Abstract
Aims: The current study sought to evaluate the clinical impact of newly reported genetic variations and their association with clopidogrel high on-treatment platelet reactivity (HTPR) in acute coronary syndrome (ACS) patients after drug-eluting stent (DES) implantation.
Methods and results: The study enrolled 1,016 consecutive patients with ACS undergoing DES implantation. A total of 19 tag single nucleotide polymorphisms (SNPs) were selected from CYP3A4/5, CYP2C19, P2Y12 and ABCB1 genes. ADP-induced light transmittance aggregometry (LTA) was performed to test the post-procedure maximum platelet agglutination (MPA). The primary endpoint was a composite of cardiovascular death, non-fatal myocardial infarction (MI), stent thrombosis, and ischaemic stroke at one-year follow-up after DES placement. The secondary endpoint was the incidence of bleeding events. The post-procedure MPA was calculated and the cut-off point was determined for the HTPR. Using multivariate logistic regression analysis, the carriage of two CYP2C19 LOF alleles was an independent predictor of the post-procedure HTPR (OR: 2.8, 95% CI: 1.70-7.23, p<0.001). Through multivariate Cox regression analysis, the carriage of two CYP2C19 LOF alleles and the post-procedure HTPR were independent predictors of the primary endpoint (HR: 2.3, 95% CI: 1.40-4.97, p<0.001; HR: 2.9, 95% CI: 1.52-5.57, p<0.001, respectively). However, post-procedure MPA did not predict a bleeding event (HR: 0.9, 95% CI: 0.44-1.59, p=0.532).
Conclusions: In patients with ACS, the CYP2C19 LOF allele was associated with post-procedure HTPR and a subsequently increased risk of adverse clinical events at one-year follow-up following DES implantation and clopidogrel administration.
Introduction
Clopidogrel and aspirin inhibit platelet function, prevent ischaemic events and therefore improve clinical outcomes in patients with acute coronary syndrome (ACS) following percutaneous coronary intervention (PCI) in the drug-eluting stent (DES) era1-3. Both the American College of Cardiology and American Heart Association guidelines recommend dual antiplatelet therapy with aspirin and clopidogrel as the standard treatment strategy to avoid thrombotic events after implantation of a DES4,5. Despite the introduction of dual antiplatelet therapy treatment, it should be noted that a patient treated by DES remains at risk of an ischaemic event at clinical follow-up. Moreover, persistent high platelet reactivity following PCI is reported to occur, even with adequate pre-treatment of clopidogrel, and is related to an increased risk of adverse cardiovascular events. Indeed, platelet activity in response to clopidogrel treatment is highly variable, with clinical, cellular, and genetic factors considered as important reasons for the variability6.
Clopidogrel, an inactive prodrug, is metabolised to its active thiol metabolite form by the hepatic cytochrome P450 (CYP) family of isoenzymes, including CYP3A4/5, 2C19, 1A2, 2B6, and 2C97-9. Accumulating evidence now indicates that the polymorphically expressed isoenzymes CYP3A4/5 and CYP2C19 play an integral role in the metabolism of clopidogrel8,10,11. The active clopidogrel metabolite directly blocks adenosine diphosphate (ADP) binding to platelet P2Y12 receptors, subsequently inhibiting ADP-mediated activation of the glycoprotein IIb/IIIa complex. Exploration on this issue has demonstrated that several P2Y12 polymorphisms (e.g., C34T, G52T and i-T744C) potentially influence ADP-induced platelet activation12. Similarly, in vitro and clinical studies have shown that ABCB1 polymorphisms, particularly C3435T, might alter clopidogrel metabolism and its efficacy13,14. However, the majority of these studies were primarily carried out in Caucasian populations, with a paucity of large sample size and clinical follow-up results in patients with ACS receiving DES treatment. Therefore, the present study sought to assess the impact of gene polymorphisms (CYP3A4/5, CYP2C19, P2Y12 and ABCB1) on ADP-induced platelet reactivity, and subsequent clinical follow-up outcomes in Chinese high-risk ACS patients receiving clopidogrel after DES implantation.
Methods
PATIENTS
Between March 2009 and April 2010, 1,127 consecutive patients with ACS following coronary DES implantation were prospectively enrolled in the present study (Figure 1). Patients were considered eligible regardless of the clinical presentation, including unstable angina pectoris (UAP), ST-elevation myocardial infarction (STEMI), and non-ST-elevation myocardial infarction (NSTEMI). Patients were excluded from the study if they had known allergies to antiplatelet therapy, a history of taking clopidogrel within seven days, a left ventricular ejection fraction <30% or New York Heart Association functional Class III or IV, active bleeding and bleeding diatheses, oral anticoagulation therapy with Coumadin, a leukocyte count <3,000/mm3 and/or a platelet count <100,000/mm3, an aspartate aminotransferase level or an alanine aminotransferase level ≥3 times the upper normal limit, a serum creatinine concentration ≥2.5 mg/dl, a stroke within six months, non-cardiac disease with a life expectancy of <1 year, or if they were unable to undergo a DES implantation.
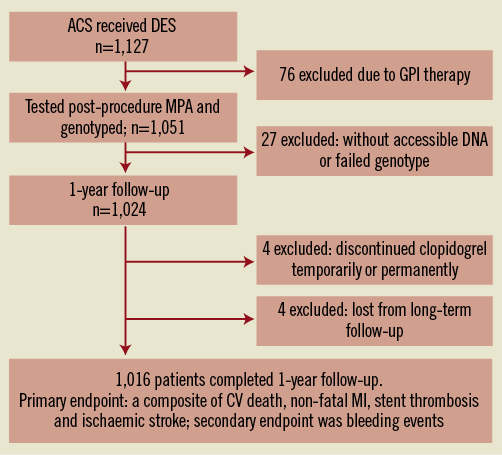
Figure 1. Flowchart of the study. ACS: acute coronary syndrome; CV: cardiovascular; DES: drug-eluting stent; GPI: glycoprotein IIb/IIIa receptor inhibitor; MI: myocardial infarction; MPA: maximum platelet agglutination
INTERVENTIONAL PROCEDURES AND MEDICATIONS
All enrolled patients were pre-treated with aspirin (300 mg) and a loading dose of 600 mg clopidogrel before PCI. Stents were deployed according to the standard techniques. Maintenance doses of antiplatelet agents taken for at least 12 months included aspirin (100 mg per day) and clopidogrel (75 mg per day). During the PCI procedure, almost all patients received intravenous unfractionated heparin; however, patients who received a glycoprotein IIb/IIIa receptor inhibitor (GPI) intravenously were excluded (n=76). Compliance with clopidogrel and aspirin therapy was verified by nursing staff in the hospital and ensured through structured or telephone interview during follow-up. Hypertension was defined as a blood pressure of ≥140/90 mmHg taken from a mean of three independent blood pressure measurements, or the use of antihypertensive drugs15. Dyslipidaemia and diabetes were defined according to the Third Report of the National Cholesterol Education Program, and the American Diabetes Association, respectively16,17.
SELECT tagSNPs
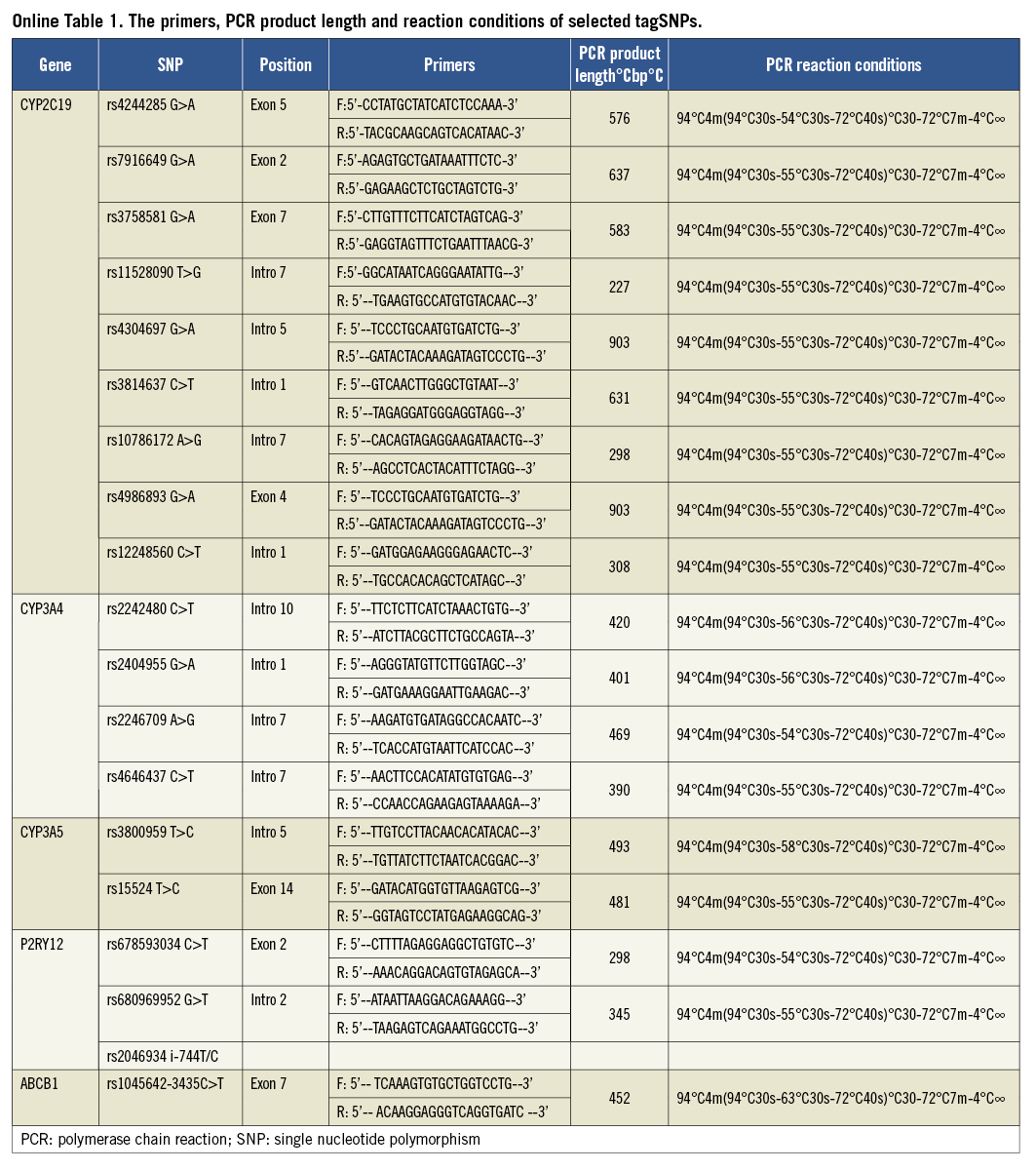
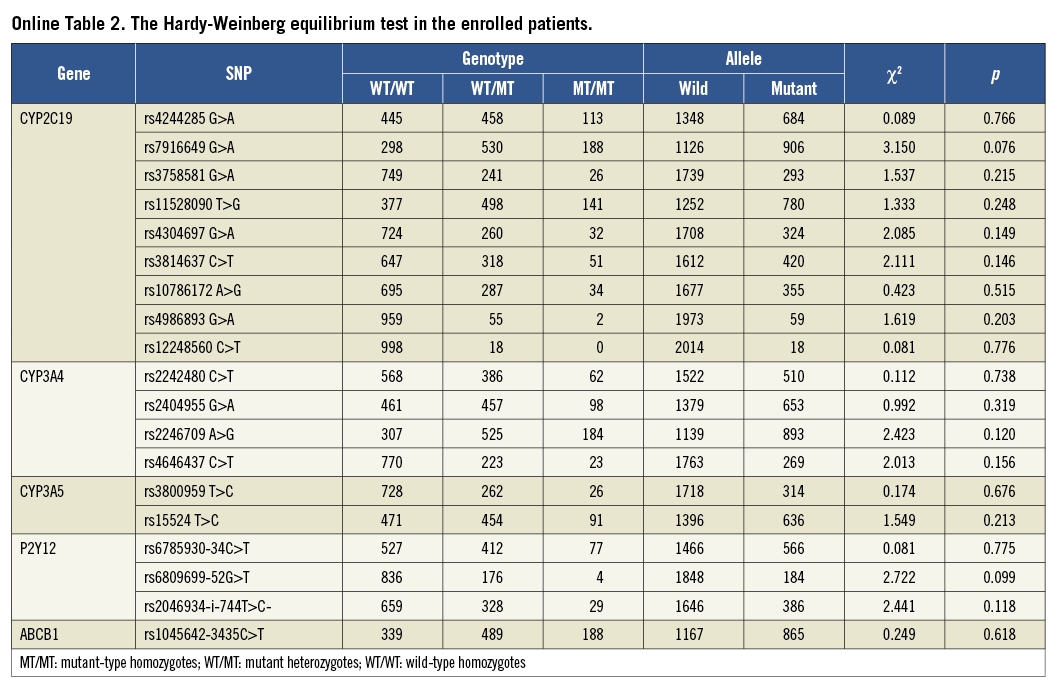
Using the pairwise tagging approach, tag single nucleotide polymorphisms (SNPs) were selected from the HapMap CHB databank (public data release #2/phase III, Feb 2009) with the aid of tagSNPs’ online software (http://hapmap.ncbi.nlm.nih.gov/cgi-perl/gbrowse/ hapmap 3r2_B36/, Broad Institute, Cambridge, MA, USA). TagSNPs covered the complete CYP3A4 region, from 3,000 bp upstream to 1,000 bp downstream. Common variants were defined as those with a minor allele frequency cut-off of 0.05, with a linkage disequilibrium (LD) measure r2 threshold of 0.8. All selected tagSNPs were ensured from different haplotype regions using this protocol. In total, four tagSNPs (rs2242480C>T, rs2404955G>A, rs2246709A>G and rs4646437C>T) were identified over the entire CYP3A4 gene. In addition, two tagSNPs (rs3800959T>C and rs15524T>C) from the CYP3A5 gene and seven haplotype tagging SNPs (CYP2C19*2-rs4244285G>A, rs7916649G>A, rs3758581G>A, rs11528090T>G, rs4304697G>A, rs3814637C>T and rs10786172A>G) from the entire CYP2C19 gene were selected. On this basis, we also enrolled CYP2C19*3-rs4986893G>A, CYP2C19*17 -rs12248560C>T, P2Y12 (C34T -rs6785930, G52T -rs6809699 and i-T744C -rs2046934), and ABCB1 (rs1045642-3435C>T) (Online Table 1). No deviation from Hardy-Weinberg equilibrium was detected in any enrolled tagSNPs (Online Table 2).
BLOOD SAMPLING AND GENOTYPING
The study protocol was approved by the Institutional Ethics Committee of Shenyang Northern Hospital (No.2011C02), China. Written informed consent was obtained from each participant. DNA was extracted from peripheral blood using TIANamp Blood DNA kits (Tiangen Biotech Co., Ltd., Beijing, China). DNA concentration and quality were assessed by absorbance spectrophotometry and agarose gel electrophoresis. All 19 selected SNPs were genotyped using standard polymerase chain reaction (PCR) techniques. PCR cycling conditions are shown in detail in Online Table 1. Each PCR amplification was performed using 0.1 μg DNA, 15 pmol of each primer, 0.4 mmol/L dNTPs, 5 μl of 10× reaction buffer with mgCl2, and 4U rTaq DNA polymerase (total volume 50 μl). DNA sequencing was performed on an ABI Prism 3730 genetic analyser (Applied Biosystems, Foster City, CA, USA) using an ABI dye terminator cycle sequencing kit (Applied Biosystems). There were 27 patients without accessible DNA or failed genotyping.
PLATELET FUNCTION TESTING
Venous blood samples were obtained at least 12 hours after DES implantation, then added to tubes containing 3.2% trisodium citrate. All blood samples were processed within 90 minutes after collection. Platelet aggregation was analysed in platelet-rich plasma (PRP) by optical turbidimetric aggregometry using a four-channel Platelet Aggregation Chromogenic Kinetic System (Helena Laboratories, Beaumont City, TX, USA). PRP was prepared by centrifugation at 200 g for 10 minutes. After adjustment from baseline, 20 μmol/L ADP was added, and aggregation was recorded for five minutes. Results are expressed as a percentage of maximal light transmission from platelet-poor plasma obtained from the same patient. The coefficient of variation of this optical aggregometry assay was 5.2%.
STUDY ENDPOINTS AND DEFINITIONS
The primary endpoint of the present study was a composite of cardiovascular (CV) death, non-fatal myocardial infarction (MI), stent thrombosis, and ischaemic stroke during the initial year after admission. The diagnosis of myocardial infarction was defined as ischaemic symptoms with electrocardiogram (ECG) abnormalities and above the normal limits of elevated troponin18. The estimated stent thrombosis (definite) was defined according to the Academic Research Consortium19. The secondary endpoint was bleeding events. Bleeding was quantified according to Thrombolysis in Myocardial Infarction (TIMI) criteria20. All events were reported to and judged by an independent committee blinded to the genotype and platelet function of the patients.
CLINICAL FOLLOW-UP
Patients were interviewed by telephone every 30 days. Patients exhibiting cardiac symptoms underwent a complete clinical, ECG, and laboratory check-up in an outpatient clinic. All clinical information, from referring physicians, relatives and hospital readmissions, was collated. Source documentation was checked to ensure high-quality data. During the initial year of follow-up, four patients were lost to follow-up study. There were four patients excluded from the study due to discontinued aspirin and/or clopidogrel temporarily or permanently. The reasons for the discontinuation were as follows: two patients received non-cardiac surgery, one patient experienced a severe gastrointestinal reaction, and one patient required dental treatment.
STATISTICAL METHODS
Under three models (codominant, dominant and recessive), the relationships between all enrolled SNPs and the post-procedure high on-treatment platelet reactivity (HTPR) were analysed. A combined receiver operator curve (ROC) analysis was used to determine the cut-off point of post-procedure maximum platelet agglutination (MPA) for the HTPR (MedCalc software, Mariakerke, Belgium). Patients above the cut-off value were considered to exhibit HTPR. Comparisons were performed with the Kaplan-Meier method and the log-rank test. The odds ratio (OR) was estimated by logistic regression analysis. Categorical variables are expressed as n (%) and continuous variables as mean±SD. The Fisher’s exact test and Mann-Whitney rank sum test were used for comparison of categorical and continuous variables.
Multivariate logistic regression analysis was used to evaluate the following risk factors on the occurrence of post-procedure HTPR: gene polymorphism, age, gender, body mass index, diabetes mellitus, left ventricular ejection fraction (LVEF), hypercholesterolaemia, hypertension, active smoker, previous MI, previous PCI and proton pump inhibitors. Multivariate Cox regression analysis was used to evaluate the prognostic significance of the following risk factors on the occurrence of ischaemic events or bleeding events within one year of discharge: post-procedure HTPR, gene polymorphism, LVEF, diabetes mellitus, hypercholesterolaemia, hypertension, active smoker, previous MI and previous PCI. The measure of effect was the hazard ratio (HR). A probability value of <0.05 was considered statistically significant. All abovementioned analyses were performed using SigmaStat software (Systat Software Inc., Point Richmond, CA, USA).
Results
STUDY POPULATION
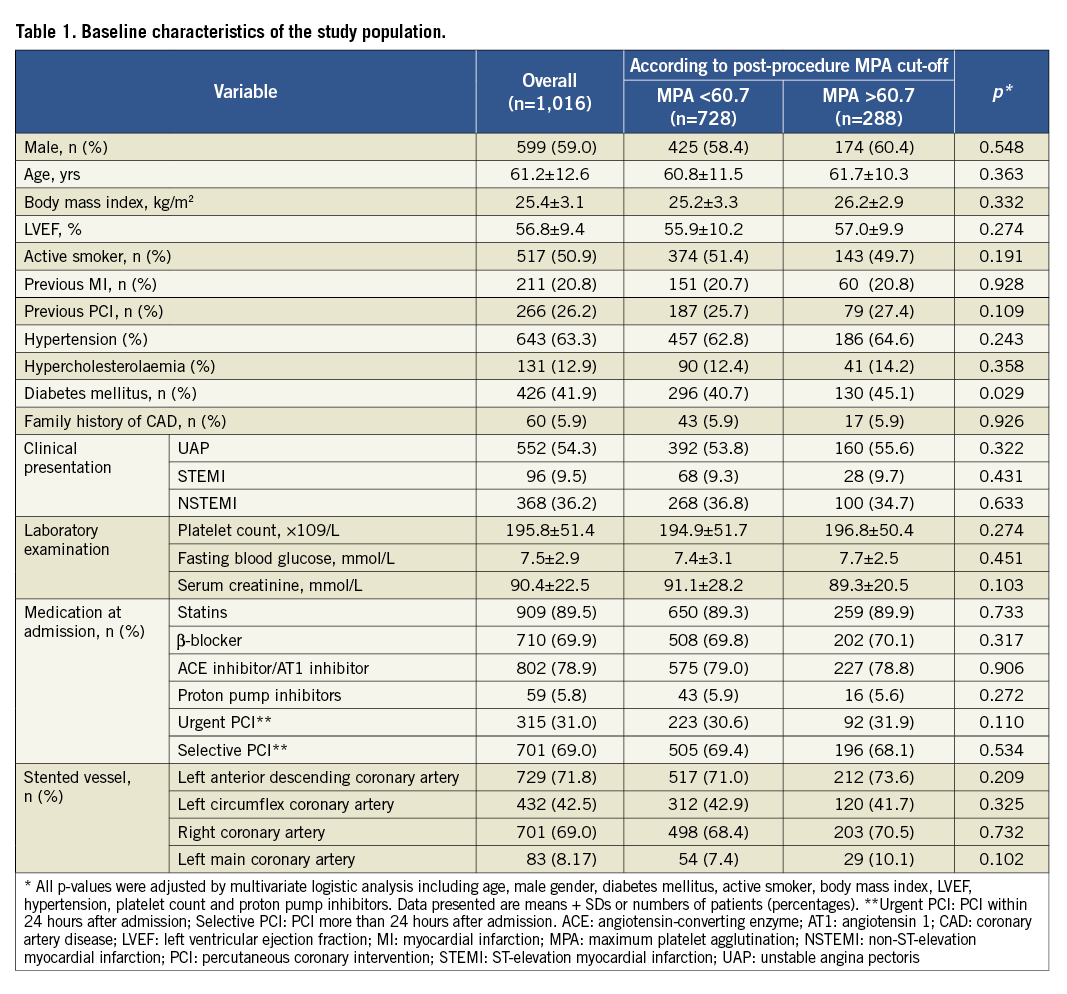
Within the present study, 1,016 patients with ACS treated by DES were assessed. All patients were from the Chinese Han population. Clinical and procedural characteristics according to the cut-off point of post-procedure HTPR are shown in Table 1. The proportion of diabetes mellitus in the HTPR group was higher than that in the non-HTPR group (45.1% vs. 40.7%, p=0.029).
At one-year follow-up, a total of 78 ischaemic events occurred (7.68%): 15 (1.48%) CV deaths, 38 (3.74%) non-fatal MIs, 16 (1.57%) stent thromboses and 9 (0.89%) ischaemic strokes. Sixty-five bleeding events (6.40%) occurred, which included four (0.39%) cases of major bleeding and 61 (6.01%) cases of minor bleeding.
ROC ANALYSES
We calculated the cut-off value (namely, MPA >60.7, as the HTPR) using the ROC curve analysis in accordance with or without the composite primary endpoints (Figure 2A). Thus, 288 patients (28.3%) above the HTPR cut-off point were identified. The area under the ROC curve is equal to 0.783 (95% CI: 0.757-0.808, p<0.001). We also calculated the cut-off point (MPA <50.5) according to the bleeding event; however, the area under the curve is only 0.498 (95% CI: 0.515-1.273, p=0.059, Figure 2B).
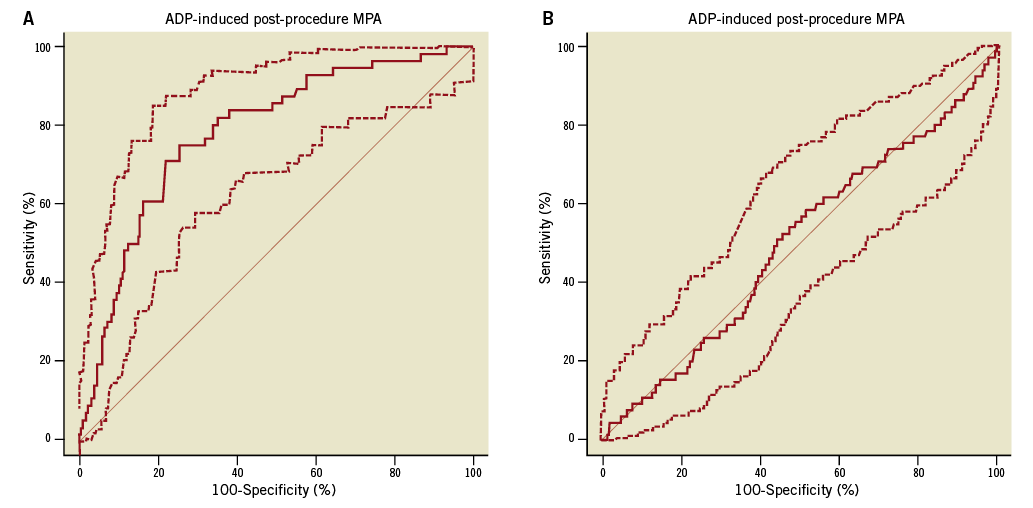
Figure 2. ROC curve of MPA for the enrolled patients. A) Combined receiver operator curve for 20 mM ADP-induced post-procedural platelet aggregation measured by LTA. A criterion value of >60.7% aggregation provided the HPR-ADP cut-point. Dashed lines represent 95% confidence intervals. Sensitivity: 75.0 (95% CI: 61.6-85.6), specificity: 74.4 (95% CI: 61.6-85.6), area under the ROC curve (AUC): 0.783 (95% CI: 0.757-0.808, p<0.001). B) Combined receiver operator curve for 20 mM ADP-induced post-procedural platelet aggregation measured by LTA. A criterion value of <50.5% aggregation provided the bleeding cut-point. Dashed lines represent 95% confidence intervals. Sensitivity: 58.46 (95% CI: 45.6-70.6), specificity: 48.26 (95% CI: 45.0-51.5), area under the ROC curve (AUC): 0.498 (95% CI: 0.515-1.273, p=0.059). ADP: adenosine diphosphate; CAD: coronary artery disease; HPR: high platelet reactivity; LTA: light transmittance aggregometry; MPA: maximum platelet agglutination; ROC: receiver operator curve
GENOTYPING AND HTPR
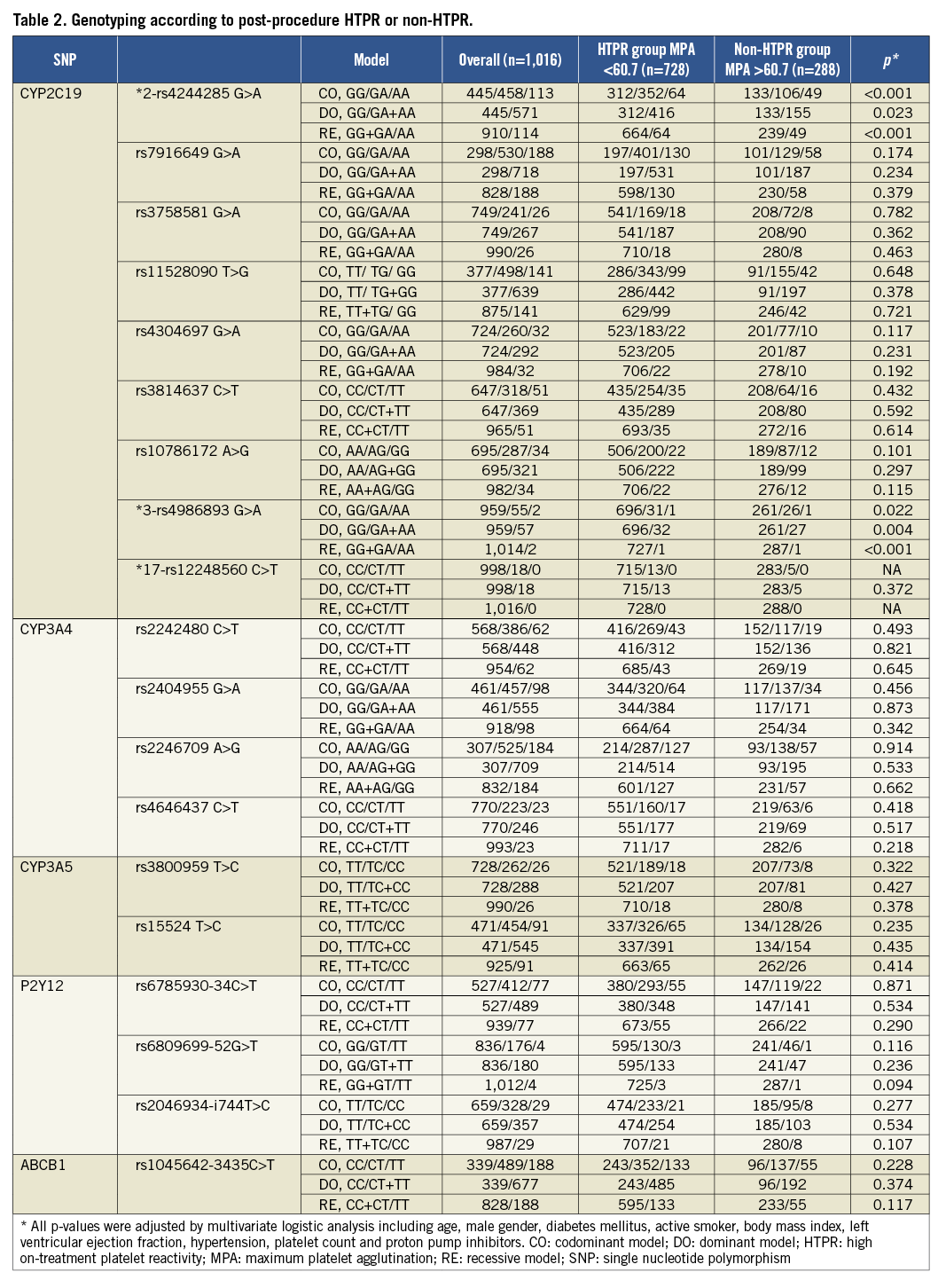
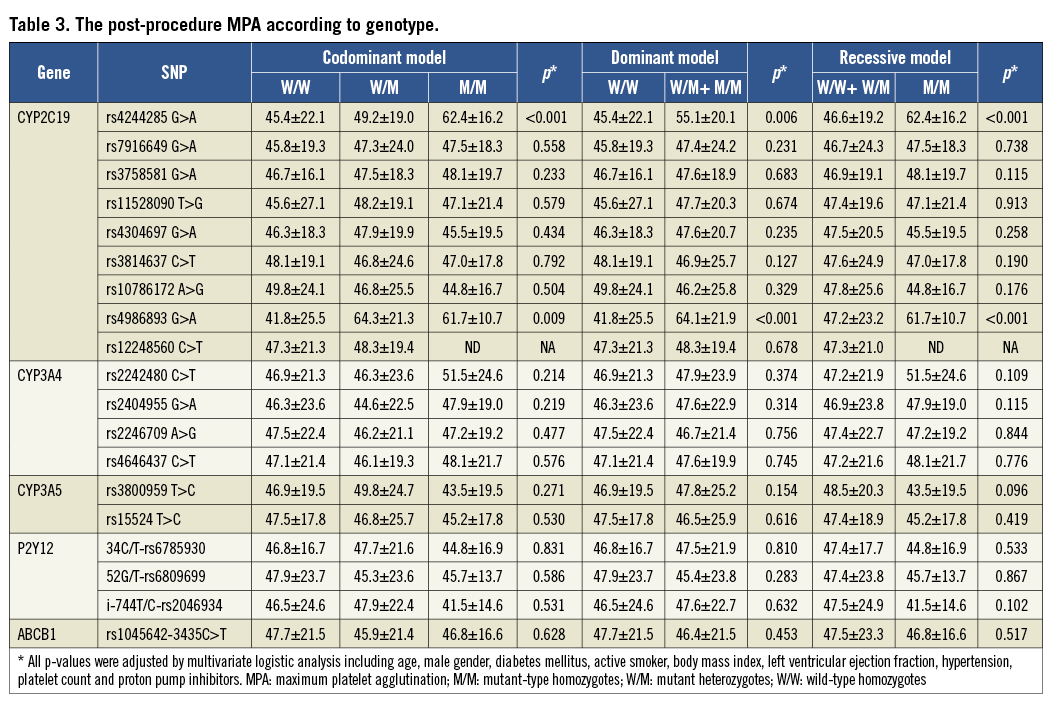
In the HTPR group, the proportion of LOF alleles of CYP2C19*2 and *3 is higher than that in the non-HTPR group. No correlation was found between other individual tagSNPs and HTPR (Table 2). The post-procedure platelet aggregation was calculated according to three models (Table 3).
GENOTYPING, HTPR AND OUTCOMES
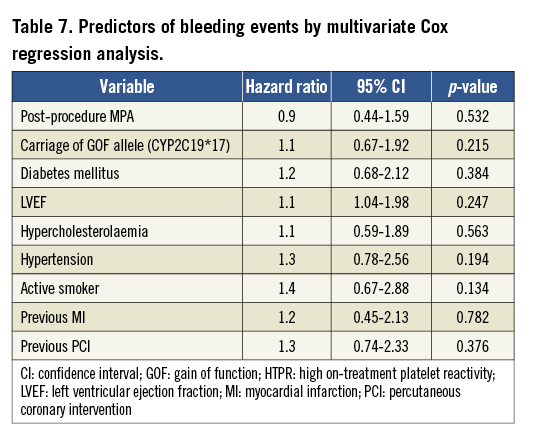
Patients with post-procedure HTPR had a significantly high risk of ischaemic events compared to the patients without HTPR (Figure 3). Meanwhile, patients carrying two CYP2C19 LOF alleles were associated with a higher risk of ischaemic events compared to those carrying no or one CYP2C19 LOF allele at one-year follow-up (Figure 4). There was no significant difference in the bleeding risk between the groups (Table 4).
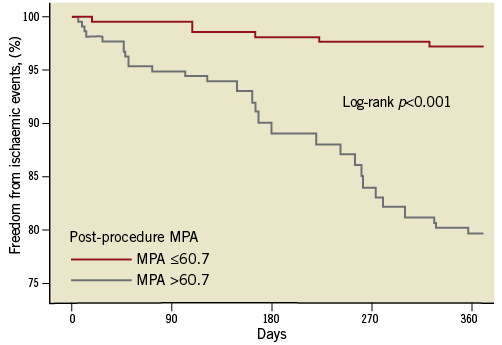
Figure 3. Kaplan-Meier analysis of the clinical endpoint between the HTPR group and non-HTPR group.
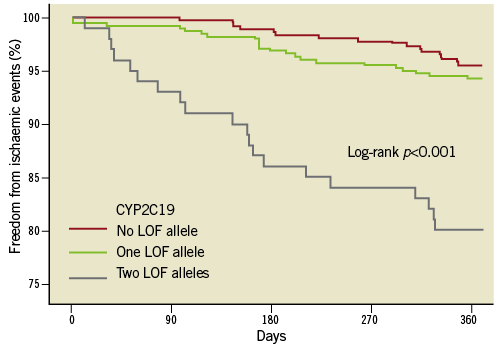
Figure 4. Kaplan-Meier analysis of the clinical ischaemic endpoint for the presence of the CYP2C19 LOF alleles polymorphism.
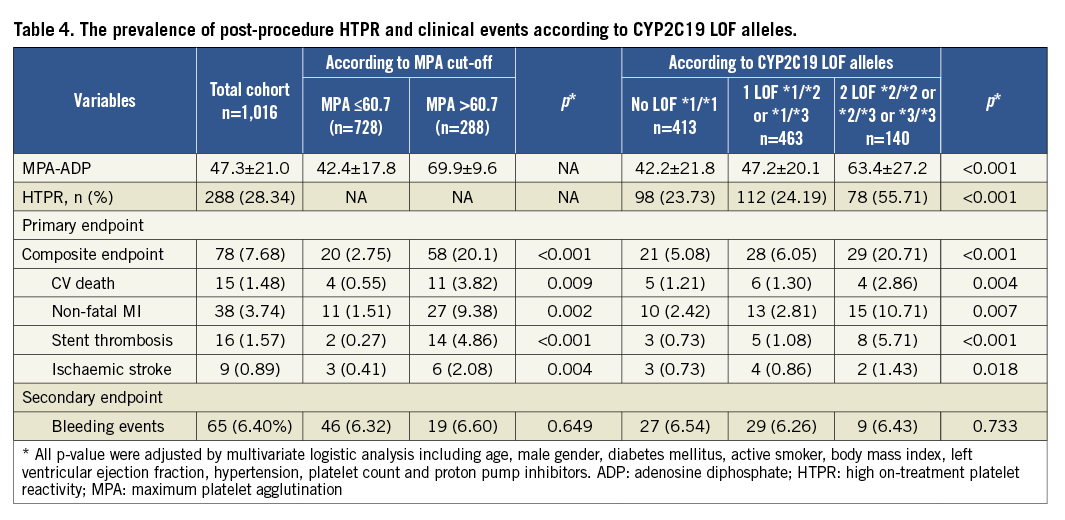
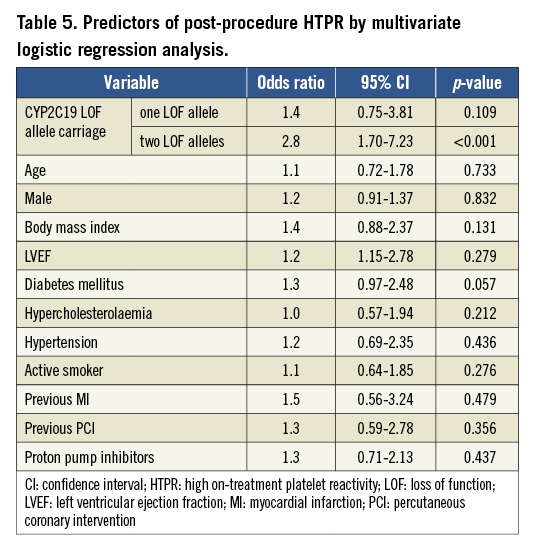
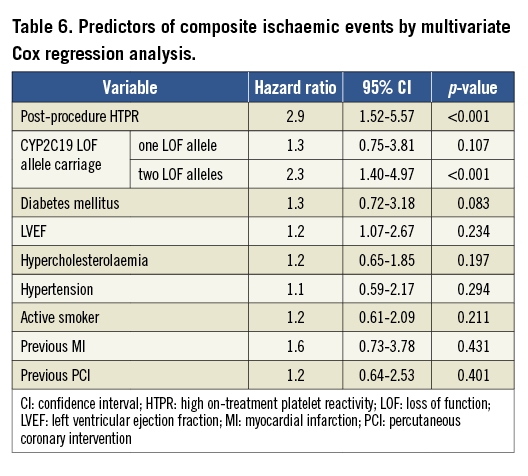
The carrying of two CYP2C19 LOF alleles was an independent predictor for the risk of post-procedure HTPR (OR: 2.8, 95% CI: 1.70-7.23, p<0.001). Diabetes mellitus was associated with a risk trend of post-procedure HTPR (OR: 1.3, 95% CI: 0.97-2.48, p=0.057, Table 5). The post-procedure HTPR was an independent predictor for the composite of ischaemic events (HR: 2.9, 95% CI: 1.52-5.57, p<0.001). Compared with the carriage of no or one LOF allele, the carriage of two CYP2C19 LOF alleles predicted the composite risk of ischaemic events (HR: 2.3, 95% CI: 1.40-4.97, p<0.001; Table 6). There were no variables predicting the risk of a bleeding event (Table 7).
Discussion
To our knowledge, this is the first study in East Asia to investigate the correlation among post-procedure platelet reactivity, SNPs (genes of liver metabolic enzymes, platelet surface receptors and intestinal absorption), and clinical outcomes in ACS patients with clopidogrel administration and coronary DES treatment. The following are the main findings of the present study: (1) the post-procedure HTPR was associated with a significantly increased risk of adverse clinical events; (2) the carrying of two CYP2C19 LOF alleles was associated with an increased post-procedure platelet reactivity and a significantly high incidence of ischaemic events.
Individual HTPR is an emerging issue in interventional cardiology as several studies have reported widely variable response to the therapeutic actions21,22. The absolute level of platelet reactivity during treatment has been proposed as a better measure of thrombotic risk than responsiveness to clopidogrel, and any definition of HTPR will only be meaningful when a cut-off or target value is identified23. The ROC curve analysis has been used extensively to define the optimal cut-off point for the definition of HTPR according to the clinical endpoint23. In this study, the post-procedure HTPR was defined as the cut-point of 60.7% 20 μmol/l ADP-induced MPA which correlated with the occurrence of ischaemic events, with an area under the curve of 0.783.
Studies have consistently demonstrated that HTPR is an independent predictor for the occurrence of thrombotic/ischaemic events after PCI24,25. A previous meta-analysis of 4,564 patients with coronary artery disease undergoing PCI showed that the high residual platelet reactivity was significantly associated with an increased risk of death or recurrent cardiovascular events in patients with a poor response to clopidogrel22. Similarly, the present study confirmed that post-procedure HTPR was strongly associated with the incidence of ischaemic events (HR 2.9, 95% CI: 1.52-5.57, p<0.001).
Furthermore, Siller-Matula et al found that personalised antiplatelet treatment resulted in an improved efficacy with an equal safety compared to the standard treatment26. Recently, Collet et al randomly assigned 2,440 patients scheduled for coronary stenting at 38 centres to a strategy of platelet-function monitoring, with drug adjustment (double dose of clopidogrel or shift to prasugrel) in patients who had a poor response to antiplatelet therapy, or to a conventional strategy without monitoring and drug adjustment27. Collet’s result showed no significant improvements in clinical outcomes with platelet-function monitoring and treatment adjustment for coronary stenting. Thus, the association between optimised high platelet reactivity and the prognosis of patients after PCI remains unsettled. In the present study, all enrolled patients were high-risk ACS (UAP: 54.3%, NSTEMI: 36.2%, STEMI: 9.5%) and had a high prevalence of diabetes (41.9%), and 31.0% received urgent DES implantation. These factors were different from Collet’s study population, and may be attributed to the different clinical prognostic. It should be noted that the reactivity of prasugrel was potentially influenced by the gene polymorphism in Collet’s study28.
Combining clinical studies and investigations into CYP metabolism in vitro suggested that polymorphism was related to reduced CYP function involving the conversion of clopidogrel to its active metabolite, subsequently altering the degree of clopidogrel-induced platelet inhibition29,30. Frere et al identified the CYP2C19*2 allele to be more frequent in patients without an efficient response to clopidogrel31. Mega et al demonstrated that tripling the maintenance dose of clopidogrel (225 mg daily) in patients carrying CYP2C19*2 heterozygotes achieved equivalent levels of platelet reactivity compared to those seen with a standard dose (75 mg daily) in non-carriers. Conversely, doses as high as 300 mg daily did not result in comparable degrees of platelet inhibition for patients carrying CYP2C19*2 homozygotes32. The findings of the current study are consistent with these previous reports even though there are differences of ethnicity between the subject populations, Chinese and Caucasian.
In a cohort of 1,477 clopidogrel-treated patients with ACS, carriers of at least one CYP2C19 LOF allele had a 53% increased risk of death from CV death, MI or ischaemic stroke, and a threefold increased risk of stent thrombosis when compared with non-carriers33. The present study found that patients carrying two CYP2C19 LOF alleles were 2.3 times more at risk of suffering adverse ischaemic events during the first year after DES implantation (HR: 2.3, 95% CI: 1.40-4.97, p<0.001). Of note, a directionally consistent hazard was observed among carriers of two CYP2C19 LOF alleles for clinical ischaemic events when compared with carriers of no or one CYP2C19 LOF allele. These findings indicate that gene polymorphisms are capable of affecting the efficacy of clopidogrel, and subsequently of patient outcomes. However, a recent meta-analysis by Holmes et al concluded that the CYP2C19 genotype is not a significant predictor of clinical outcomes in patients treated with clopidogrel, which was contrary to a previous meta-analysis34,35. Although the finding was interesting, several methodological issues were of concern. The analysis by Holme et al included patients in whom there was relatively little or no benefit of clopidogrel, thus curtailing the ability to observe any pharmacokinetic effects, and it included outcomes that occurred in patients who were no longer taking clopidogrel. Also, testing the association of the CYP2C19 genotype with responsiveness to clopidogrel in patients not taking clopidogrel was perhaps problematic, and the meta-analysis included outcomes such as elective target lesion revascularisation and non-CV death, in which clopidogrel had no definite effect. Therefore, we speculate that the CYP2C19 genotype could influence platelet function and clinical events in strict conditions (such as DES, taking clopidogrel, cardiovascular adverse events and so on).
Pharmacological approaches have consistently identified the P2Y12 receptor as being involved in dense granule secretion, fibrinogen receptor activation, P-selectin expression, and thrombus formation36-39. Despite this, our cohort exhibited no significant association between ADP-induced platelet reactivity and P2Y12 polymorphisms (C34T, G52T and i-T744C). The ABCB1 gene encodes the intestinal efflux transporter P-glycoprotein, which modulates the absorption of clopidogrel. The ABCB1 C3435T has been extensively studied and some researches have shown that the ABCB1 C3435T genotype influenced the impaired function of P-glycoprotein which could hinder the absorption of clopidogrel14. Though a number of investigators have evaluated the relationship between the ABCB1 polymorphism and clopidogrel response, the results were inconclusive13,40,41. In the present study, the ABCB1 C3435T genotype did not significantly influence the antiplatelet effect and clinical outcomes of clopidogrel in our patients. Recent evidence suggests that harbouring the CYP2C19 gain of function (GOF) allele (*17) might increase the risk of bleeding42. However, the frequency of the CYP2C19 GOF allele (*17) (0.894%) in our population is lower than that in Western populations (18-28%), and the CYP2C19*17 polymorphisms did not significantly influence the clopidogrel pharmacodynamics and long-term clinical outcomes42,43.
The safety and efficacy of altering therapy in response to genotype is not entirely known. Whereas neither genotyping nor platelet function tests alone adequately describe the global risk profile of an individual patient treated with clopidogrel, point-of-care platelet function testing to identify high-risk patients combined with CYP2C19 genetic testing may be more effective in identifying high-risk individuals for alternative antiplatelet therapies. Ultimately, prospective randomised clinical trials will be needed to test specific personalised antiplatelet algorithms to provide the evidence base necessary for widespread adoption into clinical practice.
Study limitations
The current study contains some limitations. First, single assessment of platelet function combined with only one method of testing platelet function may be regarded as insufficient to diagnose the response to antiplatelet therapy comprehensively. Future studies will incorporate multiple tests to improve determination of platelet function in response to clopidogrel. Second, the results gathered may be specific to the patient cohort and the way their cases were clinically managed. The difference in results from previous studies would not rule out the reason for the selective migration. Even though there is no reason to suspect, a priori, that there would be different results in other populations, further studies in diverse populations are required. Finally, the low allele frequency of other known alleles should not alter our conclusions, but may be relevant for individual patients.
Conclusions
In Chinese ACS patients with clopidogrel administration after DES implantation, the post-procedure HTPR and carriage of two CYP2C19 LOF alleles were significantly associated with an increased risk of adverse ischaemic events at one-year follow-up, and the carriage of two CYP2C19 LOF alleles was an independent predictor of post-procedure HTPR.
Acknowledgements
This study was supported by the National Key Technology R&D Program in the 12th Five-Year Plan of China (No.2011BAI11B07), Major High-Tech Clinical Army Projects (No.2010gxjs001).
Conflict of interest statement
The authors have no conflicts of interest to declare.
Online Figure 1. Change in intravascular ultrasound assessment from post-procedure to six months. Matched analysis of area and volume changes at the in-stent segment for the PROMUS Element (n=68 pairs), SYNERGY (n=62 pairs) and SYNERGY ½ dose (n=63 pairs) arms of the EVOLVE trial between post-procedure examination and six-months