Abstract
Aims: Subacute, late, and very late stent thrombosis (ST) may occur after stent implantation, but they are characterised by different underlying pathophysiological mechanisms. We sought to appraise differences between subacute and late/very late ST at the thrombus site by optical coherence tomography (OCT). The Mechanism Of Stent Thrombosis (MOST) study was a prospective multicentre non-randomised registry which enrolled six subacute ST and six controls (subacute ST study), and 17 late/very late ST and 17 controls (late/very late ST study).
Methods and results: Patients with subacute ST had a minimum stent area at the thrombus site of 2.1 mm2 (1st-3rd quartile 1.3-4.5) vs. 2.9 mm2 (2.4-5.0) in the matched control (p=0.05). Uncovered struts were 26.2% (16.5-35.9) vs. 13.9% (8.9-18.9), p=0.001. Malapposed struts were 18.8% (13.1-24.5) vs. 15.2% (12.8-17.6), p=0.001. In patients with late/very late ST, uncovered struts were 23.6% (13.9-33.3) vs. 5.2% (0.5-10.2), p=0.001. Malapposed struts were 12.1% (6.4-17.8) vs. 2.8% (0.4-5.2), p=0.001, and maximum malapposition distance was 0.45 mm (0.32-0.62) vs. 0.12 mm (0-0.25), p=0.01. Notably, all patients with ST had previously discontinued dual antiplatelet therapy (n=14) or showed high residual platelet reactivity on clopidogrel therapy.
Conclusions: Subacute ST had a significant stent underexpansion while late/very late ST had a greater stent strut malapposition distance at the thrombus site. These findings explain how procedure-related complications and vessel remodelling have a specific impact on the segment characterised by thrombus. High platelet reactivity also seems a necessary cofactor for both subacute and late/very late ST. Clinical Trial Registration: http://www.clinicaltrials.gov unique identifier NCT01410539.
Introduction
Stent thrombosis (ST) is a major complication of percutaneous coronary intervention (PCI) with either bare metal (BMS) or drug-eluting stents (DES). Although ST is relatively rare, it may be fatal1-4. The mechanism of ST is still not clear1,2, depending as it does on: 1) patient characteristics and risk factors, 2) stent, treated lesion and procedure-related factors, and 3) platelet reactivity and other unknown factors5-7.
ST includes four different categories: acute, subacute, late and very late ST. Different pathophysiological mechanisms are involved in each category and, in particular, procedure-related and mechanical-related factors seem to play a role in acute/subacute ST, while uncovered stent struts and inflammation have a role in late/very late ST.
Optical coherence tomography (OCT) is a new intracoronary imaging modality that enables a detailed analysis of the stented segment. The use of OCT in patients with ST may therefore be a valid approach to help understand the impact of stent-related features that can lead to ST.
We sought to assess stent strut coverage and malapposition at the thrombus site between subacute ST compared to matched controls and, similarly, between late/very late ST and matched controls. Finally, we evaluated ongoing antiplatelet treatment and residual platelet reactivity using the VerifyNow® System (Accumetrics Inc., San Diego, CA, USA) in patients on clopidogrel therapy.
Methods
The Mechanism Of Stent Thrombosis (MOST) study was a prospective multicentre non-randomised registry. Stent thromboses were defined according to the Academic Research Consortium (ARC) definitions8. Subacute ST patients (>3 days and within 30 days) were all the patients presenting to one of the participating centres with a previous PCI with BMS or DES. Late/very late ST (beyond 30 days) patients were all the patients with a previous PCI with DES.
Study exclusion criteria were: a) development of ST within 72 hours of stent implantation (acute and early subacute ST); b) late and very late ST of BMS because the mechanisms of late ST in BMS seem to be associated more with in-stent neoatherosclerosis as compared to DES9; c) age less than 18 years; d) creatinine values greater than 2.5 g/dl (this was to avoid the negative effects related to the contrast medium necessary to perform the OCT evaluation); and e) ST of either left main or aorto-ostial involvement and heavily calcified lesion.
After thrombectomy the stent with ST was assessed by OCT before further interventions. Moreover, in patients who were still on clopidogrel therapy at the time of ST, a VerifyNow P2Y12 assay (Accumetrics Inc.), a turbidimetry-based optical detection system that measures platelet-induced aggregation by the level of P2Y12 receptor blockade, was performed immediately after angiographic diagnosis of ST, but before the administration of further antiplatelet agents, in order to assess residual platelet reactivity (RPR) to ADP (VerifyNow P2Y12). According to a previous report10, high RPR was defined as P2Y12 reaction units (PRU) ≥240. Genomic DNA of patients was extracted from peripheral blood leukocytes using the GeneCatcher™ gDNA Blood Kit (Invitrogen, Life Technologies, Carlsbad, CA, USA). CYP2C19*2 (rs4244285) loss-of-function polymorphism genotyping was performed using a TaqMan® SNP assay with the 7900HT Sequence Detection System (Applied Biosystems®, Life Technologies).
The study protocol was approved by the coordinating centre ethics committee (#554/10). Written informed consent in accordance with the Declaration of Helsinki and the applicable laws of our country was obtained from all patients prior to any study-related procedure.
OCT assessment
OCT PROCEDURE
After preliminary manual or mechanical thrombectomy at the ST site, with or without the use of glycoprotein IIb/IIIa inhibitors11,12, the target stented segment was assessed by either frequency-domain (FD) OCT (18 out of 23 ST patients and 16 out of 23 matched controls), or time-domain (TD) OCT.
The FD OCT (C7 XR) system (St. Jude Medical, St. Paul, MN, USA) has been previously described13, and proved its safety14. After administration of intracoronary nitrates (about 100 γ), the FD OCT imaging catheters were delivered over a 0.014 inch guidewire through a 6 Fr or larger guiding catheter. For an effective clearing of blood from the imaging field, an angiographic contrast medium was injected through the guiding catheter adopting an automated modality15.
Injection of a 14 ml volume of contrast at a rate of <4 mL/sec was sufficient to achieve an imaging period of two to three seconds consistently in all of the major coronary branches. At a pullback rate of 20 mm/sec, an imaging period of two seconds was long enough to scan a 4 cm vessel segment.
The TD OCT examinations were carried out using a dedicated optical wire (Image Wire; St. Jude Medical) connected to either the M2 or M3 OCT console (St. Jude Medical).
The OCT image wire was advanced distally to the region of interest by an over-the-wire catheter (Transit; Cordis, Johnson & Johnson, Miami Lakes, FL, USA). The automated pullback (at 3.0 mm/sec) was then commenced during simultaneous flushing of viscous iso-osmolar contrast (Visipaque™) through the guiding catheter by use of an automated power injector.
OCT images were calibrated adjusting the Z-offset. This critical step was performed before image acquisition so as to obtain accurate measurements.
OCT IMAGE ANALYSIS
OCT and angiographic images were stored on CDs and then sent to an independent coronary imaging core lab for evaluation (Rome Heart Research, Rome, Italy), blinded to clinical data.
All cross-sectional images were screened for quality and excluded from analysis if >25% of the image was out of the screen, if a side branch was present in the cross-section, or for inadequate image quality due to some artefacts. Patients were excluded by reason of OCT analysis if the percentage of analysable cross-section was less than 95.1%16.
OCT analysis was conducted on three levels: cross-section level analysis, strut level analysis and patient level analysis. OCT findings were separately presented based on their clinical classification as subacute ST or late/very late ST (Figure 1).
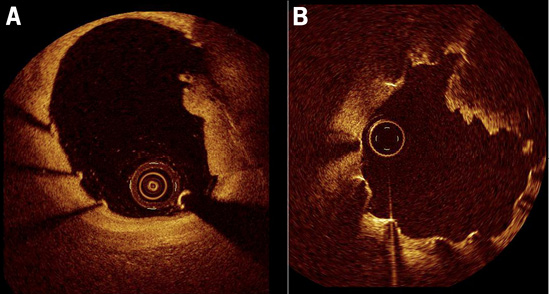
Figure 1. Intra-stent thrombus assessed by OCT. A) OCT findings in a patient with subacute stent thrombosis after bare metal stent implantation. B) OCT findings in a patient with very late stent thrombosis after drug-eluting stent implantation.
In the absence of residual thrombus a covered stent strut was defined when the distance between the centre of the reflection of the stent and the surface of tissue was more than 20 μm; otherwise it was defined as an uncovered strut. In the presence of thrombus remnants, despite the use of thrombo-aspiration, struts were judged uncovered if: 1) the thrombus was not contiguous with the vessel wall and the presence of neointima could reasonably be excluded and 2) in sequential cross-sections the studied strut was located on the surface of the residual thrombus in at least one frame where it appeared uncovered.
A malapposed strut was classified when the distance between the centre of the reflection and the vessel wall was more than the thickness of the stent strut for BMS and more than the thickness of the stent strut+polymer thickness for DES17.
Statistical analyses
Statistical analysis was performed using SPSS 17.0 (SPSS Inc., Chicago, IL, USA).
Categorical variables are presented as frequencies and analysed with the chi-square test or Fisher’s exact test. Most continuous variables were not normally distributed as assessed by the Kolmogorov-Smirnov test for normality, so they were presented as median and interquartile range (IQR), and logarithmic transformation was applied to the data to allow the Student’s test to be applied. Comparisons were performed between the thrombus site of subacute ST and the stented segment in the entire length of matched control patients. The same approach was applied for late/very late ST. An alpha level of 0.05 was considered statistically significant.
Sensitivity analyses based on random-effect mixed models without any log transformation were also conducted, yielding similar results in terms of statistical magnitude and direction. Given the similar findings obtained with both analytical approaches, results stemming from standard bivariate analyses are reported throughout.
To compare the OCT findings in patients with either subacute or late/very late ST included in the present registry, a case-matched (1:1) control group of patients without ST undergoing OCT stent follow-up was selected from the Rome Heart Research OCT core lab database (control group). Matching was performed through an automatic query on the database, blinded to OCT findings. For each ST patient, the first patient in the database satisfying the matching parameters and fulfilling inclusion/exclusion criteria was chosen. The matching parameters in order of sequential selection were as follows: 1) stent type; 2) days from stent implantation to OCT assessment (±7 days for subacute ST or ±30 days for late ST); 3) ST-elevation myocardial infarction as admission diagnosis; and 4) stent length (±5 mm). We chose 1:1 matching instead of 1:N matching as OCT enables very detailed per-lesion, per-segment and per-cross-section analyses, and thus adding additional controls would not have increased statistical power and precision meaningfully.
Results
Between January 2010 and June 2011, 30 consecutive patients presenting with an acute coronary syndrome due to a definite ST were screened for entry to the study. A study flow chart is depicted in Figure 2. Seven patients were excluded, four because of acute ST, two because of suboptimal recorded OCT images due to a poor blood clearing process during OCT acquisition (both cases were performed using TD OCT), and one because of unavailable OCT system. Thus, data regarding the remaining six patients with subacute ST and 17 patients with late/very late ST are reported in the present manuscript and compared with the six and 17 control patients, respectively. The baseline characteristics of the subacute ST and the late/very late ST patients and their matched control subjects are reported in Table 1. There were no significant clinical differences among the study groups, but left ventricular ejection fraction was numerically lower in patients with ST (42%±14% versus 45%±10%, p=0.061). Rheolytic thrombectomy was used in 13 patients and manual aspiration in 10 patients according to the operator’s experience and centre availability.
Figure 2. Study flow chart.
OCT findings in subacute stent thrombosis
OCT findings for subacute ST are described in Table 2.
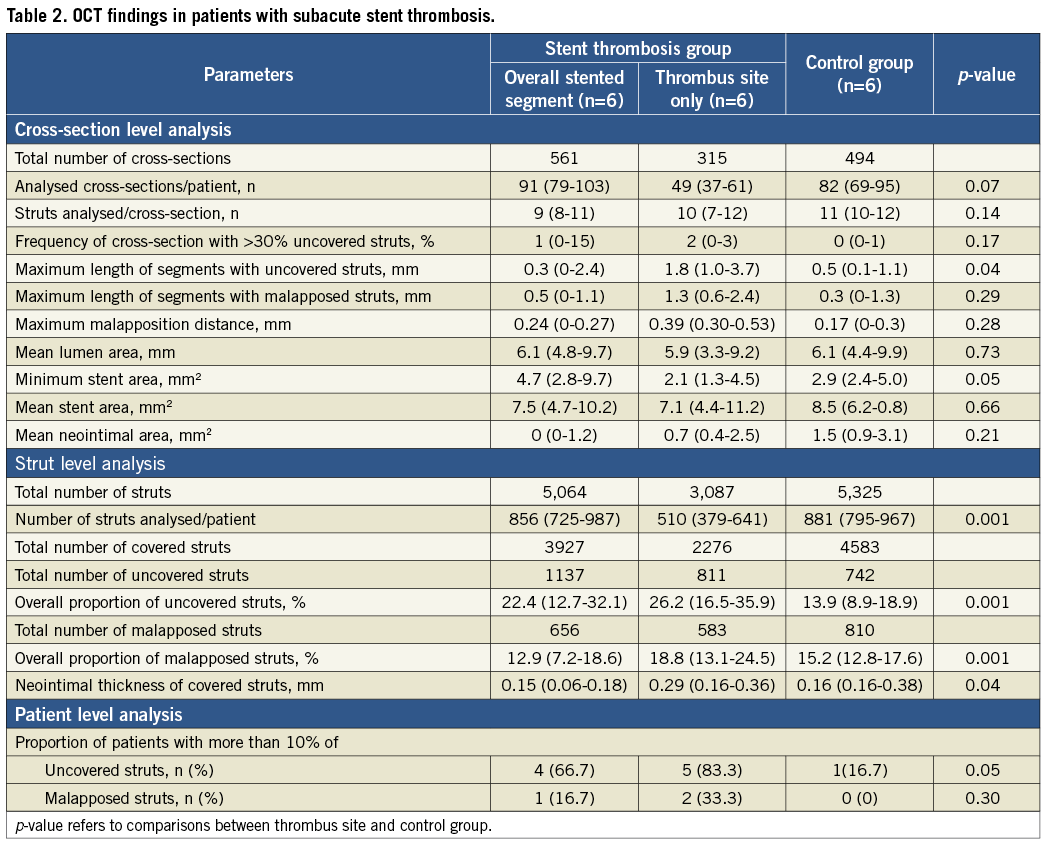
In the cross-section level analysis, minimum stent area (MSA) was significantly lower at the thrombus site compared with matched control patients, 2.1 mm2 (1.3-4.5) vs. 2.9 mm2 (2.4-5.0), respectively (p=0.05). Mean stent area did not differ in the two groups due to the focal extension of underexpansion.
In the strut level analysis the percentage of both uncovered struts and malapposed struts was significantly higher at the thrombus site compared with matched control patients.
In the patient level analysis the proportion of patients with more than 10% of uncovered struts at the thrombus site was 83.3% vs. 16.7% in matched control patients (p=0.05).
OCT findings in late/very late stent thrombosis
OCT findings for late and very late ST are described in Table 3. At the cross-section level analysis maximum malapposition distance was higher at the thrombus site compared with matched control patients, 0.45 mm (0.32-0.62) vs. 0.12 mm (0-0.25), (p=0.019).
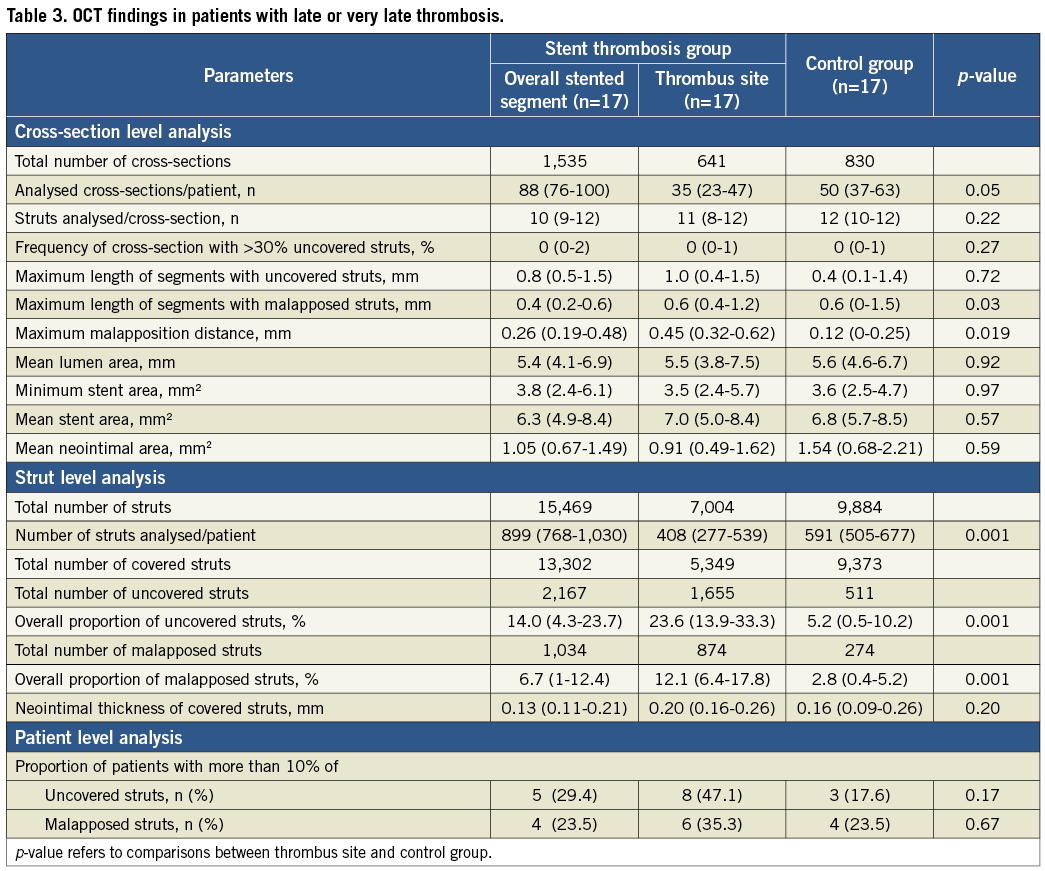
In the strut level analysis the percentage of both uncovered struts and malapposed struts was significantly higher at the thrombus site compared with matched control patients.
In the patient level analysis the proportion of patients with more than 10% of uncovered struts at the thrombus site was 47.1% vs. 17.6% for matched control patients (p=0.17).
Platelet function tests and clopidogrel pharmacogenetics
Overall, 14 patients (61%) had discontinued dual antiplatelet therapy before ST (average time 398±308 days), while nine (39%) patients were reported to have taken clopidogrel until the ST day (six subacute, two late, and one very late stent thrombosis; Table 4). In eight of these patients a high RPR was documented using the VerifyNow system (Accumetrics Inc.). Moreover, four out of seven (57%) patients on clopidogrel treatment when genotyped were carriers (heterozygotes or homozygotes) of the CYP2C19*2 polymorphism.
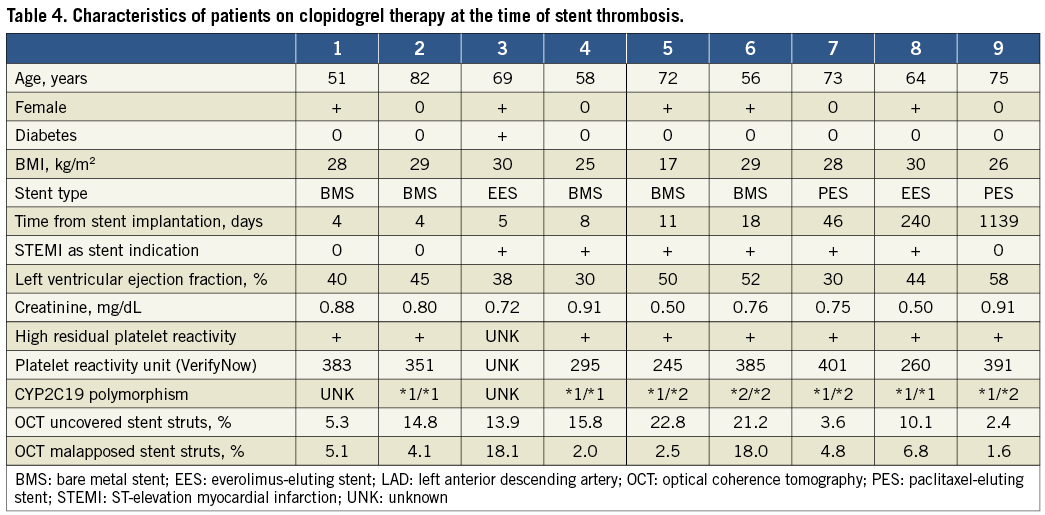
Figure 3 shows the relation between RPR and uncovered stent strut rate. At patient discharge, prasugrel was used as P2Y12 platelet receptor inhibitor in 15 patients, according to platelet function test results and drug availability.
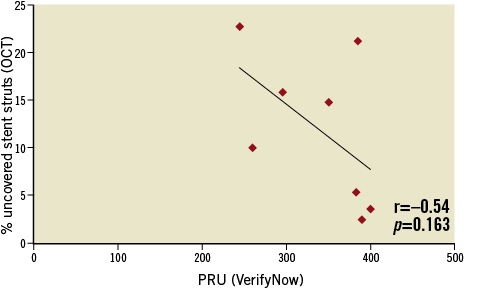
Figure 3. Relationship between residual platelet reactivity by VerifyNow and uncovered stent strut rate by OCT in eight patients taking clopidogrel at the time of stent thrombosis.
Discussion
The main findings of the present study are the following:
– Although subacute and late/very late ST were both characterised by higher malapposed and uncovered struts, these findings were mainly located at the thrombus site of the culprit stent.
– A smaller minimal stent area was found in patients with subacute ST as compared with controls, suggesting stent underexpansion as a potential mechanism in these patients.
– The fact that dual antiplatelet therapy discontinuation or high RPR was detected in all the patients with ST suggests that this phenomenon might be an important risk factor for such adverse events.
Autopsy studies of DES late thrombosis have shown that the most frequent substrate is the abnormal healing with subsequent delayed and incomplete stent strut coverage18. However, pathologic studies carry the bias of the assessment limited to subjects with the worst outcome and case control matching is not possible. Moreover, intravascular ultrasound has shown that DES use may be associated with incomplete stent apposition, vessel remodelling, and histopathologic evidence of eosinophilic inflammation19,20.
In addition, Guagliumi et al recently used both first-generation TD OCT and IVUS for assessing the percentage of uncovered struts and vessel remodelling in a cohort of late ST. Interestingly, the percentage of uncovered struts was significantly higher in the late ST group compared with the control group (12.27 vs. 4.14), as well as sections with >30% uncovered struts21.
Our study confirms and expands the results of previous studies regarding stent-related findings after ST. In particular, patients who experienced a ST showed a higher rate of uncovered stent struts by OCT as compared with patients who had the same stent implanted for the same reason at the same time interval from the stent implantation. Unlike previous reports, we also analysed the percentage of lack of coverage/malapposition at the thrombus site, which was relatively short in length, highlighting the fact that stent-related defects are often focal in our cohort of ST patients.
The number and percentage of uncovered stent struts were particularly high at the ST site, reaching a 24% uncovered stent strut rate that seems very similar to the pathologic findings18. Thus, the incomplete endothelialisation (or generally, coverage) of the stents exposes the metallic material to the bloodstream and consequent thrombosis. Similarly, malapposed stent struts were clearly more frequent in patients with late ST as compared to controls. In patients with late ST, a three times higher malapposed strut rate was detected as compared to controls (nearly four times higher at the stent thrombus site). The difference between the ST group and the control group was particularly evident in late ST, probably due to the impact of late acquired stent malapposition.
We also noticed a significant stent underexpansion in the subacute ST group at the thrombus site. This finding further supports the concept that very early postprocedural events are frequently triggered by suboptimal PCI results and procedure-related defects, although presence of uncovered and malapposed struts can be an additional mechanism of both subacute and late/very late ST.
It is well known that arterial healing after stent implantation is a very heterogeneous and complex phenomenon influenced by the morphology and composition of the underlying plaque and the type of stent implanted. BMS subacute stent thrombosis occurs with uncovered and malapposed stent strut rates that are similar to the ones found in patients with DES thrombosis22. However, at a later stage of BMS implantation, the growth of neointimal hyperplasia or atherosclerotic lesions inside the stent is expected to contribute to a greater proportion of cases of BMS thrombosis23-25. In our study, late BMS thromboses, which are very rare events and probably have different mechanisms, were not included in our registry.
All patients with ST had previously discontinued dual antiplatelet therapy or showed high residual platelet reactivity on clopidogrel therapy. In fact, point-of-care tests, performed at the time of presentation in eight patients who continued to take clopidogrel, revealed a high RPR, according to a previously validated cut-off. Thus, we confirmed that high RPR acts as a cofactor for ST. After DES implantation, the delayed and incomplete strut coverage increases the risk of thrombosis that is probably directly proportional to the number of uncovered metallic struts facing the bloodstream.
Currently, it is unknown what extent of lack of stent coverage can be considered unsafe. Effective dual antiplatelet therapy is able to limit the increased risk related to uncovered stent struts, and in the meantime the neointima increases stent coverage. Early discontinuation of dual antiplatelet therapy, or ineffective platelet inhibition due to poor responsiveness to therapy, allows the uncovered metallic stent strut to trigger the thrombotic event. The number of uncovered stent struts is only a chronic trigger factor, requiring activated platelets to develop thrombosis. Taking into consideration the small amount of available data, it seems that, in the case of a slight lack of stent strut coverage, the occurrence of ST can be favoured by concomitant high RPR. However, these data must be considered only hypothesis-generating and not conclusive.
Intracoronary imaging is able to assess the efficacy of coronary thrombus removal procedures and to detect the prevalent stent-related factor that caused ST. Due to the important contribution of platelets to the event, intracoronary imaging should be combined with the evaluation of RPR in order to understand fully the ST causes patient by patient and to guide not only the interventional coronary therapies but also the subsequent medical management.
Limitations
Our study must be evaluated in the light of some limitations. First, the small sample size is certainly its most important limitation. However, we enrolled a study population that mirrors other similar OCT studies26,27, and the data provided may add important clinical information to the current knowledge. Since ST is associated with high mortality, it is likely that some patients died before reaching a catheterisation laboratory, and thus the reported data refer only to ST patients not complicated by sudden cardiac death or fatal cardiogenic shock. Second, identification of lack of stent strut coverage in the setting of ST is not a simple task, as thrombus remnants represent an obstacle that may underestimate the incidence of lack of strut coverage. To overcome this issue, we considered only stents imaged with OCT after thrombus aspiration, performed in the majority of cases with a powerful rheolytic thrombectomy device, with thrombus aspiration being repeated until optimal thrombus removal was achieved. Furthermore, we applied novel criteria to detect lack of coverage in case of thrombus remnants, despite the use of thrombectomy. Third, we did not assess local or systemic markers of inflammation. It is currently believed that hypersensitivity reaction around the struts coated with polymers and aneurysmal formations associated with extensive inflammatory processes in the arterial wall may be contributory factors at least in some ST cases28. In our series no ST was associated with a coronary aneurysm. Also, we did not perform a histological analysis of thrombotic material obtained after thrombectomy. Fourth, in the case of malapposed strut documentation at the time of ST, we were not able to distinguish between persistent incomplete stent apposition (from the time of stent implantation) and late acquired stent malapposition29. The lack of immediate postprocedural OCT images precludes determining the time of stent strut malapposition. Unfortunately, the evaluation of the degree of stent apposition at the time of stent implantation by intracoronary imaging, either IVUS or OCT, is not routinely used because it is expensive and time-consuming, and no study has proved a clear benefit on clinical outcomes. Fifth, we did not include acute ST due to the absence of a matching control group. Sixth, despite the innovative assessment of lack of stent coverage using novel definitions, it was not possible to analyse all cross-sections. As a consequence, significant differences in the numbers of struts and frames analysed were observed among groups (Table 3). In addition, no baseline OCT was performed and so none was available for analysis. Finally, short-term coverage data (Table 4) remain difficult to interpret, given that endothelial coverage is most likely going to require more than one week in any given stent. However, the relatively high rate of uncovered/malapposed struts suggests that this might be a potential safety threat early after stenting.
Conclusions
The above-mentioned limitations notwithstanding, the present study provides several unique and potentially important insights into the mechanisms responsible for ST. Patients with ST show a higher rate of uncovered and malapposed stent struts as compared with control subjects. High platelet reactivity seems a necessary cofactor. In the acute phase, intracoronary imaging findings and platelet function tests give the opportunity for individualised and cost-effective treatments of ST. The prevention of ST represents a great challenge for the interventional cardiologist, whose goal is to provide more effective and safe options of revascularisation in patients with coronary artery disease. Without doubt, the complete and definitive prevention of this dramatic complication of percutaneous management of coronary artery disease will be possible only if we achieve a better understanding of the mechanisms involved in the pathophysiology of ST.
Acknowledgements
This study was supported by the CLI Foundation, Rome, Italy, and by the A.R. CARD Foundation, Florence, Italy.
Conflict of interest statement
G. Parodi reports having received lecture fees from Daiichi Sankyo/Eli Lilly and Company and AstraZeneca. M. Valgimigli has received honoraria for lectures, has served on the advisory board, and has received research grants from Merck, Iroko, Eli Lilly and Company, and Medtronic; has received honoraria for lectures and served on the advisory board for The Medicines Company and Eli Lilly and Company, Daiichi Sankyo Inc., St. Jude Medical and Abbott Vascular; and has received honoraria for lectures from Cordis, CID, and Terumo. M. Fineschi reports having received lecture fees from St. Jude Medical and AstraZeneca. The other authors have no conflicts of interest to declare.

