Abstract
Aims: Cangrelor is a new antiplatelet agent that has been used in percutaneous coronary intervention (PCI) with mixed results. We aimed to review the evidence on the efficacy of cangrelor in comparison to clopidogrel in reducing ischaemic endpoints at 48 hours in patients undergoing PCI in large randomised trials.
Methods and results: In three large clinical trials involving 25,107 participants, the risk of the primary composite efficacy endpoint of death, MI and ischaemia-driven revascularisation at 48 hours, (pooled OR 0.94; 95% CI: 0.77-1.14, p=0.51, I2=68%), death from all cause (pooled OR 0.72, 95% CI: 0.36-1.43, p=0.34, I2=52%), myocardial infarction (pooled OR 0.94, 95% CI: 0.77-1.14, p=0.51, I2=68%) was not significantly different between cangrelor and clopidogrel. Likewise, severe or life-threatening bleeding was similar between cangrelor and clopidogrel (pooled OR 1.21, 95% CI: 0.70-2.12, p=0.50, I2=0%). The risk of stent thrombosis (pooled OR 0.59, 95% CI: 0.43-0.81, p=0.001, I2=0%), Q-wave myocardial infarction (pooled OR 0.53, 95% CI: 0.30-0.92, p=0.02, I2=0%) and ischaemia-driven revascularisation (pooled OR 0.71, 95% CI: 0.52-0.98, p=0.04, I2=0%) was lower in the cangrelor group.
Conclusions: Based on this meta-analysis, we did not find any difference in the risk of the primary composite efficacy endpoint of all-cause death, ischaemia-driven revascularisation, and myocardial infarction at 48 hours between cangrelor and clopidogrel use. Given that cangrelor was associated with a lower risk of stent thrombosis, ischaemia-driven revascularisation and Q-wave myocardial infarction compared to clopidogrel, cangrelor can be considered as a suitable alternative during PCI.
Introduction
Adjunctive therapy with antithrombotic agents plays an important role in reducing adverse cardiac events during percutaneous coronary intervention (PCI)1. Most commonly, aspirin and clopidogrel are used during PCI. Pharmacokinetic and pharmacodynamic properties2,3 and genetic polymorphisms leading to clopidogrel resistance4 can sometimes limit the efficacy of clopidogrel. Oral administration can also be challenging in sick patients presenting with acute coronary syndromes undergoing PCI in settings where nausea, vomiting, and intubated patients are managed. Two other oral antiplatelet agents, prasugrel and ticagrelor, both P2Y12 inhibitors, have been shown to be useful alternatives in a selected patient population undergoing PCI5-8. However, their use is limited to the oral route of administration. Hence, a newer antiplatelet agent, cangrelor, which is quickly reversible9, rapid acting, and with a shorter half-life of less than six minutes, has been tried in PCI with mixed and inconclusive results10-12.
Therefore, we sought to review systematically the evidence on the efficacy of cangrelor in comparison to clopidogrel in reducing ischaemic endpoints at 48 hours in patients undergoing PCI in large randomised trials.
Methods
A protocol for this meta-analysis was prospectively devised that details the background, the objectives, and eligibility criteria of studies, outcomes and statistical method. This is available for review upon request to the investigators.
STUDY SELECTION
The PRISMA statement for reporting systematic reviews13 recommended by the Cochrane Collaboration was followed for the conduct of this meta-analysis. This qualitative systematic review and meta-analysis included studies published up to May 2013. PubMed, Cochrane Library, EMBASE, CINAHL and Web of Knowledge were searched using the search terms “Cangrelor” and “Clopidogrel” and “PCI”. The search was limited to clinical trials. Hand searching for additional relevant studies was done until no further references were found.
The following search string was used to find articles suitable for inclusion (“cangrelor” OR “cangrelor”[All Fields]) AND PCI [All Fields] AND (“clopidogrel” OR “clopidogrel”[All Fields]) AND (“randomised controlled trial”[Publication Type] OR “randomised controlled trials as topic”[MeSH Terms] OR “randomised controlled trial”[All Fields] OR “randomised controlled trial”[All Fields]). Comparing papers from the same authors eliminated data duplication due to multiple reporting. We also searched www.clinicaltrials.gov for studies. Two authors (MRA and LJ) screened and retrieved reports and excluded irrelevant studies. One author (MRA) extracted and another author (LJ) crosschecked the data. An additional author (AP) participated in the review process when uncertainty about eligibility was encountered. Mean age, gender, dose of clopidogrel, type of stent used, and loading dose before or after PCI as well as the indication for PCI, primary and secondary efficacy endpoints were extracted and tabulated.
SELECTION OF STUDIES FOR THE REVIEW
We selected studies that were randomised, and compared the use of post- or pre-PCI cangrelor and/or clopidogrel. The steps of the literature search process are summarised in Figure 1. The eligibility criteria for this meta-analysis were: 1) human subjects older than 18 years with acute coronary syndromes (STEMI/NSTEMI/UA) and/or stable or unstable angina undergoing PCI; 2) post or pre-PCI treatment with clopidogrel and/or cangrelor; 3) phase III and higher randomised clinical trial. We did not include conference abstracts.
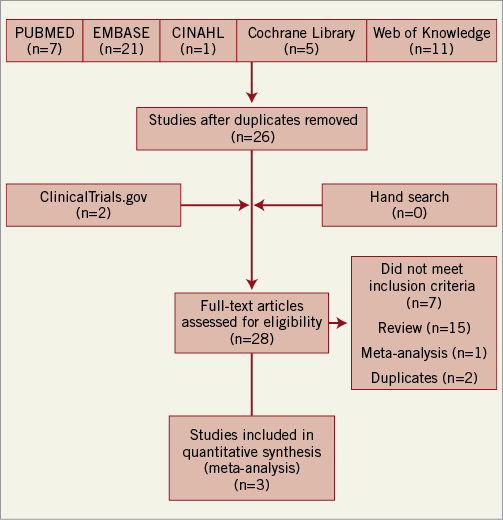
Figure 1. Flow chart of study selection protocol.
DEFINITION OF OUTCOMES FOR THIS META-ANALYSIS
The composite of death from any cause, myocardial infarction or ischaemia-driven revascularisation at 48 hours and definite stent thrombosis were two pre-specified primary efficacy endpoints for this meta-analysis. We also examined death from all cause, ischaemia-driven revascularisation and myocardial infarction as secondary endpoints. The Global Utilisation of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries (GUSTO) trial-defined severe or life-threatening bleeding was defined as the primary safety endpoint. GUSTO major bleeding, TIMI major and minor bleeding were also assessed. The data on endpoints were extracted from the published full text articles and, if the data were not published in the main full text articles, then supplemental appendices were searched. In the Cangrelor versus Standard Therapy to Achieve Optimal Management of Platelet Inhibition (CHAMPION) PHOENIX trial, the primary endpoint was different compared to the other two RCT. It included death, myocardial infarction, ischaemia-driven revascularisation or stent thrombosis. However, a supplemental appendix is published online providing incidence of death from all cause, myocardial infarction or ischaemia-driven revascularisation at 48 hours. Incidence of definite stent thrombosis was reported in the supplemental appendix of the trial so we extracted data from the supplemental appendix in the case of the CHAMPION PHOENIX trial11.
STATISTICAL ANALYSIS
All outcome comparisons and treatment effects were calculated with RevMan version 5.2 (Cochrane Collaboration, Oxford, United Kingdom). Intention-to-treat patient cohorts were used for this meta-analysis. The summary odds ratio and 95% confidence intervals were estimated using a random effects method. To control for heterogeneity, random effect models were used for this meta-analysis as their assumptions account for the presence of variability among the studies. We also performed a sensitivity analysis using the inverse variance method to pool odds ratios that were adjusted for baseline differences between the two treatment arms. We calculated the I2 statistic to evaluate the percentage of heterogeneity among the trials. Suggested thresholds for heterogeneity were used with I2 values of 25% to 49%, 50% to 74% and ≥75% indicative of low, moderate and high heterogeneity14. Because the sample was limited to three studies, publication bias was not assessed. A p-value of <0.05 was used as the level of significance.
Results
CHARACTERISTICS OF INCLUDED STUDIES
A total of 26 trials were screened for eligibility. Of these, twenty-three studies were excluded for the following reasons: seven studies did not meet the inclusion criteria, fifteen were reviews, and one study was excluded as it was a meta-analysis of all P2Y12 as one arm and clopidogrel as the other arm (Online Appendix 1). Only three trials met the inclusion criteria (Figure 1) and were included in the final analyses10-12. A risk of bias assessment was performed at study level for all the included studies (Online Figure 1). There was an unclear risk of bias with regard to blinding at outcome level for all studies as this was not explicitly mentioned in the study methods. On the other hand, there was a low risk of bias for all other assessments within the included studies. However, they differed slightly in the intervention and patient population. The CHAMPION PCI trial used clopidogrel 600 mg oral loading dose before PCI and compared with placebo, while the CHAMPION PLATFORM trial included only patients with unstable angina and non-ST-segment elevation myocardial infarction. CHAMPION PCI included patients with stable angina, unstable angina and acute coronary syndrome. The CHAMPION PHOENIX included patients with stable angina, unstable angina and acute coronary syndrome11. The baseline characteristics of the trial are shown in Table 1. The results from these three trials were pooled. A total of 25,107 patients were analysed in this meta-analysis for various outcomes.
EFFICACY ENDPOINTS
COMPOSITE OF DEATH, MI AND ISCHAEMIA-DRIVEN REVASCULARISATION
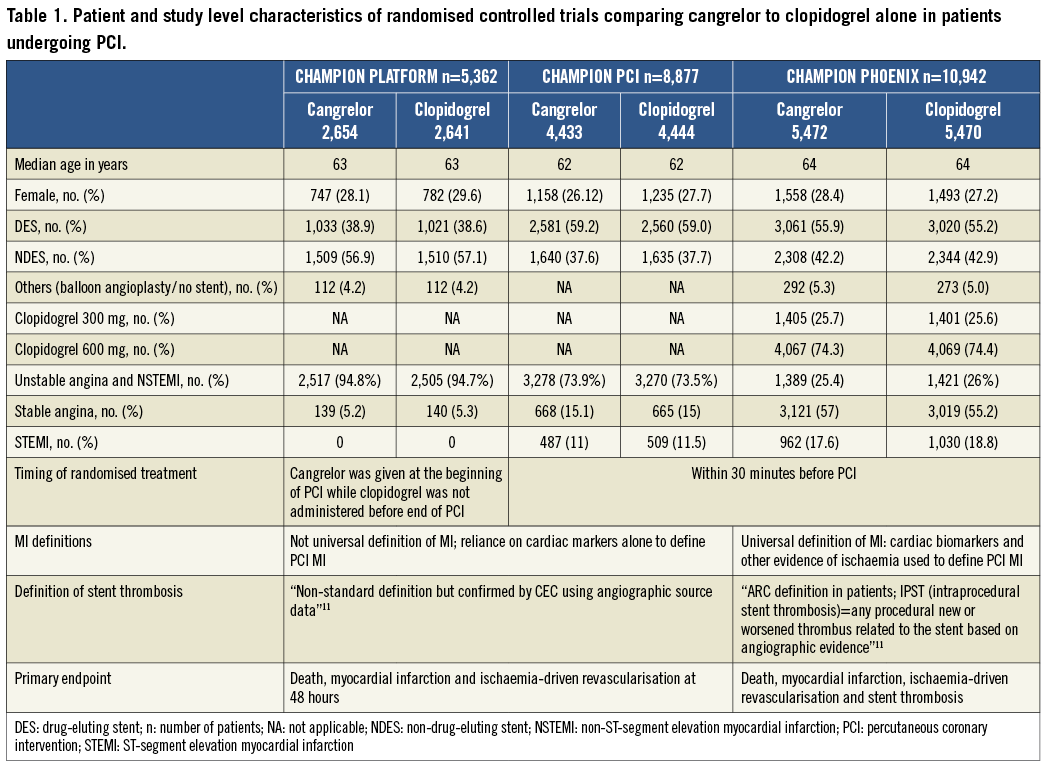
The composite of death, MI and ischaemia-driven revascularisation occurred in 723 of 12,475 (5.8%) treated with cangrelor and in 789 of 12,435 (6.3%) patients treated with clopidogrel (Figure 2A) (pooled OR 0.9; 95% CI: 0.76-1.07, p=0.23, I2=61%).
DEFINITE STENT THROMBOSIS
Stent thrombosis occurred in 62 of 12,475 (0.5%) treated with cangrelor and in 105 of 12,435 patients treated with clopidogrel (0.84%) (Figure 2B). Cangrelor was associated with a statistically significant reduction in stent thrombosis (pooled OR 0.54, 95% CI: 0.34-0.85, p=0.008, I2=0%). The absolute risk reduction was 0.34% (95% CI: 0.1%-0.6%). The number needed to treat to prevent one definite stent thrombosis was 287 (95% CI: 689-180).
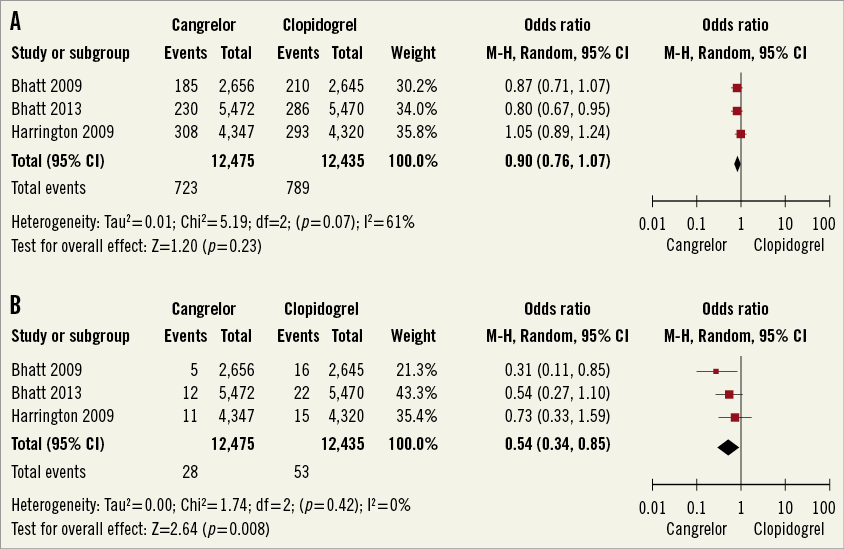
Figure 2. A) Meta-analysis of composite endpoint of all-cause death, MI, and IDR. B) Meta-analysis of stent thrombosis. (Modified intention to treat cohort). MI: myocardial infarction; IDR: ischaemia-driven revascularisation
MYOCARDIAL INFARCTION
Periprocedural myocardial infarction occurred in 676 of 12,475 (5.4%) patients treated with cangrelor and in 710 of 12,435 (5.7%) patients treated with clopidogrel (Figure 3A) (pooled OR 0.94, 95% CI: 0.77-1.14, p=0.51, I2=68%). Q-wave myocardial infarction occurred in 19 of 12,475 (0.15%) patients treated with cangrelor and in 36 of 12,435 (0.29%) patients treated with clopidogrel (Figure 3B) (pooled OR 0.53, 95% CI: 0.30-0.92, p=0.02, I2=0%). The absolute risk reduction was 0.14%. The number needed to prevent one Q-wave myocardial infarction was 729 (95% CI: 394-4,859). The majority of periprocedural myocardial infarctions which occurred in the study population were due to biomarker-driven myocardial infarction.
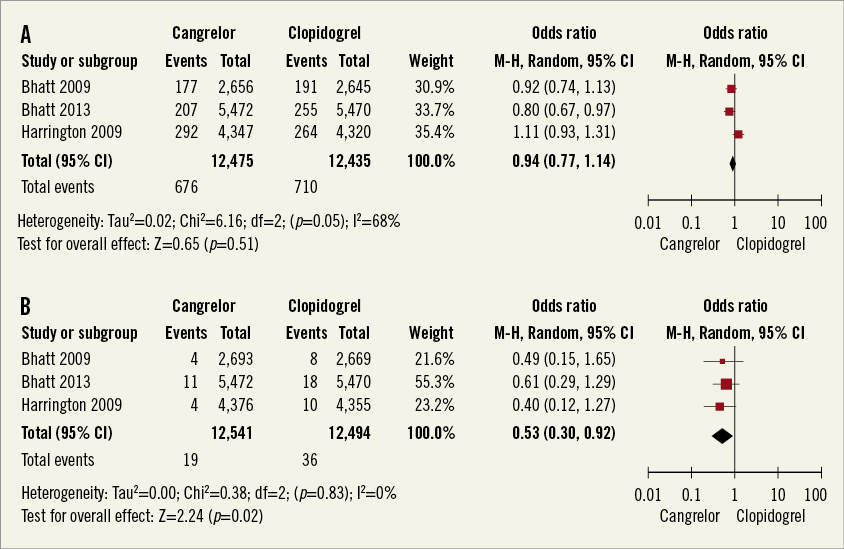
Figure 3. A) Meta-analysis of myocardial infarction. B) Meta-analysis of Q-wave myocardial infarction. (Modified intention to treat cohort).
ISCHAEMIA-DRIVEN REVASCULARISATION
Ischaemia-driven revascularisation occurred in 66 of 12,475 (0.52%) patients treated with cangrelor and in 92 of 12,435 (0.74%) patients treated with clopidogrel (Figure 4A) (pooled OR 0.71, 95% CI: 0.52-0.98, p=0.04, I2=0%). The absolute risk reduction was 0.21% (95% CI: 0.01%-0.41%). The number needed to treat to prevent one ischaemia-driven revascularisation was 475 (95% CI: 245-7,334).
DEATH FROM ALL CAUSE
Death from all cause occurred in 33 of 12,475 (0.26%) treated with cangrelor and in 45 of 12,435 (0.36%) patients treated with clopidogrel (Figure 4B) (pooled OR 0.72, 95% CI: 0.36-1.43, p=0.34, I2=52%).
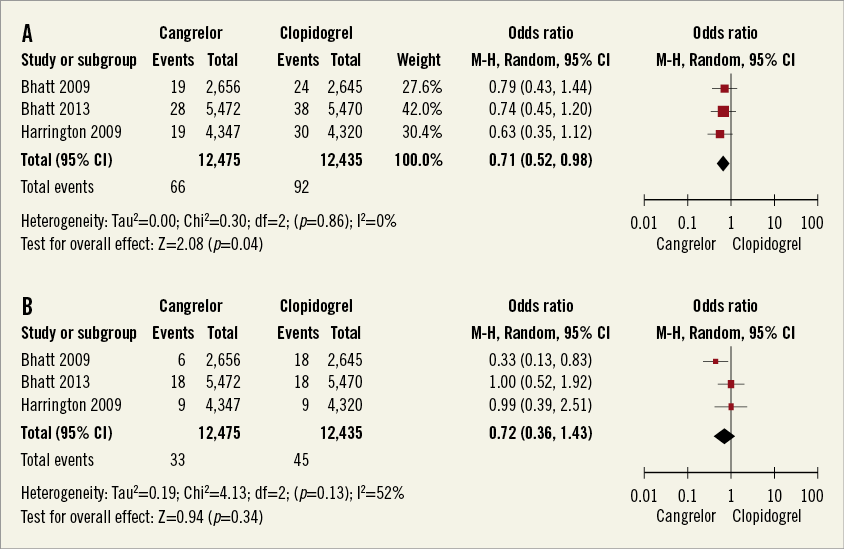
Figure 4. A) Meta-analysis of ischaemia-driven revascularisation. B) Meta-analysis of any cause death. (Modified intention to treat cohort).
SAFETY ENDPOINT
The primary safety endpoint, defined as severe or life-threatening bleeding based on GUSTO criteria, occurred in 28 of 12,565 (0.22%) patients treated with cangrelor and in 23 of 12,542 (0.18%) patients treated with clopidogrel (Figure 5A) (pooled OR 1.21, 95% CI: 0.7-2.11, I2=0%). The risk of GUSTO moderate bleeding was not different between the groups (Figure 5B) (pooled OR 1.38, 95% CI: 0.99-1.93, p=0.06, I2=0%). In terms of ACUITY (Acute Catheterization and Urgent Intervention Triage Strategy) bleeding criteria, there was no difference in the risk of major and minor bleeding (Figure 6A and Figure 6B). Sensitivity analyses pooling adjusted ORs showed similar results (data not shown).
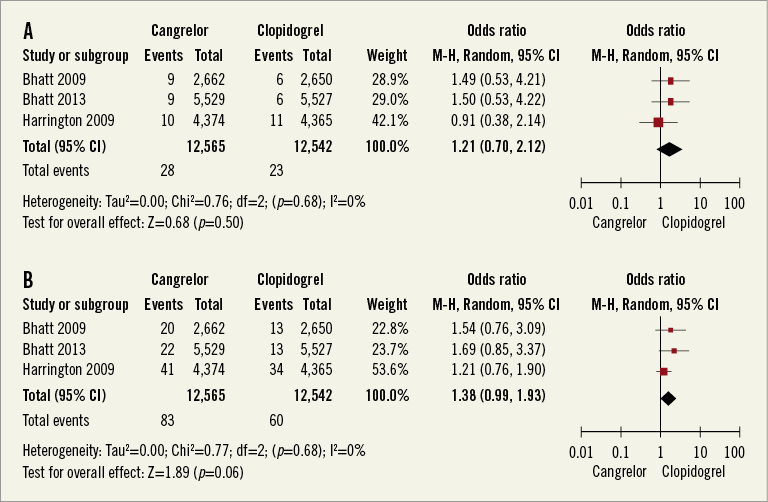
Figure 5. A) Meta-analysis of GUSTO severe or life-threatening bleeding. B) Meta-analysis of GUSTO moderate bleeding. (Safety cohort).
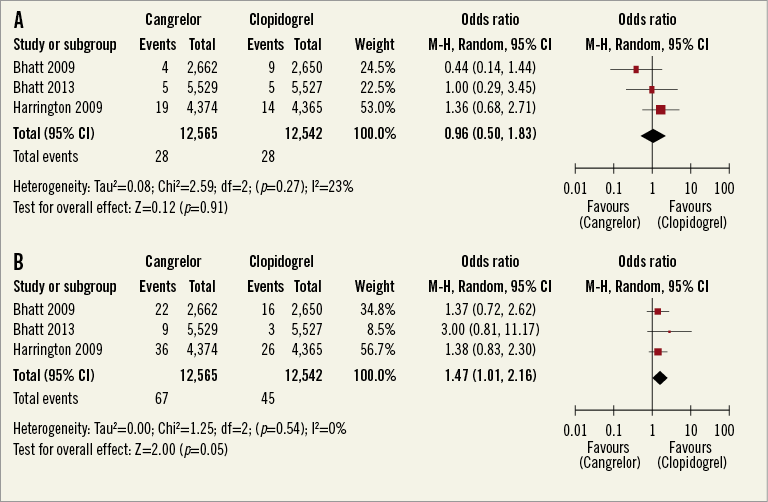
Figure 6. A) Meta-analysis of TIMI major bleeding. B) Meta-analysis of TIMI minor bleeding. (Safety cohort).
Discussion
FINDINGS
In this meta-analysis that included 25,107 patients, we did not find a difference in the composite endpoint of death from all causes, myocardial infarction or ischaemia-driven revascularisation at 48 hours between cangrelor and clopidogrel use. In comparison with clopidogrel, cangrelor was associated with a lower risk of stent thrombosis, Q-wave myocardial infarction, ischaemia-driven revascularisation, but no significant difference was seen between all-cause mortality and risk of myocardial infarction at 48 hours. There was no significant increased risk of GUSTO-defined severe or life-threatening bleeding in the cangrelor group.
IMPLICATIONS
Cangrelor was associated with a lower risk of stent thrombosis, ischaemia-driven revascularisation and Q-wave myocardial infarction at 48 hours. Given lower stent thrombosis, ischaemia-driven revascularisation and Q-wave myocardial infarction at 48 hours, cangrelor could be considered as an alternative to clopidogrel. We compared the number needed to treat of cangrelor with that of prasugrel and ticagrelor. The data from TRITON TIMI 38 show that the absolute risk reduction of definite stent thrombosis compared with clopidogrel is 1.15% giving a number needed to treat of 869 for prasugrel7. The data from PLATO trial show that the absolute risk reduction of definite stent thrombosis for ticagrelor is 0.6%, giving a number needed to treat of 1665. The magnitude of number needed to treat for cangrelor to prevent definite stent thrombosis is similar to the number needed to treat of ticagrelor while preventing definite stent thrombosis. However, one should be mindful that the data for cangrelor were assessed at 48 hours after randomisation while the rate of definite stent thrombosis for prasugrel and ticagrelor was assessed on long-term follow-up. Dual antiplatelet therapy has been the standard of care for patients presenting with acute coronary syndromes15. The present guidelines recommend adding either clopidogrel or ticagrelor or eptifibatide as an additional antiplatelet agent for moderate and high-risk acute coronary syndrome. The TRITON TIMI 38 trial demonstrated the benefit of prasugrel in patients with high-risk ACS who are undergoing coronary intervention but at the expense of an increased risk of bleeding. Retrospective analysis suggested that prasugrel was more effective in patients with diabetes and patients with STEMI. The TRILOGY ACS trial showed that prasugrel had no more benefit compared with clopidogrel in patients with unstable angina and NSTEMI who did not undergo revascularisation. The PLATO trial randomised patients with ACS with or without ST-elevation to clopidogrel or ticagrelor. Ticagrelor showed benefit for the primary endpoint without an increase in major bleeding. The benefit of pre-treatment was also seen in the group of patients who underwent a non-invasive strategy (HR 0.85; p=0.04). Pre-treatment with clopidogrel on the other hand has consistently been shown to improve ischaemic endpoints in patients undergoing PCI both for stable coronary artery disease and for acute coronary syndromes16. However, the use of clopidogrel during PCI has been limited by its route of administration (i.e., oral), and about 30% of white patients may have clopidogrel resistance due to genetic mutation leading to suboptimal platelet inhibition leading to increased thrombotic complications17. Cangrelor, being a rapid acting, intravenous and reversible P2Y12 inhibitor, can fill that gap. Regarding the primary efficacy endpoint and myocardial infarction, no major conclusion can be drawn from our data as the pooled estimate has moderate heterogeneity. This moderate heterogeneity could be related to combining data from three trials which included patients with different clinical presentation, modes of drug administration and definition of the primary endpoint. Of particular note, the CHAMPION PHOENIX trial used the universal definition of myocardial infarction, which is a better definition of myocardial infarction. This limitation could have been overcome by combining patient level data from all three trials using the universal definition of myocardial infarction. Our data are limited because of the lack of covariate analysis and time-to-event analysis. However, we believe that the pooled estimates of ischaemia-driven revascularisation, Q-wave myocardial infarction and definite stent thrombosis in our study are valid as there is no heterogeneity in the pooled estimates, as reflected in I2=0%. In terms of safety, cangrelor and clopidogrel seem to be equally safe when compared on the basis of GUSTO-defined life-threatening or severe bleeding criteria. However, it should be remembered that GUSTO criteria are less sensitive than ACUITY criteria. We also compared bleeding based on TIMI and ACUITY criteria and did not find any difference in risk of major or minor bleeding between cangrelor and clopidogrel (Figure 6).
UNDERSTANDING THE CONFLICTING RESULTS OF INDIVIDUAL TRIALS
Current ACC and AHA guidelines for PCI recommend dual antiplatelet therapy to minimise the risk of periprocedural complications1. Dual antiplatelet therapy is also recommended for patients presenting with acute coronary syndromes15. At present, all available P2Y12 inhibitors are oral agents, which take a longer time to act, and whose antiplatelet effects last longer. There is an unmet need for antiplatelet agents that are rapid acting and have a shorter duration of action with quick reversibility. Cangrelor seems promisingly to fill that gap. Previous studies involving cangrelor in patients undergoing PCI (CHAMPION PCI and CHAMPION PLATFORM) failed to show any additional benefit in reducing the primary composite endpoints using cangrelor during PCI. In CHAMPION PCI, the study drugs were given within 30 minutes of PCI, while in CHAMPION PLATFORM a loading dose of cangrelor was given before PCI and clopidogrel was loaded after the completion of PCI. The study design of these trials has come under some criticism18. The CHAMPION PHOENIX study showed that cangrelor was superior to clopidogrel in patients undergoing PCI. However, the benefit seen was mainly due to a reduction in stent thrombosis and myocardial infarction. However, one should note that there was no difference in Q-wave MI between the cangrelor and clopidogrel groups, which would have been a clinically meaningful outcome. Furthermore, in this study, about one quarter of the patients in the clopidogrel group received a 300 mg load, which is inferior to the 600 mg loading dose in preventing periprocedural MI1. More than one third of patients in the clopidogrel group received clopidogrel during or after PCI, which could lead to suboptimal efficacy of clopidogrel during PCI. Regarding safety endpoints, there was no real difference between cangrelor and clopidogrel with respect to both GUSTO and ACUITY criteria.
STRENGTHS AND LIMITATIONS
This is, to the best of our knowledge, the first systematic review and meta-analysis of cangrelor vs. clopidogrel in patients undergoing PCI. There was moderate between-study heterogeneity in primary efficacy endpoints. A certain amount of heterogeneity is to be expected in the meta-analysis19. Heterogeneity may be partly due to combining results from trials involving a wide spectrum of patient presentations ranging from stable angina, non-ST-segment elevation myocardial infarction to ST-segment elevation myocardial infarction. This limitation could potentially have been overcome by doing a sensitivity analysis based on presentation. However, we were not able to perform a sensitivity analysis based on presentation because of the lack of patient-level data. Another important limitation of this study-level meta-analysis is the lack of patient-level data, and therefore we were unable to perform covariate-adjusted or time-to-event analysis. Since CHAMPION PHOENIX used the universal definition of MI while CHAMPION PCI/CHAMPION PLATFORM did not, stratifying results based on baseline biomarker positivity versus negativity would have given us a better insight into the efficacy of cangrelor for periprocedural myocardial infarction. Though the universal MI definition was used for periprocedural myocardial infarction, the CHAMPION PHOENIX trial did not discriminate between spontaneous myocardial infarction and periprocedural myocardial infarction. Moreover, the median time from hospital admission to PCI was 4.4 hours. Currently, guidelines recommend getting at least two samples of biomarkers six hours apart. It is very unlikely that, in the CHAMPION PHOENIX trial, most patients had two biomarkers obtained six hours apart.
Conclusions
Based on this meta-analysis, we did not find any difference in the risk of the composite primary efficacy endpoint of all-cause death, ischaemia-driven revascularisation, and myocardial infarction at 48 hours between cangrelor and clopidogrel use. However, the interpretation of the primary efficacy endpoint in our meta-analysis is limited by moderate heterogeneity among the studies. Given that cangrelor was associated with a lower risk of stent thrombosis, ischaemia-driven revascularisation and Q-wave myocardial infarction compared to clopidogrel, cangrelor can be considered as a suitable alternative during PCI.
Acknowledgements
The authors have no relationships that might have an interest in the submitted work and have no financial or non-financial interests. There was no role of any funding agency in the conduct of this meta-analysis.
Conflict of interest statement
The authors have no conflicts of interest to declare.
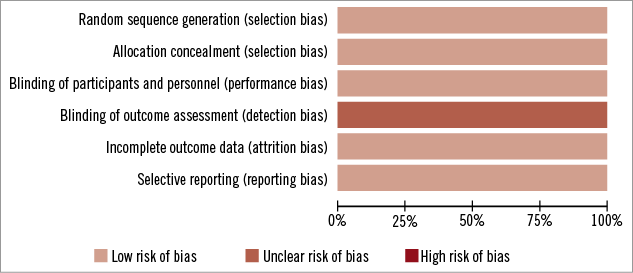
Online Figure 1. Risk of bias graph.
Appendix 1. Lists of excluded studies.
DID NOT MEET INCLUSION CRITERIA
REVIEWS
META-ANALYSIS

