAPT, trials of adequately dosed GP IIb/IIIa inhibitors in patients undergoing balloon angioplasty and coronary stent implantation demonstrated a lower incidence of composite ischaemic events in favour of GP IIb/IIIa treatment in combination with UFH, than with UFH alone, primarily through a reduction in myocardial infarction.813 In the ISAR-REACT 2 trial, this benefit –according to the primary endpoint of death, myocardial infarction, or urgent TVR within 30 days– was maintained despite clopidogrel pre-treatment with a loading dose of 600 mg in patients with NSTEMI (13.1% vs. 18.3%; RR 0.71; 95% CI 0.54-0.95; P=0.02), but not in unstable angina without biomarker protein elevation (4.6% vs. 4.6%; RR 0.99; 95% CI 0.56-1.76; P=0.98).814
The ACUITY trial –which compared a regimen of bivalirudin alone (with bail-out GP IIb/IIIa inhibitors in 7.4%) against UFH plus GP IIb/IIIa inhibitors– found a significant benefit of bivalirudin alone with respect to the primary 30-day composite endpoint of ischaemic and bleeding complications (10.1% vs. 11.7%, respectively; RR 0.86; 95% CI 0.77-0.97; P=0.02), driven by a reduction in major bleeding complications (3.0% vs. 5.7%, respectively; RR 0.53; 95% CI 0.43-0.65; P<0.001) without a significant increase in ischaemic complications (7.8% vs. 7.3%, respectively; RR 1.08; 95% CI 0.93-1.24; P=0.32).815 This benefit of bivalirudin was found regardless of whether GP IIb/IIIa inhibitors were administered downstream or upstream and was maintained during 1-year follow-up.816 The more recent ISAR-REACT 4 trial in PCI patients with NSTEMI did not find a significant benefit of UFH with abciximab, compared with bivalirudin alone. The primary endpoint of death, recurrent myocardial infarction, urgent TVR, or major bleeding within 30 days occurred in 10.9% of patients in the heparin-plus-abciximab group, as opposed to 11.0% in the bivalirudin group (RR 0.99; 95% CI 0.74-1.32; P=0.94).817 However, heparin plus abciximab was associated with significantly more major bleeding than bivalirudin (4.6% vs. 2.6%, respectively; RR 1.84; 95% CI 1.10-3.07; P=0.02).
Consistent with ACUITY and ISAR-REACT 4, the EARLY-ACS trial did not confirm a benefit from upstream eptifibatide, with or without clopidogrel pre-treatment (9.3% vs. 10.0%, respectively; OR 0.92; 95% CI 0.80-1.06; P=0.23), but was associated with a higher bleeding risk with eptifibatide (TIMI major haemorrhage 2.6% vs. 1.8%, respectively; OR 1.42; 95% CI 1.07-1.89; P=0.02).357
In TRITON-TIMI 38, 7414 patients (54.5% of the total study population) received a GP IIb/IIIa inhibitor and, in terms of reducing the risk of cardiovascular death, myocardial infarction or stroke, a consistent advantage was observed from prasugrel when compared with clopidogrel, irrespective of the use of GP IIb/IIIa inhibitors (with GP IIb/IIIa inhibitors: HR 0.76; 95% CI 0.64-0.90; without GP IIb/IIIa inhibitors: HR 0.78; 95% CI 0.63-0.97; P-value for interaction 0.83). The risk of TIMI major or minor bleeding was not significantly different with either prasugrel or clopidogrel, regardless of whether or not patients were treated with GP IIb/IIIa inhibitors (P-value for correlation 0.19).818 Overall, there is no evidence for an additional benefit of routine upstream use of GP IIb/IIIa inhibitors in NSTE-ACS patients scheduled for coronary angiography.
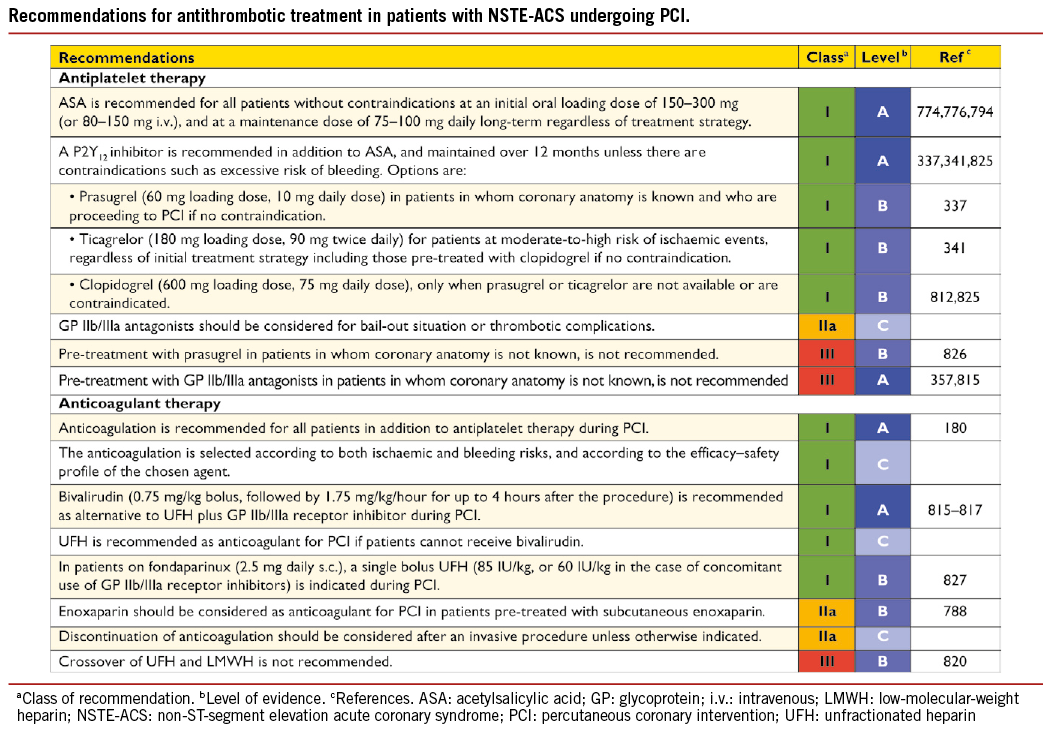
18.2.3 ANTICOAGULATION
A general rule is to avoid crossover between antithrombins (with the exception of adding UFH to fondaparinux) –especially between UFH and low-molecular-weight heparin (LMWH)819,820– and to discontinue antithrombins after PCI except in specific situations (e.g. LV aneurysm and/or thrombus, AF, prolonged bed rest, deferred sheath removal).
Among patients with high-risk ACS –as evidenced by positive biomarkers, ST-segment changes, or a GRACE risk score >140 with an intended urgent or early invasive strategy– bivalirudin plus provisional GP IIb/IIIa receptor inhibitors is recommended as an alternative to UFH plus GP IIb/IIIa receptor inhibitors, particularly in patients with a high risk of bleeding. ACUITY demonstrated the superiority of bivalirudin over UFH or low molecular weight heparin (LMWH) plus GP IIb/ IIIa inhibitor, a regimen previously shown to be superior to heparin alone.815 For patients with NSTEMI undergoing PCI, ISAR-REACT 4 presented additional evidence in favour of bivalirudin, with a better safety profile than the combination of UFH and abciximab. The use of bivalirudin preserves the option for bail-out GP IIb/IIIa inhibition.817 However, in lower-risk patients pre-treated with clopidogrel, bivalirudin does not appear to offer an advantage over heparin.821 We acknowledge that most of the evidence in support of bivalirudin is derived from trials in which the comparator was UFH plus GP IIb/ IIIa inhibitor, a combination that is no longer routinely applied.
A substantial number of patients will undergo catheterization after a conservative treatment phase. Many of these patients will be on fondaparinux, an indirect factor Xa inhibitor, as recommended by current guidelines based on the Optimal Antiplatelet Strategy for Interventions (OASIS)-5 trial.180,822 In this trial, the combined ischaemic event rate was similar, but severe bleeding complications were significantly lower with fondaparinux than with enoxaparin. This favourable net clinical outcome included reduced long-term mortality and stroke rates. Because of a higher rate of catheter thrombosis in patients undergoing PCI treated with fondaparinux alone, full-dose intravenous UFH (85 U/kg) must be added to prevent formation of catheter thrombi.823
Earlier studies on ACS patients receiving predominantly conservative treatment demonstrated the superiority of enoxaparin over UFH.824 The more recent studies in the setting of PCI did not find an advantage of enoxaparin over UFH when pre-randomization anticoagulation was not consistent with the study treatment or when there was a post-randomization cross-over.819,820 A benefit of enoxaparin over UFH in reducing mortality and bleeding complications was recently reported in a meta-analysis covering NSTE-ACS patients.788
18.3 ST-SEGMENT ELEVATION MYOCARDIAL INFARCTION
Patients undergoing primary PCI should receive a combination of DAPT with ASA and a P2Y12 receptor blocker as early as possible before angiography, and a parenteral anticoagulant.
18.3.1 ORAL ANTIPLATELET THERAPY
An oral loading dose of ASA 150-300 mg (or i.v. 80-150 mg) fol- lowed by 75-100 mg p.o. daily should be given to ensure inhibition of TXA2-dependent platelet aggregation.887
The preferred P2Y12 inhibitors are prasugrel (60 mg p.o. loading dose; 10 mg maintenance dose) and ticagrelor (180 mg p.o. loading dose; 90 mg maintenance dose b.i.d.).341,518 In the pre-specified subgroups of patients with STEMI undergoing PCI in the TRITON-TIMI 38 trial, the benefit of prasugrel was consistent for the primary endpoint at 15 months (prasugrel 10.0% vs. clopidogrel 12.4%; HR 0.79; 95% CI 0.65-0.97; P=0.02), without a significant increase in non-CABG-related bleeding risk (2.4% vs. 2.1%, respectively; HR 1.11; 95% CI 0.70-1.77; P=0.65). There was a lower risk of stent thrombosis (1.6% vs. 2.8%, respectively; HR 0.58; 95% CI 0.36-0.93; P=0.02), as well as of cardiovascular mortality (1.4% vs. 2.4%, respectively; HR 0.61; 95% CI 0.37-1.00; P=0.047)828 in favour of prasugrel at 30-day and 15-month follow-up (2.4% vs. 3.4%, respectively; HR 0.74; 95% CI 0.50-1.09; P=0.129).
Notably, two-thirds of STEMI patients underwent PCI as the primary revascularization strategy and one-third underwent late or secondary PCI after fibrinolysis or lack of early revascularization. Prasugrel is contraindicated in patients with prior stroke or TIA. Treatment with prasugrel is generally not recommended for patients aged 75 years or more. In the ≥75 years age group –if treatment is deemed necessary after a careful, individual risk-benefit evaluation by the prescribing physician– then, following a loading dose of 60 mg, a reduced maintenance dose of 5 mg should be prescribed.811 In patients with body weight less than 60 kg, a maintenance dose of 5 mg is also recommended; this was shown to result in lower platelet reactivity –to a similar extent to prasugrel 10 mg/day in high body weight patients– and in greater platelet inhibition and lower HPR than with clopidogrel 75 mg/day, with similar bleeding rates.829
In the subset of patients with STEMI randomized in the PLATO trial, the benefit of ticagrelor over clopidogrel for the primary endpoint (9.4% vs. 10.8%, respectively; HR 0.87; 95% CI 0.75-1.01; P=0.07; P for correlation 0.29),823 was consistent with the overall results, without higher risk of bleeding (TIMI non-CABG major 2.5% vs. 2.2%, respectively; HR 1.09; 95% CI 0.80-1.48; P=0.60) but with a trend towards a lower risk of cardiovascular mortality at 1 year (4.7% vs. 5.4%, respectively; HR 0.84; 95% CI 0.69-1.03; P=0.07). In a pooled analysis of 48 599 patients, of whom 94% presented with acute coronary syndrome and 84% had PCI, novel P2Y12 inhibitors –including prasugrel and ticagrelor– have been associated with a mortality benefit and no significant excess of major bleeding among STEMI patients.830
Importantly, the more potent agents (prasugrel and ticagrelor) should not be used in patients with prior haemorrhagic stroke or with moderate-to-severe liver disease. When neither of these agents is available (or if they are contraindicated), clopidogrel 600 mg p.o. should be given instead, according to the pre-specified PCI analysis of CURRENT-OASIS 7.812
18.3.2 INTRAVENOUS ANTIPLATELET THERAPY
Several trials –performed before the use of pre-loading with thienopyridines and mostly using abciximab (i.v. bolus followed by infusion of 0.125 mg/kg/min up to a maximum of 10 mg/min for 12 hours)– documented clinical benefits from GP IIb/IIIa inhibitors as adjunct to primary PCI performed with UFH,242,831-833 including a significant 1-year survival benefit that was revealed in a meta-analysis of GP IIb/ IIIa inhibitors with abciximab.831
The large Facilitated Intervention with Enhanced Reperfusion Speed to Stop Events (FINESSE) study tested whether or not upstream administration at the time of first medical contact might improve the clinical efficacy of GP IIb/IIIa inhibitors, compared with administration at the time of primary PCI. In this trial, patients were randomly assigned to upstream abciximab vs. abciximab in the catheterization laboratory.271 Upstream vs. in-cath-lab administration of abciximab had no significant effect on the primary endpoint of death, recurrent myocardial infarction, and heart failure, but significantly increased the risk of bleeding. In subgroup analyses, a benefit was observed with early use of abciximab in patients recruited by the ambulance system or in high-risk patients presenting rapidly at ‘spoke’ centres and requiring transfer for primary PCI.834 The randomized, double-blind Continuing TIrofiban in Myocardial infarction Evaluation (On-TIME-2) trial, using high-dose tirofiban, demonstrated a significant benefit of upstream compared with downstream provisional administration on the primary surrogate endpoint of ST-segment resolution and on the primary composite clinical endpoint of death, recurrent myocardial infarction, urgent target vessel re-intervention or thrombotic bail-out.835 However, the clinical benefit was related predominantly to a reduction in the perceived need for bail-out tirofiban. After pooling the On-TIME-2 data with the 414 patients of an open-label run-in phase, using the same inclusion and exclusion criteria and concomitant treatment, the rate of MACE was significantly reduced by systematic high-dose tirofiban versus no tirofiban or placebo (5.8% vs. 8.6%; P=0.043), with reduced mortality (2.2% vs. 4.1%, respectively; P=0.051) and no increased risk of major bleeding (3.4% vs. 2.9%, respectively; P=0.58).836 It remains unclear whether the effects observed in On-TIME-2 are due to upstream vs. downstream administration or due to systematic vs. provisional administration. However, time from symptom onset to study drug in FINESSE was twice as long as in On-TIME 2;837 only about 40% of patients needed to be transferred from a hospital without a catheterization facility to a hospital with such a facility, and a handful were recruited by the ambulance system. This may account for the differences between the two trials.
Intracoronary –as compared with intravenous– administration of GP IIb/IIIa inhibitors has been tested in several small studies and was associated with some benefits, which have not been confirmed in larger trials.838,839
In the event of angiographic evidence of large thrombus, slow- or no-reflow, and other thrombotic complications, use of GP IIb/IIIa inhibitors as bail-out therapy appears reasonable, although this has not been tested in a randomized trial.
18.3.3 ANTICOAGULATION
In the Harmonizing Outcomes with Revascularization and Stents in Acute Myocardial Infarction (HORIZONS-AMI) trial, an RCT involving 3602 patients with STEMI, bivalirudin with bail-out GP IIb/IIIa inhibitors (in 7.2% of patients) was found superior to systematic GP IIb/ IIIa inhibitors (mostly abciximab) plus UFH in respect of the two primary endpoints of net adverse clinical events (9.2% vs. 12.1%, respectively; RR 0.76; 95% CI 0.63-0.92; P=0.005) and major bleeding (4.9% vs. 8.3%, respectively; RR 0.60; 95% CI 0.46-0.77; P<0.001).840 The clinical benefit comprised a significant survival benefit from bivalirudin as compared with the GP IIb/IIIa inhibitor arm, both at 30 days and at 3 years (2.1% vs. 3.1%, respectively; P=0.049 and 5.9% vs. 7.7%; P=0.03; respectively). However, there was a higher incidence of stent thrombosis during the first 24 hours in the bivalirudin group (1.3% vs. 0.3%; P<0.001), which diminished during follow-up, while pre-randomization UFH and 600 mg clopidogrel loading dose were independent predictors of lower risk of acute and subacute stent thrombosis. The more recent, open-label European Ambulance Acute Coronary Syndrome Angiography (EUROMAX) trial compared a strategy of pre-hospital bivalirudin with UFH or LMWH with optional use of glycoprotein IIb/ IIIa inhibitors (69%) in 2218 STEMI patients, with frequent use of radial access (47%) and pre-treatment with P2Y12 inhibitors (98%).841 The primary endpoint of death or non-CABG major bleeding at 30 days was significantly lower with pre-hospital administration of bivalirudin than with UFH plus optional GP IIb/IIIa inhibitors (5.1% vs. 8.5%, respectively; RR 0.60; 95% CI 0.43-0.82; P<0.001). There were no differences in death (2.9% vs. 3.1%, respectively; RR 0.96; 95% CI 0.60-1.54; P=0.86), but there was a lower risk of major bleeding (2.6% vs. 6.0%, respectively; RR 0.43; 95% CI 0.28-0.66; P<0.001) mainly driven by differences in blood transfusion, whereas rates of TIMI major bleeding were not significantly reduced (1.3% vs. 2.1%, respectively; RR 0.62; 95% CI 0.32-1.20; P=0.15). Sensitivity analyses showed results to be consistent without significant interactions with arterial access site; however, stent thrombosis was more frequent in the bivalirudin group (1.6% vs. 0.5%, respectively; RR 2.89; 95% CI 1.14-7.29; P=0.02) at 30 days, solely driven by a difference during the first 24 hours, which was paralleled by a trend towards a higher rate of re-infarction (1.7% vs. 0.9%, respectively; RR 1.93; 95% CI 0.90-4.14; P=0.08) despite use of novel P2Y12 inhibitors in more than half of the patients. The mortality benefit observed in the HORIZONS-AMI trial was not confirmed by EUROMAX, and the excess of stent thrombosis remained despite prolonged infusion of bivalirudin. The How Effective are Antithrombotic Therapies in PPCI (HEAT-PCI) study is a single-centre randomised trial comparing bivalirudin and unfractionated heparin in 1829 STEMI patients planned to undergo primary PCI.842 The study represents contemporary practice with restriction of GPIIb/IIIa inhibitors to bail-out situations (in 15% of the randomised patients population), the frequent use of novel P2Y12 inhibitors (89% of the patients), radial approach and predominant DES implantation. Among the 1812 patients included in the final analysis, 1491 actually underwent primary PCI. The primary efficacy outcome measure, a composite of all-cause mortality, stroke, recurrent infarction and unplanned target lesion revascularization, was higher in the bivalirudin than in the UFH group (8.7% vs. 5.7%, respectively; HR 1.52; 95% CI 1.09-2.13; P=0.01) including an increase in stent thrombosis (3.4% vs. 0.9%, respectively, RR 3.91; 95% CI 1.61-9.52; P=0.001) but no significant difference in mortality (5.1% vs. 4.3%, respectively). The primary safety outcome –defined as major BARC 3-5 bleeding– was 3.5% in the bilvalirudin group vs. 3.1% in the UFH group (P=0.59). The Bavarian Reperfusion Alternatives Evaluation (BRAVE) 4 trial examined the question of whether a strategy of prasugrel plus bivalirudin (n=269) was superior to a strategy with clopidogrel plus UFH (n=275) in primary PCI STEMI patients and was interrupted due to slow patient recruitment.843 The primary endpoint –a composite of death, myocardial infarction, unplanned revascularization of the infarct-related artery, stent thrombosis, stroke or major bleeding evaluated at 30 days– occurred in 15.6% vs. 14.5%, respectively (RR 1.09; 95% CI 0-1.79; P=0.68), the secondary composite ischaemic endpoint (death, myocardial infarction, revascularization of the infarct-related artery, stent thrombosis or stroke) was seen in 4.8% vs. 5.5%, respectively (RR 0.89; 95% CI 0.40-1.96; P=0.89) and the secondary bleeding endpoint (non-CABG related bleeding according to the HORIZONS-AMI definition) in 14.1% vs. 12.0%, respectively (RR 1.18; 95% CI 0.74-1.88 P=0.54). In summary, recent trials comparing bivalirudin with UFH without systematic use of GPIIb/IIIa antagonists uphold concerns over an excess risk for acute stent thrombosis with bivalirudin, while differences in major bleeding are small.
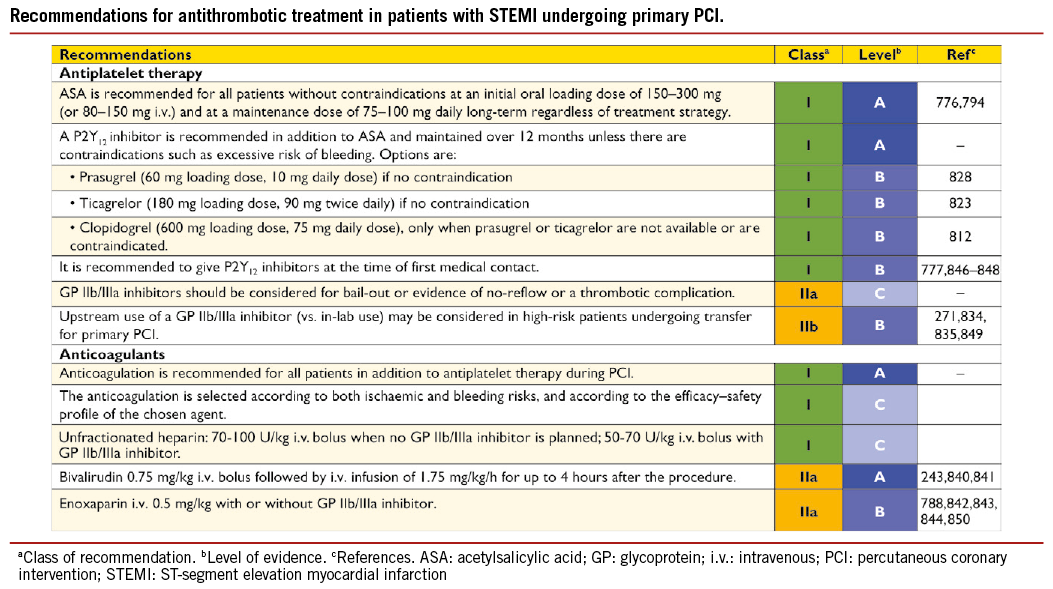
Enoxaparin [0.5 mg/kg i.v. followed by subcutaneous (s.c.) treatment] was compared with UFH in one randomized, open-label trial, known as Acute STEMI Treated with primary PCI and intravenous enoxaparin Or UFH to Lower ischaemic and bleeding events at short- and Long-term follow-up (ATOLL) trial. The primary composite endpoint of 30-day death, complication of myocardial infarction, procedural failure, and major bleeding was not significantly lower for the enoxaparin arm (–17%; P=0.063), but there were reductions in the composite main secondary endpoint of death, recurrent myocardial infarction or ACS, or urgent revascularization, and in other secondary composite endpoints –such as death, or resuscitated cardiac arrest and death, or complication of myocardial infarction. There was no indication of higher incidence of bleeding from use of enoxaparin over UFH. In the per-protocol analysis of the ATOLL trial –pertinent to more than 87% of the study population– i.v. enoxaparin was superior to UFH in reducing the primary endpoint (RR 0.76; 95% CI 0.62-0.94; P=0.012) but also ischaemic endpoints, mortality (RR 0.36; 95% CI 0.18-0.74; P=0.003) and major bleedings (RR 0.46; 95% CI 0.21-1.01; P=0.050), contributing to the improvement of the net clinical benefit (RR 0.46; 95% CI 0.3-0.74; P=0.0002) in patients undergoing primary PCI. Based on these considerations, enoxaparin may be considered as an alternative to UFH as anticoagulant to primary PCI.844
Use of fondaparinux in the context of primary PCI was associated with potential harm in the OASIS-6 trial and is therefore not recommended.845 In particular, when used alone during primary PCI, fondaparinux is associated with the risk of catheter thrombosis. Thus, an additional anticoagulant with anti-IIa activity (unfractionated heparin or enoxaparin) should be administered.
18.4 POINTS OF INTEREST AND SPECIAL CONDITIONS
18.4.1 PRE-TREATMENT WITH P2Y12 INHIBITORS
Clopidogrel
The concept of pre-treatment with P2Y12-receptor blockers is based on the observation that the risk of PCI depends on the intraprocedural level of platelet inhibition. The three largest clinical studies supporting this concept are (i) Clopidogrel in Unstable Angina to Prevent Recurrent Events (CURE), with the PCI-CURE subset, (ii) Clopidogrel for the Reduction of Events During Observation (CREDO) with the subset of patients with sufficient delay between intake of clopidogrel 300 mg and PCI, and (iii) Do Tirofiban and ReoPro Give Similar Efficacy Outcome Trial (TARGET), with its non-randomized pre-treatment towards a background of GP IIb/IIIa inhibition.791,825,851
Additional circumstantial evidence for pretreatment with P2Y12-receptor blockers comes from the notion that benefit of GP IIb/IIIa inhibition over placebo in historic studies is mitigated in more recent studies with systematic upstream P2Y12- receptor inhibition.269,817,821
A recent meta-analysis evaluated the relationships of clopidogrel pre-treatment vs. no treatment with mortality and major bleeding among patients undergoing PCI. Pre-treatment with clopidogrel had no effect on death (OR 0.80; 95% CI 0.57-1.11) or the risk of major bleeding (OR 1.18; 95% CI 0.93-1.50) but the risk of major cardiac events was significantly reduced (OR 0.77; 95% CI 0.66-0.89; P<0.001).777 There was substantial heterogeneity according to the type of clinical presentation of SCAD, NSTE-ACS, and STEMI, suggesting the lack of a consistent treatment effect –especially with respect to mortality– across the entire clinical spectrum. The benefit of pre-treatment was greater with increasing severity of clinical presentation. In particular, clopidogrel pre-loading did not improve ischaemic outcomes in PCI for SCAD, with a trend towards more bleedings.777 In NSTE-ACS, there was a significant reduction in major cardiovascular events (OR 0.78; 95% CI 0.66-0.91; P=0.002) driven mainly by myocardial infarction, with a trend towards more TIMI major bleeds (OR 1.28; 95% CI 0.98-1.67; P=0.07).
In primary PCI for STEMI, a single trial has evaluated the administration of DAPT before hospital admission, rather than in hospital, and has been terminated prematurely due to slow recruitment, with a trend towards a higher proportion of TIMI 2 or 3 flow and fewer ischaemic events in the pre-treatment group.846 However, this common practice in Europe is supported by a lower mortality (OR 0.50; 95% CI 0.26-0.96) without significant excess in major bleedings (OR 0.78; 95% CI 0.42-1.45).777
Prasugrel and ticagrelor
A Comparison of Prasugrel at the Time of Percutaneous Coronary Intervention (PCI) Or as Pre-treatment At the Time of Diagnosis in Patients With Non-ST-Elevation Myocardial Infarction (NSTEMI) (the ACCOAST study) is the largest and the only pre-treatment study that has investigated the use of prasugrel (30 mg) vs. placebo before PCI in 4033 NSTE-ACS patients. Overall, 69% of patients underwent PCI and 5% CABG. When PCI was performed, an additional dose of 30 mg prasugrel was given after diagnostic coronary angiography in the pre-treatment group and 60 mg prasugrel was given in the placebo group. The primary endpoint –a composite of cardiovascular death, myocardial infarction, stroke, urgent revascularization, and bail-out GP IIb/IIIa inhibitor use at 7 days– was similar for both groups (HR with pre-treatment, 1.02; 95% CI 0.84-1.25; P=0.81). The rate of the safety endpoint of TIMI major bleeding, through day 7, was higher with pre-treatment (HR 1.90; 95% CI 1.19-3.02; P=0.006). The study was stopped 1 month before the end of enrolment due to an excess of major bleeding, and further highlights the lack of benefit of pre-treatment in NSTE-ACS patients.826 Pre-treatment with 30 mg prasugrel, with an average time delay of 6 hours before angiography, led to a much faster and more profound inhibition of platelet aggregation thana 600 mg clopidogrel loading dose as given in Antiplatelet therapy for Reduction of MYocardial Damage during Angioplasty (ARMYDA)-5.789 Within one hour after PCI, there was a catch-up phenomenon of the pharmacodynamic profile of pre-treatment and in-lab treatment group with 60 mg prasugrel. These very different pharmacodynamic profiles may account for the excess of periprocedural major bleedings reported in the pre-treatment group, namely access site-related bleeds and pericardium drainage. No such dramatic differences were observed with 600 mg clopidogrel, with which safety profiles of in-lab vs. pre-treatment were similar.789
A pre-treatment strategy, compared with a delayed administration of ticagrelor, has not so far been tested. In PLATO, all patients had received pre-treatment with clopidogrel or ticagrelor, irrespective of treatment strategy (invasive vs. non-invasive) and patients undergoing PCI had received P2Y12 receptor inhibitors at a median of 4 hours prior to the intervention. Therefore, the risk-benefit ratio of pre-treatment using ticagrelor prior to diagnostic coronary angiography is not known.
18.4.2 INTRAVENOUS P2Y12 INHIBITORS
Cangrelor is a direct reversible, short-acting (half-life 3 min) P2Y12 inhibitor that does not require metabolic conversion, although it is not available for oral administration. It has been used during PCI with mixed results. In Cangrelor vs. Standard Therapy to Achieve Optimal Management of Platelet Inhibition (CHAMPION)- PHOENIX, a double-blind, placebo-controlled trial, 11 145 patients who were undergoing either urgent or elective PCI and received guideline-recommended therapy, were randomized to receive a bolus and infusion of cangrelor (30 mg/kg; 4 mg/kg/min) or a loading dose of 300 mg or 600 mg of clopidogrel. The rate of the primary efficacy endpoint –defined as a composite of death, myocardial infarction, ischaemia-driven revascularization, or stent thrombosis at 48 hours after randomization– was 4.7% in the cangrelor group and 5.9% in the clopidogrel group (adjusted OR 0.78; 95% CI 0.66-0.93; P=0.005).852 Stent thrombosis developed in 0.8% of the patients in the cangrelor group and in 1.4% in the clopidogrel group (OR 0.62; 95% CI 0.43-0.90; P=0.01). Severe bleeding at 48 hours did not differ significantly. Although the universal definition of myocardial infarction was used, the incidence of Q-wave myocardial infarction did not differ between the study groups.852 The pre-specified pooled analysis of patient-level data from the three cangrelor trials (CHAMPION-PCI, CHAMPION-PLATFORM, and CHAMPION-PHOENIX) confirmed the lower rates of PCI periprocedural thrombotic complications (3.8% for cangrelor vs. 4.7% for control; OR 0.81; 95% CI 0.71-0.91; P=0.0007) and of stent thrombosis (0.5% vs. 0.8%, respectively; OR 0.59; 95% CI 0.43-0.80; P=0.0008) with no difference in Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries (GUSTO) major bleeding.853 These early benefits were maintained at 30 days and found to be consistent across all the pre-specified subgroups. There was no correlation between treatment effect and clinical presentation and there was a significant lower incidence of Q-wave myocardial infarction in favour of cangrelor. Altogether, cangrelor seems to be a good therapeutic option in P2Y12 inhibitor-naïve patients undergoing coronary stent implantation. It should be pointed out that there was no effect on mortality and that the benefit of cangrelor was mainly derived from preventing intraprocedural stent thrombosis.853
In addition, the use of cangrelor allows platelet inhibition to be maintained up to surgery in patients discontinuing oral antiplatelet therapy, without any excess of perioperative bleeding, in contrast to interruption of oral P2Y12 several days before CABG surgery.854
Cangrelor has not yet been approved by the European Medical Agency or the Federal Drug Administration and therefore no specific recommendation about its use can be given.
18.4.3 ANTICOAGULATION AFTER PERCUTANEOUS CORONARY INTERVENTION IN ACUTE CORONARY SYNDROME PATIENTS
The recent trial known as Anti-Xa Therapy to Lower cardiovascular events in Addition to Standard therapy in subjects with Acute Coronary Syndrome – Thrombolysis In Myocardial Infarction 51 (ATLAS ACS 2 – TIMI 51) demonstrated that the addition of rivaroxaban –either 2.5 mg or 5.0 mg, twice daily– to ASA and clopidogrel among ACS patients lowered the composite primary efficacy endpoint of cardiovascular death, myocardial infarction, and stroke (9.1% vs. 10.7%, respectively; HR 0.84; 95% CI 0.74-0.96; P=0.008) but was associated with a near four-fold increased risk of non-CABG-associated major bleeding (2.1% vs. 0.6%, respectively; HR 3.96; 95% CI 2.46-6.38; P<0.001) and an increased risk of intracranial haemorrhage.855 The twice-daily 2.5 mg dose of rivaroxaban resulted in significantly lower rates of all-cause and cardiovascular mortality, which was not observed with the twice-daily 5.0 mg dose. The composite of definite and probable stent thrombosis was lower in the pooled (1.9% vs. 1.5%, respectively; HR 0.65; P=0.017) and 2.5 mg twice-daily groups (1.9% vs. 1.5%, respectively; HR 0.61; P=0.023) with a trend towards lower incidences in the 5 mg twice-daily treatment group (1.9% vs. 1.5%, respectively; HR 0.70; P=0.089).856 The ATLAS ACS 2-TIMI 51 trial did not test the combination of rivaroxaban with prasugrel or ticagrelor, which might be associated with an even higher bleeding risk. This trial suggests that low-dose rivaroxaban (2.5 mg twice daily) may be considered in patients who receive ASA and clopidogrel after ACS, particularly after STEMI.857 However, a phase III trial of apixaban, another factor Xa antagonist, the Apixaban for Prevention of Acute Ischemic and Safety Events (APPRAISE-2),858 which compared full-dose apixaban (5 mg b.i.d.) in combination with DAPT against DAPT alone, was stopped early due to safety concerns related to an excess bleeding risk in the absence of a benefit in ischaemic outcomes in high-risk ACS patients. Notably, the study population carried higher comorbidities and the apixaban dose regimen was the full dose used to prevent cardioembolic stroke in non-valvular atrial fibrillation. Finally, darexaban and dabigatran were both tested in phase II dose-ranging trials in post-ACS patients.859,860 In both cases, dose-dependent increases in major bleeding were observed, but there was no sign of added efficacy when adding anticoagulant therapy to antiplatelet therapy in this setting. Conversely, the phase II dose-ranging trials with rivaroxaban and apixaban demonstrated a dose-dependent higher incidence in major bleeding but a significantly lower rate of death, myocardial infarction or stroke than with placebo for revaroxaban and a trend for apixaban.861,862 Pharmacological features of direct oral anticoagulants are summarized in Table 14. In conclusion, the role of direct oral anticoagulants in combination with DAPT in secondary prevention of ACS is promising, but interpretation of the totality of evidence for the class of oral anticoagulants is inconclusive and requires further study.
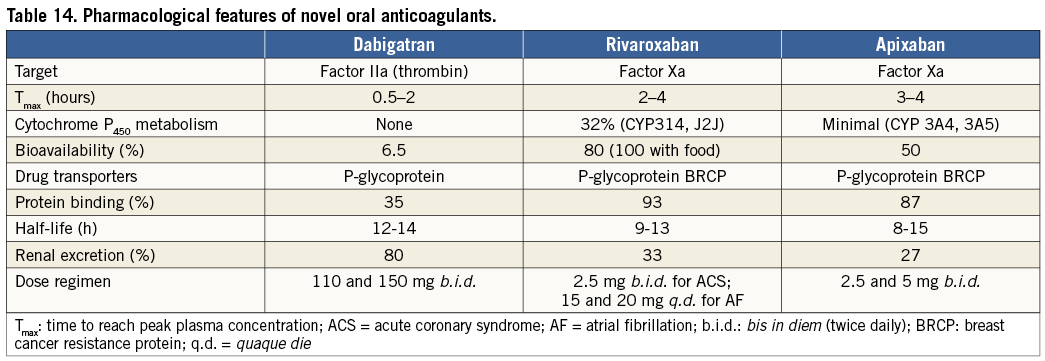
18.4.4 ANTICOAGULATION DURING PERCUTANEOUS CORONARY INTERVENTION IN PATIENTS ON ORAL ANTICOAGULATION
A sizeable proportion of patients (6-8%) undergoing PCI have an indication for long-term oral anticoagulation with a vitamin K antagonist (VKA) or NOAC, due to various conditions such as moderate-to-high embolic risk AF, mechanical heart valves, or venous thromboembolism. Interruption of VKA therapy may expose the patient to an increased risk of thromboembolic episodes.863 Percutaneous coronary intervention may be a delicate process under full VKA anticoagulation or NOAC.
In elective PCI, no additional anticoagulation is needed if the international normalized ratio (INR) is >2.5. Radial access should be the preferred choice, to reduce the risk of periprocedural bleeding. PCI without interruption of VKAs, to avoid bridging therapy that may lead to more bleeding or ischaemic complications, should be the preferred strategy. The use of GP IIb/IIIa inhibitors, unless for bail-out, should be also avoided.
Primary PCI in patients on therapeutic oral anticoagulation should be performed via a radial approach with use of additional parenteral anticoagulation, regardless of the timing of the last dose of oral anticoagulant. Given its short-term action of 25 minutes and lower bleeding risk bivalirudin –used during the procedure and discontinued immediately after primary PCI– may be preferred over UFH or enoxaparin, especially when patients are exposed to dabigatran. Enoxaparin should be the preferred parenteral anticoagulant in cases of prior exposure to direct anti-Xa inhibitors (rivaroxaban or apixaban) to avoid cross-over. Unless for bail-out situations, glycoprotein IIb/IIIa inhibitors should generally be avoided.
18.4.5 ANTITHROMBOTIC THERAPY AFTER PERCUTANEOUS CORONARY INTERVENTION IN PATIENTS REQUIRING ORAL ANTICOAGULATION
Long-term exposure of patients to triple therapy is associated with a high risk of bleeding.864 Fatal bleeds represent 1 in 10 of all bleeds, of which half are of intracranial origin and half from the gastrointestinal tract.865 Evidence is too weak to provide clear guidance.866,867 Triple therapy, consisting of ASA, clopidogrel, and (N)OAC after PCI, should only be given if a compelling indication exists (i.e. paroxysmal, persistent, or permanent AF with Cardiac failure, Hypertension, Age ≥75 [Doubled], Diabetes, Stroke [Doubled] - Vascular disease, Age 65-74 and Sex category [Female] (CHA2DS2-VASc) score ≥2; mechanical valves; recent or recurrent history of deep venous thrombosis or pulmonary embolism).
Triple therapy should be limited in duration, depending on the clinical setting, thromboembolic (CHA2DS2-VASc score) and bleeding risks Hypertension, Abnormal renal/liver function, Stroke, Bleeding history or predisposition, Labile INR, Elderly, Drugs/alcohol (HAS-BLED) score. The use of prasugrel or ticagrelor as part of triple therapy should be avoided, given the lack of established benefit and the greater risk of major bleeding compared with clopidogrel (HR 4.6; 95% CI 1.9-11.4; P<0.001) in an observational study.868 Gastric protection should be implemented with a proton pump inhibitor. The dose intensity of oral anticoagulation should be carefully monitored with a target INR of 2.0-2.5 in the case of vitamin K antagonists and use of lower tested dose for stroke prevention in the case of NOACs (dabigatran 100 mg b.i.d.; rivaroxaban 15 mg once daily, etc.).
Recommendations on stent type (DES vs. BMS) are difficult in the absence of conclusive data. Although DAPT is routinely recommended for a duration of at least 1 month after BMS and for 6 months after DES, the risk of stent thrombosis (and other ischaemic endpoints) between 1 and 12 months after stenting appears similar with both stent platforms.124,352,869 In addition, recent data on the risk of adverse events among patients who have ceased DAPT medication648 and patients undergoing non-cardiac surgery suggest no differences between BMS and DES.663 Until data from randomized trials become available, this task force recommends the use of new-generation DES over BMS in patients requiring oral anticoagulation who are at low bleeding risk (HAS-BLED score ≤2). Among patients undergoing PCI who require oral anticoagulation and have a high bleeding risk (HAS-BLED score ≥3) the choice between BMS and new-generation DES needs to be decided on an individual basis.
Omission of ASA while maintaining clopidogrel has been evaluated in the What is the Optimal antiplatElet and anticoagulant therapy in patients with oral anticoagulation and coronary StenTing (WOEST) trial, which randomized 573 patients either to dual therapy with oral anticoagulation and clopidogrel (75 mg daily) or to triple therapy with oral anticoagulation, clopidogrel, and ASA 80 mg daily. Treatment was continued for 1 month after BMS placement in 35% of the patients and for 1 year after DES placement in the remaining 65%; follow-up was for 1 year.870 Percutaneous coronary intervention was performed on VKA in half of the patients and one-third presented with NSTE-ACS. The primary endpoint of any TIMI bleeding was significantly lower in the dual therapy arm (19.5% vs. 44.9%; HR 0.36; 95% CI 0.26-0.50; P<0.001). The rates of myocardial infarction, stroke, TVR, or stent thrombosis did not differ significantly, but all-cause mortality was lower in the dual therapy group (dual 2.5% vs. triple 6.4%; P=0.027) at 1 year. However, differences were driven by minor bleeding as major bleeding was not significantly lower, femoral access was used in the majority of patients (74%), and triple therapy was extended to 1 year. Although the trial was too small to assess ischaemic outcomes, dual therapy with clopidogrel and oral anticoagulants may be considered as an alternative to triple therapy in patients with high bleeding risk.
18.4.6 DURATION OF DUAL ANTIPLATELET THERAPY AFTER PERCUTANEOUS CORONARY INTERVENTION
In the pivotal studies establishing the value of early-generation DES, the duration of DAPT was 2-3 months for the sirolimus-eluting stent and 6 months for the paclitaxel-eluting stent. Following concerns of a greater risk of stent thrombosis and ischaemic adverse events,651 several guideline documents recommended DAPT for 1 year or longer after DES implantation.779 Detailed analyses comparing early-generation DES with BMS confirmed no safety issue, with similar rates of death, and myocardial infarction, during long-term follow-up throughout 5 years with heterogeneous duration of DAPT, ranging from 2 months up to 1 year.124,649,650 Although very late stent thrombosis was more frequent, this infrequent event was offset by a somewhat lower rate of early stent thrombosis and a lower risk of myocardial infarction related to repeat revascularization. More recently, new-generation DES have been shown to have a safety profile similar to or even better than BMS, including the risk of very late stent thrombosis.125,129-132
Currently available data do not support prolonging DAPT following DES beyond 1 year. A randomized trial called The Zotarolimus-Eluting Stent, Sirolimus-Eluting Stent, or PacliTaxel-Eluting Stent Implantation for Coronary Lesions - Late Coronary Arterial Thrombotic Events/REAL-world Patients Treated with Drug-Eluting Stent Implantation and Late Coronary Arterial Thrombotic Events (ZEST-LATE/REAL-LATE) assigned stable patients, 1 year after DES implantation, to continuation with clopidogrel plus ASA or to ASA alone.871 After a median follow-up of 19 months, there was a non-significantly higher rate of myocardial infarction, stroke, and death in the patients who had continued clopidogrel treatment than in those who stopped clopidogrel at random assignment 1 year after implantation.
Several randomized trials including Efficacy of Xience/Promus vs. Cypher in reducing Late Loss After stenting (EXCELLENT),803 Real Safety and Efficacy of a 3-month Dual Antiplatelet Therapy Following Zotarolimus-eluting Stents Implantation (RESET),805 Optimized Duration of Clopidogrel Therapy Following Treatment With the Zotarolimus-Eluting Stent in Real-World Clinical Practice (OPTIMIZE)804 and PROlonging Dual Antiplatelet Treatment In Patients With Coronary Artery Disease After Graded Stentinduced Intimal Hyperplasia studY (PRODIGY),799 compared short duration (3-6 months) of DAPT against extended duration (12-24 months) and consistently showed a lack of benefit in terms of ischaemic outcome but a higher risk of bleeding. A recent meta-analysis of data comparing brief vs. prolonged DAPT (beyond 12 months) duration concluded that extension of DAPT beyond 6 months increased the risk of bleeding without reducing ischaemic events.802 It should be pointed out that none of these trials were powered for ischaemic endpoints; all were open-label and the time from stenting to randomization varied. Therefore, weighing the quality of available evidence is difficult and these inferences need be confirmed by continuing large-scale trials including Intracoronary Stenting and Antithrombotic Regimen: Safety And eFficacy of a 6-month DAT after drug-Eluting stenting (ISAR-SAFE; NCT00661206) and DAPT (NCT00977938).
In view of the well-established risks of bleeding associated with DAPT beyond 12 months, and the lack of evidence of a benefit in the prevention of ischaemic complications, routine extension of DAPT beyond 6 months after new-generation DES implantation in SCAD cannot be recommended based on currently available data. Observational data from new-generation zotarolimus-eluting and everolimus-eluting stents suggest that even shorter durations of DAPT may be sufficient.872,873 In the OPTIMIZE trial, clinical non-inferiority of 3 months vs. 12 months of DAPT was assessed in patients undergoing PCI with zotarolimus-eluting stents.804 The rate of net adverse clinical events did not differ between short-term DAPT and extended-duration DAPT (6.0% vs. 5.8%, respectively; risk difference, 0.17; 95% CI –1.52 to 1.86). Rates of bleeding, major or otherwise, were not statistically different. Owing to the paucity of high-quality data for a 3-month (or shorter) duration of DAPT with new-generation DES, this regimen should be reserved for patients at high risk of bleeding or requiring oral anticoagulation.
In patients undergoing myocardial revascularization for high-risk ACS, DAPT is recommended for 1 year, irrespective of stent type. This recommendation is based on the early CURE study –which demonstrated a continuously increasing benefit of DAPT over ASA during the entire study follow-up period– as well as the more recent results of TRITON-TIMI 38 and PLATO, which showed a continuously increasing benefit of DAPT with the new more potent P2Y12-receptor blockers. After stenting for ACS, particularly STEMI, extended DAPT reduces the risk of stent thrombosis, re-infarction, and cardiovascular mortality,825 and more potent DAPTs are associated with the greatest post-ACS clinical benefits of any type.830 It is important to inform patients and their physicians about the need to avoid premature discontinuation of DAPT.
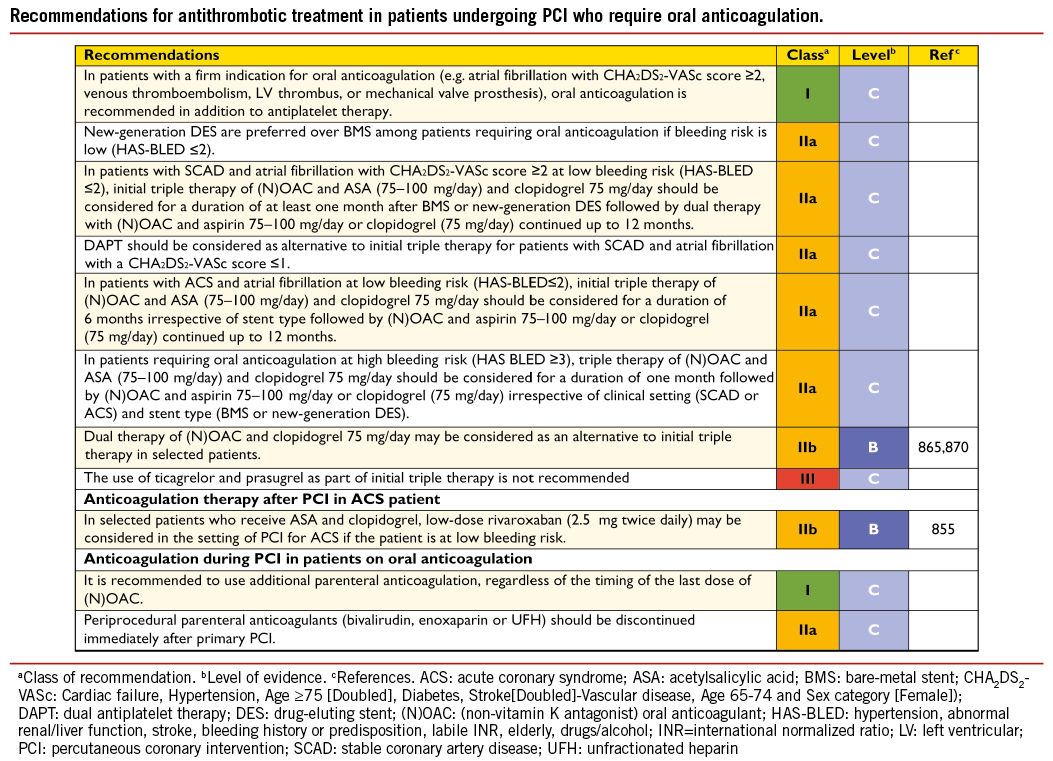
In summary, it is recommended that DAPT be administered for at least 1 month after BMS implantation in SCAD,86 for 6 months after new-generation DES implantation in SCAD,86 and for up to 1 year in patients after ACS, irrespective of revascularization strategy.180
18.4.7 DRUG INTERACTIONS: A CLOPIDOGREL-RELATED TOPIC
Those statins which are substrates of the CYP3A4 isoform (i.e, simvastatin, atorvastatin and lovastatin) may interact with clopidogrel metabolism, a drug interaction that has little, if any, clinical relevance.
European and USA regulatory agencies have issued warnings about diminished clopidogrel action when combined with proton pump inhibitors (especially omeprazole and esomeprazole). Treatment with proton pump inhibitors should be carefully considered in patients with previous gastrointestinal complications or risk factors for gastro-intestinal bleedings (e.g. the elderly, concomitant use of warfarin, glucocorticoids, non-steroidal anti-inflammatory drugs, or Helicobacter pylori infection) who require DAPT. Several studies have shown a proton pump inhibitor-related impact on the pharmacodynamics of antithrombotic drugs, whereas few studies support significant effects on clinical outcomes. There is insufficient data to discourage the use of proton pump inhibitors in patients treated with ASA, prasugrel, ticagrelor, dabigatran, or one of the oral factor Xa inhibitors (rivaroxaban and apixaban). By far the most extensively investigated proton pump inhibitor interaction is with clopidogrel. Notwithstanding, potential interactions between the antiplatelet effect of clopidogrel and proton pump inhibitors are controversial, without firm conclusions on clinical implications. Clopidogrel is most often prescribed with ASA, and patients on DAPT have an increased risk of gastrointestinal bleeding; however, proton pump inhibitors should not be used automatically in these patients but should be prescribed to patients with previous gastrointestinal complications or who are at an increased risk of bleeding. Pharmacodynamic studies –but not clinical outcome studies– support the use of newer proton pump inhibitors such as pantoprazole instead of omeprazole.874
18.4.8 RENAL DYSFUNCTION
Renal dysfunction is present in 30-40% of patients with CAD and the extent of CKD is strongly related to the risk of in-hospital adverse outcomes. Impaired clinical outcomes of patients with CKD are possibly explained by more frequent pre-existing cardiovascular disease, more extended atherothrombosis, a more serious presentation of ACS, lower revascularization rates, and under-utilization of evidence-based therapies, with potential overdosing of medication in patients whose metabolism and excretion depend on renal function. Creatinine clearance should be calculated with the Cockroft-Gault formula, to comply with drug labelling and avoid overdosing with antithrombotics –a frequent situation in patients with CKD– leading to increased bleeding risk.875,876 In patients referred for acute PCI, the first dose of an antithrombotic drug does not usually add to the risk of bleeding in the case of CKD. Repeated infusion or intake might lead to drug accumulation and increased bleeding risk. Accordingly, in the absence of contraindications, patients with CKD should receive the same first-line treatment as any other patient. Thereafter, dose adaptation with respect to kidney function is essential and specific antithrombotic agents may be preferred (Table 15). It is important, in minimizing the risk of CIN, to ensure proper hydration during and after primary PCI and to limit the dose of contrast agents (see section 11.4).
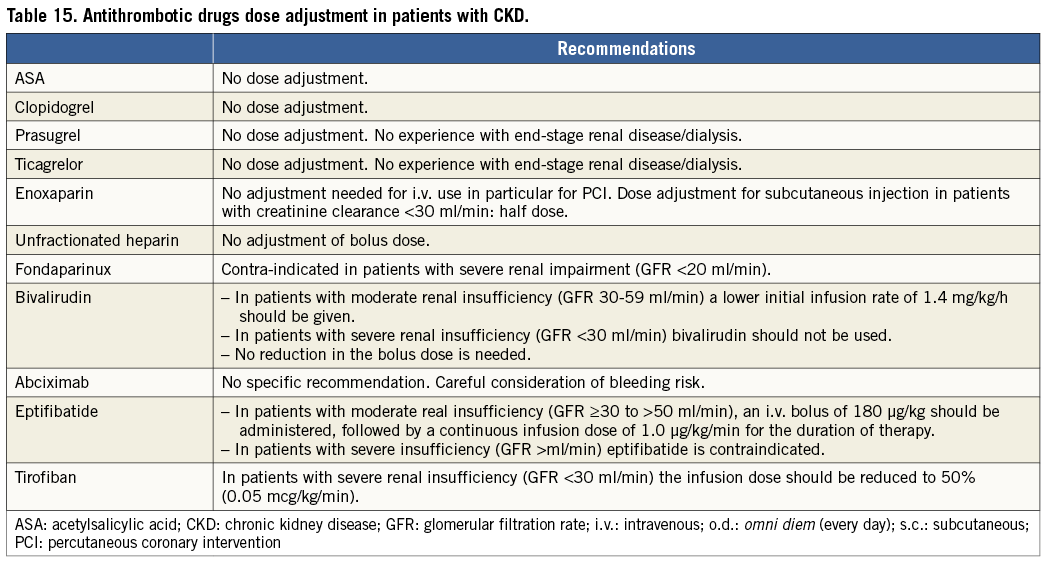
Renal dysfunction was one of several risk criteria that had to be considered in the PLATO study and only patients with end-stage renal failure requiring dialysis were excluded. Patients with CKD (21%) did particularly benefit from ticagrelor, with a 23% RRR for the primary ischaemic endpoint (compared with a non-significant 10% lower figure in patients without CKD), and an even more pronounced 4.0% absolute and 28% RRR in all-cause mortality.877
18.4.9 SURGERY IN PATIENTS ON DUAL ANTIPLATELET THERAPY
Management of patients on DAPT who are referred for surgical procedures depends on the level of emergency and the thrombotic and bleeding risk of the individual patient (Figure 4).878 Most surgical procedures can be performed on DAPT or at least on ASA alone with acceptable rates of bleeding. A multidisciplinary approach is required (cardiologist, anaesthesiologist, haematologist, and surgeon) to determine the patient’s risk (bleeding and thrombosis) and to choose the best strategy. Indeed, surgery-related bleeding increases 30-day and long-term mortality.573
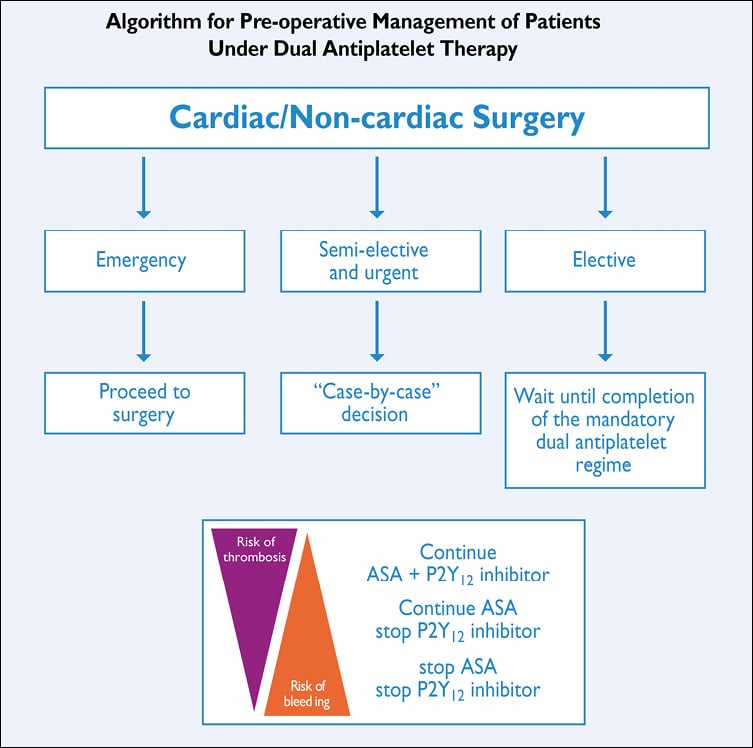
Figure 4. Pre-operative management of patients considered for/undergoing surgery under DAPT. ASA: acetylsalicylic acid; DAPT: dual antiplatelet therapy
Observational data from a large cohort study (124 844 BMS or DES implantations) indicate that the strongest risk factors for MACE following non-cardiac surgery are the need for non-elective surgery, a history of myocardial infarction within 6 months of surgery and advanced cardiac disease. While timing of surgery was associated with MACE during the first 6 months after PCI, this was no longer apparent beyond 6 months.663 Notably, stent type (BMS vs. DES) was not associated with MACE after surgery. In order to reduce the risk of bleeding and thrombosis, it is recommended that elective non-cardiac surgery be delayed until completion of the full course of recommended DAPT (ideally 6 months in SCAD and 1 year in ACS patients) and that surgery be performed without discontinuation of aspirin, if possible. Shorter duration of DAPT may be justifiable if surgery cannot be delayed.
In preparation for surgical procedures with high-to-very-high bleeding risk, it is recommended that clopidogrel be discontinued 5 days before surgery to reduce bleeding and the need for transfusion, while maintaining ASA throughout the perioperative period.879 Prasugrel should be stopped 7 days before surgery, based on its prolonged and more effective platelet inhibition than clopidogrel. Interestingly, despite higher levels of observed TIMI major bleeding (OR 4.73; 95% CI 1.9-11.8), platelet transfusion, and surgical re-exploration for bleeding, prasugrel was associated with a lower rate of death after CABG than with clopidogrel in the small subgroup of patients in the TRITON-TIMI 38 trial (2.3% vs. 8.7%, respectively; adjusted OR 0.26; P=0.025).880 Most cases of CABG were planned and undertaken after discharge from the qualifying event, and the study drug was usually resumed after CABG. In the PLATO trial, in the subgroup of patients undergoing CABG within 7 days after the last study drug intake (3-5 days), ticagrelor, compared with clopidogrel, was also associated with lower all-cause mortality (4.6% vs. 9.2%, respectively; P=0.002) without excess risk of CABG-related bleeding.881 More than half of the cases of CABG were undertaken during the qualifying event. This was accounted for by fewer deaths associated with bleeding and infection as well as fewer ischaemic events. A total of 37% did not restart study medication within 7 days of discharge.
Accordingly, withdrawal of P2Y12 inhibitors is not recommended in high-risk cohorts, such as those with continuing ischaemia and high-risk anatomy (e.g. LM or severe proximal multivessel disease). These patients should undergo CABG while maintaining P2Y12 inhibition, while paying particular attention to reducing bleeding. It may be reasonable –though only in patients whose risk of bleeding is very high– to withhold P2Y12 inhibitors before surgery, even among those with active ischaemia, and to consider bridging strategies (see below). Dual antiplatelet therapy should be resumed as soon as possible, including a loading dose for clopidogrel, ticagrelor, or prasugrel (if possible within 24 hours of surgery), although the optimal timing for resumption of medication following CABG surgery remains uncertain. Treatment monitoring, using bedside tests, has been suggested as an option for guiding interruption of treatment, rather than use of an arbitrary, specified period. Platelet inhibitory response to clopidogrel determines CABG-related bleeding,882 and a strategy based on preoperative platelet function testing, to determine the timing of CABG in clopidogrel-treated patients, led to ~50% shorter waiting time than recommended in the current Guidelines.883 For these reasons, the 2012 update of the Society of Thoracic Surgeons guidelines suggested that a delay of even a day or two is reasonable, to decrease bleeding and thrombotic risk in ACS patients.879
In very high-risk situations, such as in the first weeks after stent implantation, it has been suggested that, 5 days before surgery, a patient may be switched from clopidogrel to a reversible antiplatelet agent with a short half-life (e.g. the i.v. GP IIb/IIIa inhibitors tirofiban or eptifibatide), stopping the infusion 4 hours before surgery,884 but there is no clinical evidence, based solely on pharmacokinetic or pharmacodynamic studies, to support this approach. In the Bridging Anticoagulation in Patients who Require Temporary Interruption of Warfarin Therapy for an Elective Invasive Procedure or Surgery (BRIDGE) study, the use of cangrelor, an intravenous, reversible P2Y12 platelet inhibitor for bridging thienopyridine-treated patients to CABG surgery, was evaluated against placebo.854 Oral P2Y12 inhibitors were stopped 48 hours before CABG. Cangrelor resulted in a higher rate of maintenance of platelet inhibition (primary endpoint, P2Y12 reaction units <240; 98.8% (83/84) vs. 19.0% (16/84), respectively; RR 5.2; 95% CI 3.3-8.1; P<0.001). Bridging with a prolonged infusion of cangrelor did not increase major bleeding before surgery.
The substitution of DAPT with LMWH or UFH is ineffective.885 In surgical procedures with low-to-moderate bleeding risk, surgeons should be encouraged to operate while maintaining DAPT.
Resuming clopidogrel after CABG appears to be safe and effective according to a recent meta-analysis of five randomized trials and six observational studies that included 25 728 patients who, when clopidogrel was added to ASA, as opposed to ASA alone, showed a better early vein graft patency (RR 0.59; 95% CI 0.43-0.82; P=0.02) and lower in-hospital or 30-day mortality (0.8% vs. 1.9%; P<0.0001).886 The mortality benefit after CABG in PLATO and in TRITON-TIMI 38 suggests that ticagrelor and prasugrel may be restarted after CABG; however, the evidence is limited, with only one-third of patients restarting ticagrelor in PLATO and no randomized evaluation.881
18.4.10 ANTIPLATELET THERAPY MONITORING AND GENETIC TESTING
Platelet function testing has provided a measure of certainty to the understanding of cardiovascular diseases: agents that provide powerful and consistent inhibition of P2Y12-mediated reactivity reduce post-procedural myocardial infarction and stent thrombosis, confirming the mechanistic hypothesis that P2Y12-receptor signalling is a major component of pathophysiological thrombus formation in patients with ACS treated with PCI.774 In the Assessment of Dual AntiPlatelet Therapy with Drug-Eluting Stents (ADAPT-DES) trial –the largest observational platelet function study conducted to date– close to 50% of 30-day post-PCI stent thrombosis was attributable to high platelet reactivity, defined as a P2Y12 reaction unit value of >208 when using the VerifyNow® bedside test.887 However, even if on-treatment platelet reactivity appears as a reliable and independent measure of the risk of future events,888,889 the concept of selective, intensive antiplatelet therapy based on a measured drug effect has never been successfully proven.890 Randomized trials examining the platelet function test hypothesis, namely GRAVITAS and Testing Platelet Reactivity In Patients Undergoing Elective Stent Placement on Clopidogrel to Guide Alternative Therapy With Prasugrel (TRIGGER-PCI), have been limited by low event rates, insufficient pharmacodynamic intervention, potential selection bias for low-risk patients, and an intervention in patients deemed to be non-responders after stent placement.778,891 The recent Assessment by a double Randomization of a Conventional antiplatelet strategy vs. a monitoring-guided strategy for drug-eluting stent implantation and, of Treatment Interruption vs. Continuation 1 year after stenting (ARCTIC) trial, which randomized the use of a bedside platelet function test, with repeated measures of ASA and clopidogrel response before and after platelet function test, with numerous pharmacodynamic interventions in poor responders (including the use of GP IIb/IIIa inhibitors, reloading, and switching to more potent P2Y12 inhibitors) was neutral.892 This study was appropriately powered, with a significantly more aggressive pharmacological intervention in non-responders leading to a two-fold reduction in the rate of non-responders. In summary, measuring treatment response by platelet function assays should be limited to clinical research but should not be routinely used in clinical practice.
Genetic variability in metabolism and absorption of clopidogrel is a key factor, responsible for the inefficient generation of the active drug metabolite. The two-step hepatic cytochrome P450 (CYP)-dependent oxidative metabolism of the prodrug appears to be of particular importance. Pharmacogenomic analyses have identified loss-of-function variant alleles of CYP 2C19 –and specifically the 2C19*2 allele– as the predominant genetic mediators of the antiplatelet effect of clopidogrel. Carriers have been shown to have lower active metabolite levels of clopidogrel, higher platelet reactivity and associated poorer outcomes.893-896 Rapid and accurate point-of-care genetic tests are available to identify these alleles. There are pending questions about the role of such testing, such as patient selection and whether personalized treatment based on genotype has a positive impact on clinical outcome and economy.897 At present, genetic testing cannot be recommended in routine clinical practice, due to insufficient prospective data.
In conclusion, platelet function testing or genetic testing may be considered in specific high-risk situations (e.g. history of stent thrombosis; compliance issue; suspicion of resistance; high bleeding risk).
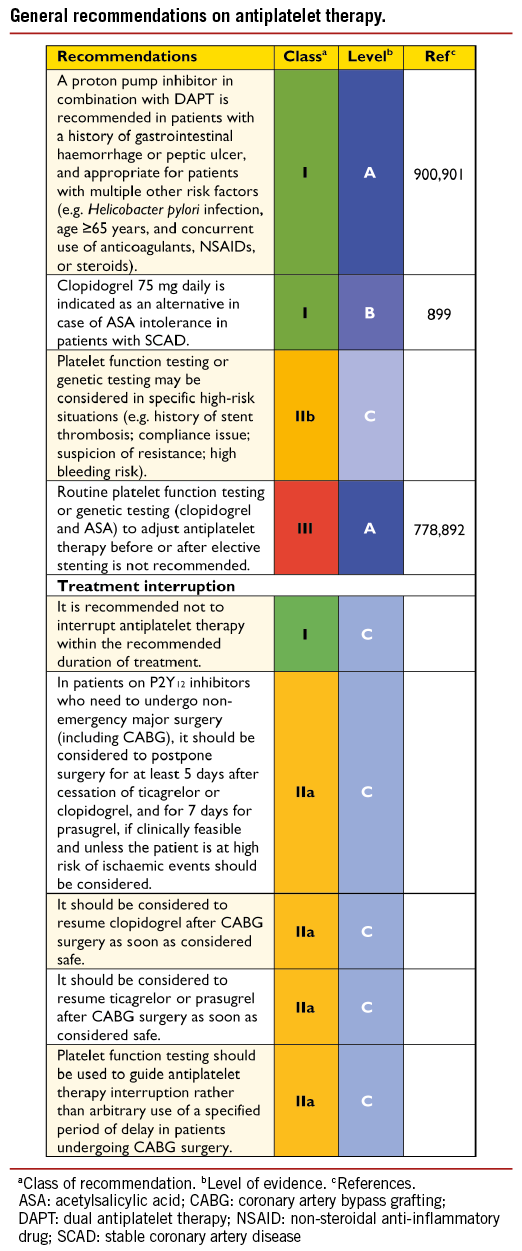
18.4.11 PATIENTS WITH HYPERSENSITIVITY TO ACETYLSALICYLIC ACID
In patients with ASA hypersensitivity, and in whom ASA therapy is necessary, a rapid desensitization procedure may be performed.898 Clopidogrel 75 mg daily is an appropriate alternative in patients who are intolerant of, or allergic to, ASA as long-term treatment.899 Alternatively, in cases of aspirin intolerance, a more potent, novel P2Y12 inhibitor (prasugrel or ticagrelor) may be preferred over clopidogrel as single antiplatelet therapy for a limited duration (1 to 6 months) after PCI.
18.4.12 Heparin-induced thrombocytopaenia
In patients with a history of heparin-induced thrombocytopaenia, neither UFH nor LMWH should be used, owing to concerns over cross-reactivity. In this case, bivalirudin is the best option for anticoagulation; other possible options are argatroban, hirudin, lepirudin, and danaparoid.
19. Volume – outcome relationship for revascularization procedures
Operator experience influences outcomes, in particular in critical, complex situations. The greater total experience of an entire hospital team –consisting of supporting members in the operating room or catheterization laboratory and those responsible for post-operative care– results in favourable outcomes. Therefore, the Leapfrog initiative has promoted PCI and CABG in high-volume centres.902
19.1 CORONARY ARTERY BYPASS GRAFTING
A meta-analysis, evaluating the impact of hospital volume in-hospital mortality, showed that among seven studies comprising 1 470 990 patients in 2040 hospitals, high-volume hospitals had lower mortality rates (OR 0.85; 95% confidence interval 0.83-0.91) even after adjustment for differences in case-mix.903 The volume of cases handled by a particular hospital may be high, but the number of procedures per surgeon may vary, making the surgeon-volume relationship a better marker. Although a recent study reported no significant difference in rates of in-hospital complications and 5-year mortality between surgical trainees and consultant surgeons after multivariable adjustment for differences in baseline characteristics (HR 1.02; 95% CI 0.87-1.20),904 the data substantiating a relationship is quite strong. Birkmeyer and co-authors found that surgeons’ case volume, as a continuous variable, was inversely related to operative mortality (adjusted OR 1.36; 95% CI 1.28-1.45).905 Moreover, when hospital case volume was taken into account, the impact of the surgeon’s case volume changed only marginally and remained a strong predictor (adjusted OR 1.33; 95% CI 1.25-1.42). Hospital volume itself had an OR of 1.13 (95% CI 1.03-1.24) if corrected for surgeon volume. It has been suggested, especially for the technically more challenging procedure of off-pump CABG, that surgical experience is of importance.906
Although the evidence accumulated over the years indicates that both surgeon and hospital case volumes matter,907 several studies suggest that quality measures are more important than volume per se and high volume does not necessarily result in better quality.908,909 Statistics on the rate of use of an IMA and on perioperative use of medication, and allowing data collection and monitoring by national registries, are several examples of these quality measures, all of which have been shown to be vital for improvement of outcomes. An observational cohort study of 81 289 CABG procedures performed by 1451 surgeons at 164 hospitals in North Carolina, USA, reported that missing quality indicators strongly predicted hospital mortality, irrespective of surgeon- or hospital case volume.910
Taking into consideration these data, the current American College of Cardiology Foundation/American Heart Association (ACCF/AHA) guidelines on CABG surgery provide a IIb recommendation that cardiac surgery programmes with less than 125 CABG procedures annually be affiliated with high-volume tertiary centres [level of evidence (LoE) C].285
19.2 PERCUTANEOUS CORONARY INTERVENTION
Numerous studies have investigated the relationship between volume of procedures and outcomes of PCI, suggesting a volume-outcome relationship at operator level, as well as institutional level.903,911-915 In a meta-analysis of 10 studies including over 1.3 million patients undergoing PCI at 1746 institutions between 1984 and 2005, treatment at high-volume centres was associated with a 13% RRR for in-hospital mortality (OR 0.87; 95% CI 0.83-0.91) compared with treatment at low-volume centres.903 Using a metaregression analysis of mean study year, the effect size did not attenuate appreciably over time. These findings are consistent with a population-based study from the PCI reporting system of New York, indicating that hospital case volumes of <400 PCIs per year and operator case volumes of <75 PCIs per year were associated with impaired outcomes.911 Some have suggested that procedural outcomes were levelled by technological improvements in PCI material, with progressive narrowing of outcome disparities and complication rates between high-volume and low-volume centres in the case of elective procedures.916 However, findings from studies carried out in the coronary stent era indicate that both operator- and hospital-volume experience continue to correlate with outcomes, with a relationship suggesting that the best outcomes are obtained with high-volume operators practising in high-volume institutions.912,917
Among patients with ACS, particularly STEMI, operator and hospital volume play an important role. A large study in the USA reported that, in a cohort of 36 535 patients undergoing primary PCI, shorter door-to-balloon times and lower in-hospital mortality resulted in institutions with higher primary PCI volumes.918 Similar results were observed in three more recent European observational studies.914,919,920 In another analysis of 29 513 patients with acute myocardial infarction who underwent primary PCI, treatment in high-volume centres was associated with a significantly lower door-to-balloon time than at medium- and low-volume centres (88 vs. 90 vs. 98 minutes, respectively; P-value for trend <0.001), although in-hospital mortality did not differ significantly (OR 1.22; 95% CI 0.78-1.91 for low-volume centres, and OR 1.14; 95% CI 0.78-1.66 for high- volume centres).921 Nallamothu and colleagues showed a direct relationship between degree of an institution’s specialization (operator and hospital experience, 24-hour/7-day availability, early activation of catheterization laboratory, written processes for emergency care) and outcomes in terms of in-hospital mortality among patients with acute myocardial infarction undergoing primary PCI.913
Current ACCF/AHA guidelines recommend that elective PCI should be performed by operators with annual case volumes of at least 75 procedures, at high-volume centres handling at least 400 procedures per year (Class I C) or, alternatively, by operators with annual volume of at least 75 procedures at centres handling at least 200 procedures per year (Class IIa C). In the case of primary PCI, it is recommended that, annually, operators should perform at least 75 elective procedures and ideally 11 primary PCI procedures in institutions that perform more than 400 elective PCIs per year and more than 36 primary procedures for STEMI.922 The ESC Guidelines on STEMI recommend that primary PCI should be performed only in centres providing 24-hour/7-day coverage.201 Owing to the continuing expansion of knowledge pertinent to PCI, increasing demands on technical skills needed to independently and expertly perform PCI, and the importance of Heart Teams in the management of patients with CAD, the ESC/EACTS Task Force on myocardial revascularization has issued recommendations on operator training and competence.
Training in interventional cardiology
A European training programme in interventional cardiology has been proposed by the European Association for Percutaneous Cardiovascular Interventions (EAPCI) in order to ensure high quality of patient care and clinical excellence. The programme should last 1-2 years at high-volume institutions that handle at least 800 PCIs per year and that have established 24-hour/7-day service for the treatment of patients with ACS.
During the programme, trainees should perform at least 200 PCI procedures as first- or only operator, acting under supervision for one-third (>66) of these procedures in emergency or ACS patients before becoming independent. Additionally, trainees are required to attend at least 30 days (240 hours) of formal learning, including attendance at accredited national and international courses in interventional cardiology.
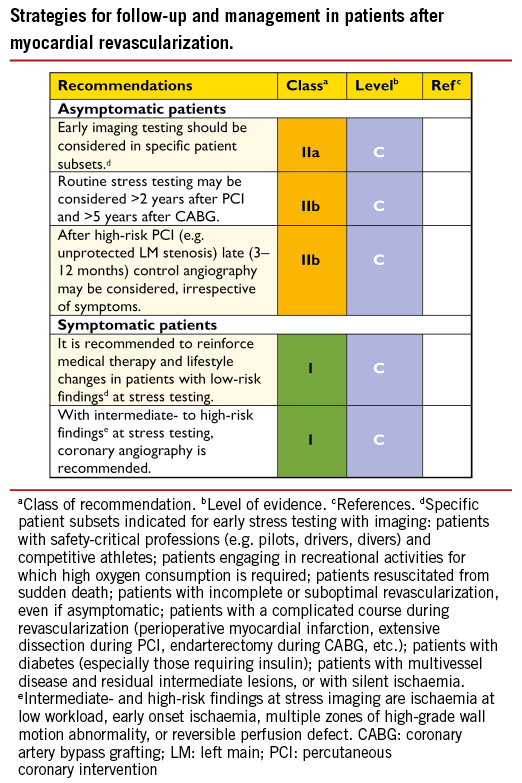
20. Medical therapy, secondary prevention, and strategies for follow-up
Myocardial revascularization must be accompanied by medical therapy and other secondary prevention strategies for risk factor modification and permanent lifestyle changes.925 Secondary prevention and cardiac rehabilitation are an integral part of the management strategy after revascularization, because such measures reduce future morbidity and mortality in a cost-effective way and can further ameliorate symptoms.
Although the need to detect restenosis has diminished in the DES era, the recurrence of symptoms due to disease progression or restenosis deserves attention. Likewise, the durability of CABG results has increased with the use of arterial grafts, and ischaemia stems mainly from SVG attrition and/or progression of CAD in native vessels.
21. Addenda
ESC National Cardiac Societies actively involved in the review process of the 2014 ESC/EACTS Guidelines on myocardial revascularization:
Austria, Austrian Society of Cardiology, Franz Weidinger; Azerbaijan, Azerbaijan Society of Cardiology, Firdovsi Ibrahimov; Belgium, Belgian Society of Cardiology, Victor Legrand; Bosnia and Herzegovina, Association of Cardiologists of Bosnia & Herzegovina, Ibrahim Terzic; Bulgaria, Bulgarian Society of Cardiology, Arman Postadzhiyan; Croatia, Croatian Cardiac Society, Bosko Skoric; Cyprus, Cyprus Society of Cardiology, Georgios M. Georgiou; Czech Republic, Czech Society of Cardiology, Michael Zelizko; Denmark, Danish Society of Cardiology, Anders Junker; Estonia, Estonian Society of Cardiology, Jaan Eha; Finland, Finnish Cardiac Society, Hannu Romppanen; France, French Society of Cardiology, Jean-Louis Bonnet; Georgia, Georgian Society of Cardiology, Alexander Aladashvili; Germany, German Cardiac Society, Rainer Hambrecht; Hungary, Hungarian Society of Cardiology, David Becker; Iceland, Icelandic Society of Cardiology, Thorarinn Gudnason; Israel, Israel Heart Society, Amit Segev; Italy, Italian Federation of Cardiology, Raffaele Bugiardini; Kazakhstan, Association of Cardiologists of Kazakhstan, Orazbek Sakhov; Kyrgyzstan, Kyrgyz Society of Cardiology, Aibek Mirrakhimov; Luxembourg, Luxembourg Society of Cardiology, Bruno Pereira; Malta, Maltese Cardiac Society, Herbert Felice; Norway, Norwegian Society of Cardiology, Thor Trovik; Poland, Polish Cardiac Society, Dariusz Dudek; Portugal, Portuguese Society of Cardiology, Helder Pereira; Serbia, Cardiology Society of Serbia, Milan A. Nedeljkovic; Slovakia, Slovak Society of Cardiology, Martin Hudec; Spain, Spanish Society of Cardiology, Angel Cequier; Sweden, Swedish Society of Cardiology, David Erlinge; Switzerland, Swiss Society of Cardiology, Marco Roffi; The Former Yugoslav Republic of Macedonia, Macedonian FYR Society of Cardiology, Sasko Kedev; Tunisia, Tunisian Society of Cardiology and Cardio-Vascular Surgery, Faouzi Addad; Turkey, Turkish Society of Cardiology, Aylin Yildirir; United Kingdom, British Cardiovascular Society, John Davies.
![]()
![]()

