Abstract
Aims: The Stent Thrombosis In Belgium (STIB) trial aimed to determine whether assessing platelet reactivity (PR) in patients with stable coronary artery disease undergoing elective percutaneous coronary intervention (PCI) could predict the risk of ischaemic complications and adverse clinical events up to 30 days post PCI.
Methods and results: PR before intervention was determined in 891 patients undergoing PCI for stable angina pectoris. Twelve to 24 hours before PCI, all patients received a 600 mg clopidogrel dose followed by 75 mg daily, and 500 mg of aspirin followed by 80-100 mg daily. Residual PR was assessed by VerifyNow point-of-care aspirin and P2Y12 assay before PCI. “Non-responders” to antiplatelet therapy were defined as aspirin reaction unit (ARU) >550 and as P2Y12 reaction unit (PRU) >230. The endpoint of the study was the composite of periprocedural myonecrosis, stent thrombosis, non-fatal myocardial infarction (MI), stroke and death at 30 days in patients with or without high PR. The endpoint was observed in 180 patients: four deaths, one stroke, 11 Q-wave MI, three non-Q-wave MI and 161 periprocedural myonecroses. At multivariate analysis, the endpoint was predicted by total stent length (OR: 1.020), GFR <60 ml/min (OR: 1.87), history of PCI (OR: 0.58), white blood cell count (OR: 1.95) and diabetes (OR: 1.83). No significant association was found between residual PR and the primary endpoint or any of its components.
Conclusions: PR measured before PCI in stable patients undergoing elective PCI who are preloaded with 500 mg of aspirin and 600 mg of clopidogrel is not predictive of periprocedural myocardial injury or adverse ischaemic complications up to 30 days.
Introduction
High platelet reactivity (PR) to clopidogrel or aspirin is associated with an increased risk of stent thrombosis and is a predictor of recurrent ischaemic events in patients with acute coronary syndrome (ACS)1,2. Conversely, a high loading dose of clopidogrel and/or optimal platelet inhibition significantly reduces ischaemic and cardiovascular complications in patients with ACS undergoing percutaneous coronary intervention (PCI)3-5. There is an unambiguous clinical benefit achieved with a 600 mg loading dose of clopidogrel against ischaemic complications following PCI performed in individuals presenting with an ACS6,7. Additionally, a seven-day double dose clopidogrel maintenance therapy after PCI offers a significant advantage over a single (75 mg) maintenance dose with respect to cardiovascular events and stent thrombosis, but carries an increased risk of bleeding8.
The efficacy of clopidogrel is, however, influenced by the large interindividual variability in the concentration of the active metabolite9,10, and the optimal dose for clopidogrel following percutaneous coronary intervention is debated. To overcome this problem, tests for diagnosing platelet responsiveness have been developed. Notably, on-treatment platelet reactivity can be evaluated by a point-of-care assay (VerifyNow®; Accumetrics Inc., San Diego, CA, USA). This test has been proposed to identify patients who are “non-responders” to antiplatelet treatment in order to adjust the dosage accurately. This strategy appears to be effective in patients who experience acute stent thrombosis as well as in patients undergoing PCI for acute coronary syndromes. Consequently, assessment of platelet reactivity to guide and tailor antiplatelet treatment in interventional procedures for patients with unstable angina or non-ST myocardial infarction is a class IIb recommendation11.
In contrast to ACS trials, the use of high-dose clopidogrel or the use of prasugrel compared with standard dose clopidogrel did not reduce the incidence of ischaemic cardiovascular events at six months in patients with high on-treatment PR after PCI for stable coronary artery disease12,13. These findings suggest that, in patients referred for the treatment of stable coronary artery disease, late thrombotic risk is not altered by a strategy aiming to reduce PR further. Nevertheless, the results of the GRAVITAS trial showed that patients achieving an on-clopidogrel reactivity <208 P2Y12 reaction units (PRU) after PCI had a lower risk for late cardiovascular events14. However, this observation may have been influenced by other factors related to the complexity of the PCI performed.
Whether the risk of ischaemic complications and unwanted clinical events during 30 days post PCI in stable patients is also determined by the level of PR at the time of PCI is as yet uncertain. We therefore designed the Stent Thrombosis In Belgium (STIB) trial to determine whether patient PR assessment before intervention, after uniformly loading patients with a standardised dual antiplatelet therapy, could determine the risk of ischaemic complications and unwanted clinical events during 30 days following PCI in stable patients.
Methods
The Stent Thrombosis In Belgium (STIB) trial was a prospective, multicentre study in patients with stable coronary artery disease eligible for PCI of one or two vessels. Its objective was to study the impact of PR to clopidogrel and aspirin on adverse clinical events potentially occurring in-hospital and up to 30 days post PCI.
STUDY DESIGN
At five medical centres patients with stable coronary artery disease planned for PCI received a clopidogrel loading dose of 600 mg and an aspirin loading dose of 500 mg, 12 to 24 hours before PCI. Following intervention, a daily dose of clopidogrel (75 mg) and aspirin (80 to 100 mg) was given. All PCIs were performed according to current standard guidelines. The type of stent was left at the discretion of the operator. Patients received unfractionated heparin 70 IU/kg during the procedure. Patients with concomitant use of antithrombotic drugs other than aspirin or clopidogrel or with the need for glycoprotein IIb/IIIa inhibitors were excluded.
PR was measured in the catheterisation laboratory before PCI by the VerifyNow point-of-care test with the P2Y12 cartridge to test the degree of platelet inhibition to clopidogrel and with the aspirin cartridge to assess the degree of platelet inhibition to aspirin (Accumetrics Inc.)15. Measures of the antiplatelet effect of clopidogrel are expressed as P2Y12 reaction units (PRU). High PR has been defined in numerous studies as >230 PRU12,16. Measures of the antiplatelet effect of aspirin are expressed as aspirin reaction units (ARU). ARU values less than 550 indicate that platelets are inhibited by aspirin17. Operators were blinded to the PRU and ARU measurements.
In all patients blood samples were drawn before and at eight and 24 hours for creatine kinase-myocardial band (CK-MB) and troponins (TnT or TnI). Hs-troponin assays were not used. Laboratory data including haematocrit, platelet count, fibrinogen, creatinine, glycated haemoglobin A1C and C-reactive protein were also obtained before PCI.
In-hospital and one-month clinical events were collected for all patients and recorded on a standardised electronic clinical case record (modified BWGIC/CARDS database of the ESC EuroHeart Survey, Heart House, Nice, France). An independent clinical events committee (JB, MC) which had access to the database reviewed and adjudicated all suspected primary endpoints every three months.
Each patient provided informed consent to the study. The study was supported by an unrestricted grant from BIOTRONIK SE & Co. KG, Berlin, Germany. The trial was approved by the institutional ethics committee of each participating institution and complied with the Declaration of Helsinki. It was not registered.
CLINICAL ENDPOINTS
The primary endpoint of the STIB study was the composite of 30-day periprocedural myonecrosis, stent thrombosis, non-fatal myocardial infarction, stroke and death in patients with or without high PR (“non-responders” or “responders”). Secondary endpoints included in-hospital major bleeding complications, subgroup analysis of patients with in-hospital myonecrosis unrelated to procedural complications and stent thrombosis.
All deaths were considered cardiovascular unless an unequivocal non-cardiovascular cause could be established. Myocardial infarction (MI) was defined as a post-procedural increase of a cardiac biomarker (Tn) >3x the upper reference limit (URL)18. The presence of new pathological Q-waves in two or more contiguous ECG leads was referred to as Q-wave MI, while myonecrosis was defined as any Tn elevation >3x URL without persistent ECG changes. Stroke included occurrence of cerebral infarction (ischaemic stroke) and intracerebral/subarachnoïdal haemorrhage (haemorrhagic stroke). Definite or probable stent thrombosis, according to the Academic Research Consortium definitions19, was a secondary endpoint unless it occurred without death or myocardial infarction. Major bleeding (haemorrhagic) complications included haematoma requiring transfusion or surgical repair, intracerebral or subarachnoid haemorrhage (haemorrhagic stroke) and any bleeding event associated with a haemoglobin drop >5 g/dL or requiring transfusion or surgical repair (e.g., retroperitoneal bleed, gastrointestinal bleed, access-site bleed).
Troponin elevation above 3x URL after PCI in survivors without Q-wave MI, acute stent thrombosis or stroke (patients with procedural myonecrosis) was further characterised by the data safety monitoring board committee as: 1) related to an obvious procedural complication such as side branch occlusion, need for urgent CABG, rotablator use, residual Type C/D coronary dissection or cardiac arrest during PCI; 2) associated with slow flow in the main vessel or side branch, or residual thrombus post PCI; or 3) without identifiable cause of Tn elevation.
STATISTICAL ANALYSIS
The sample size was calculated assuming a 21% incidence of the primary endpoint at 30 days20. We hypothesised a 30% reduction in the incidence of endpoints in patients with low PR compared with patients with a residual high degree of PR21. A sample size of at least 860 patients would be needed to fulfil these assumptions with a statistical power of 0.90 to detect the hypothesised effect size with a two-sided p-value <0.05.
Continuous variables are presented as mean value±standard deviation. Categorical data are reported as frequencies and percentages. Differences in continuous variables were compared by one-way analysis of variance. Comparisons of categorical variables were tested by the χ2 test or Fisher’s exact test, as appropriate. Multivariate stepwise logistic regression was used to identify independent predictors of the occurrence of the composite endpoint in the 30-day period. The variables (demographic, clinical, procedural, and laboratory) entered in the model were selected using stepwise regression analysis with an entry criterion of p<0.10. Odds ratio (OR) and 95% confidence intervals (CI) were calculated. All probability values reported are two-sided, and a value of p<0.05 was considered to be significant. All analysis was performed with SPSS version 13.0 software (SPSS Inc., Chicago, IL, USA).
Results
Between March 2008 and October 2010, 923 consecutive patients were screened with platelet function testing from five sites in Belgium, among 3,671 patients with stable CAD treated by PCI. Thirty-two patients were excluded because of protocol violation (abnormal Tn value before PCI in 28 and the need for glycoprotein IIb/IIIa inhibitors in four). Among the 891 eligible patients, PRU was obtained in 882 and ARU in 852 patients. Both PRU and ARU values were available in 848 patients. Baseline characteristics of the patients are presented in Table 1.
PHARMACODYNAMIC OUTCOMES
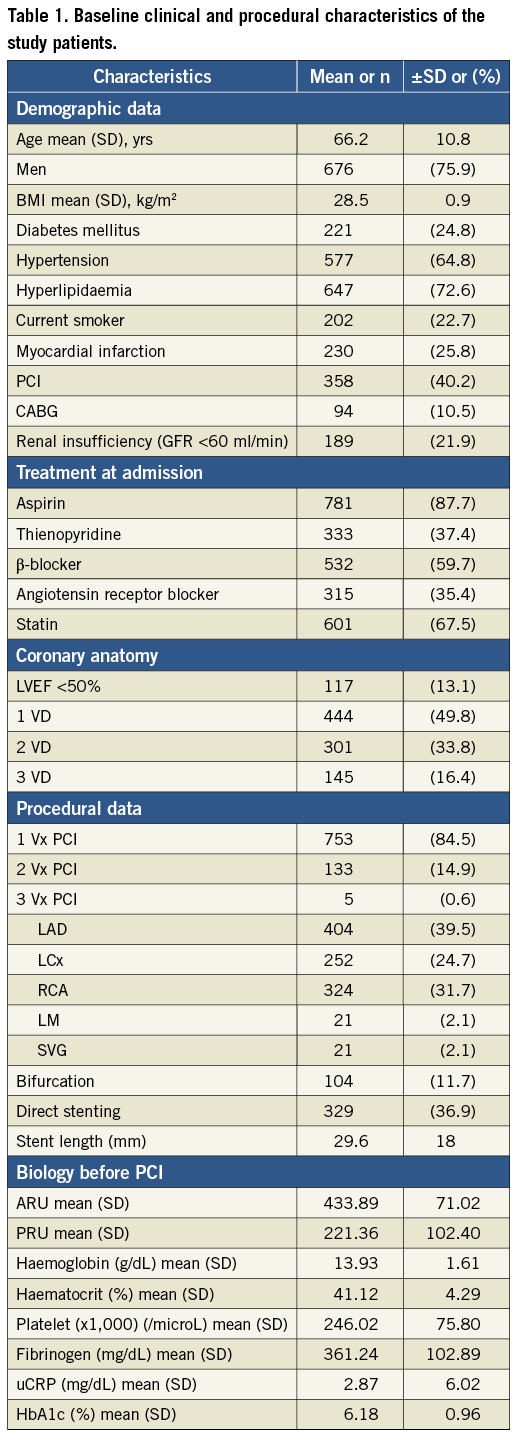
The cumulative distribution of the platelet function measured using VerifyNow is presented in Figure 1. High PR after aspirin (aspirin non-responders) represented 6.7% of the population, while high PR after clopidogrel (clopidogrel non-responders) was observed in 50.2%. Figure 2 illustrates response to both aspirin and clopidogrel in 848 patients in whom both measurements were obtained. Only 34 patients (4%) were non-responders to both aspirin and clopidogrel. Twenty-three patients (2.7%) were resistant to aspirin but responded to clopidogrel. Conversely, 60% of aspirin non-responders still had a high PR following a 600 mg loading dose of clopidogrel.
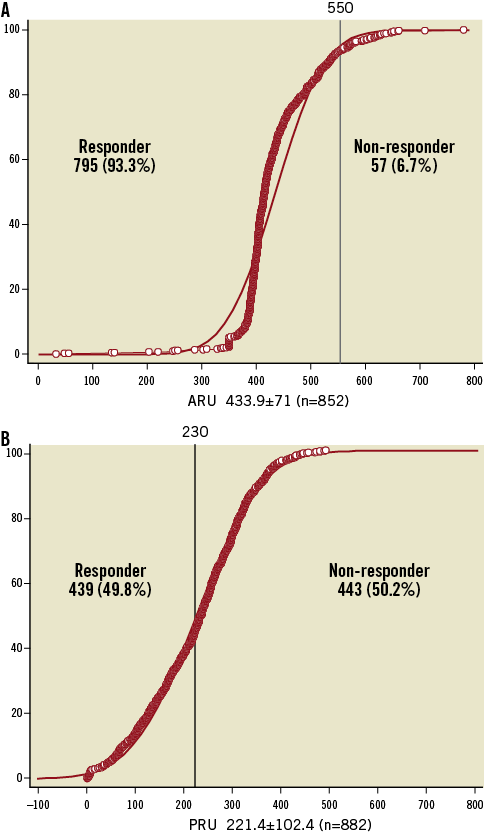
Figure 1. Cumulative distribution of the PR before PCI following aspirin (500 mg) and clopidogrel (600 mg) administration. A) Aspirin reactivity is shown and expressed in aspirin reaction units (ARU). Values above 550 indicate high residual PR to aspirin or non-responders12. B) Clopidogrel reactivity is shown and expressed in P2Y12 reaction units (PRU). Values above 230 indicate high residual PR to clopidogrel or non-responders9,11.
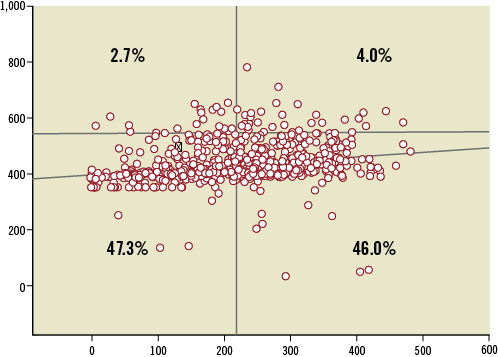
Figure 2. Plots of individual data of aspirin reaction units (ARU) in the y-axis and P2Y12 reaction units (PRU) in the x-axis. ARU values above 550 indicate high residual activity to aspirin and PRU values above 230 indicate high residual activity to clopidogrel (Figure1). Only 4% of the patients are non-responders to aspirin and clopidogrel and 47.3% are responders to both antiplatelet agents.
In order to address the influence of demographic and biological variables on the PRU value, the distribution of these variables across PRU quartiles was evaluated (Table 2). High reactivity after clopidogrel treatment was associated with increasing age and increased body mass index, poor diabetic control, low haemoglobin level and higher fibrinogen values at admission.
ENDPOINTS
The primary endpoint occurred in 180 patients (20%) and was driven by in-hospital events in 173 patients (Table 3). Four patients died. In one case, death was related to a cardiogenic shock which followed an acute anterior MI complicating a PCI of an ostial circumflex artery (ARU: 419, PRU: 223). One patient experienced a subacute stent thrombosis complicated by ST-elevation MI and died five days post PCI (ARU: 473, PRU: 386). Two other patients died suddenly at home (ARU/PRU: 383/114 and 473/67, respectively). Another patient experienced a subacute stent thrombosis and a non-Q-wave MI at day 15 (ARU: 403, PRU: 124). New Q-wave MI related to the procedure was observed in 10 cases. An additional Q-wave MI and two non-Q-wave MI unrelated to the treated vessel occurred during follow-up. According to ARC definitions, four patients suffered a definite or probable stent thrombosis. Post-procedural elevation of troponin >3x URL (myonecrosis) was diagnosed in 161 cases. The incidence of the primary endpoint was not related to treatment reactivity on either aspirin or clopidogrel (Figure 3).
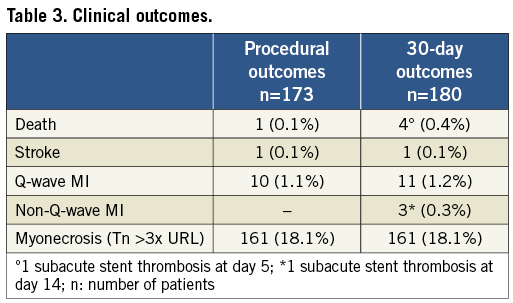
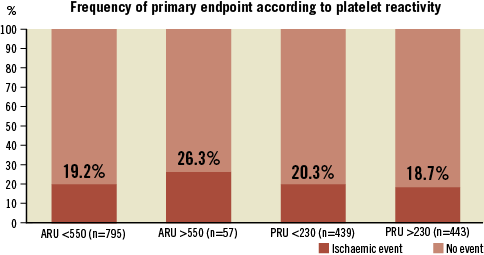
Figure 3. Frequency of primary endpoint (death, stroke, stent thrombosis, non-fatal myocardial infarction and troponin elevation >3x URL) among patients with or without high on-treatment PR. High PR on aspirin (ARU >550: non-responder to aspirin) or on clopidogrel (PRU >230: non-responder to clopidogrel) did not result in a significant increased risk of ischaemic events (p: NS).
CLINICAL, PROCEDURAL AND LESION CHARACTERISTICS AND CLINICAL OUTCOME
The association of patients’ demographic, clinical, biological and procedural characteristics with clinical outcomes was studied. On univariate analysis, neither ARU nor PRU was associated with primary endpoints. In the multivariate model, five variables emerged as independent predictors: total stent length (OR: 1.020; 95% CI: 1.009-1.031, p<0.0001), GFR <60 ml/min (OR: 1.87; 95% CI: 1.19-2.94, p=0.007), history of PCI (OR: 0.58; 95% CI: 0.38-0.88, p=0.008), white blood cell count (OR: 1.033; 95% CI: 1.001-1.052, p=0.013) and diabetes (OR: 1.83; 95% CI: 1.12-3.00, p=0.016).
PROCEDURAL MYONECROSIS, PROCEDURAL EVENTS AND PLATELET REACTIVITY
After exclusion of 12 patients who suffered in-hospital death, stroke, or Q-wave MI, and of 32 patients without determination of troponin and PRU, the cohort of patients was classified into four groups according to the assessment done by the data safety monitoring board. Group 1 comprised 54 patients in whom Tn elevation was related to an identifiable procedural complication such as side branch occlusion, need for urgent CABG, rotablator use, residual Type C/D coronary dissection or cardiac arrest during PCI. Group 2 comprised 22 patients with slow flow in the main vessel or side branch, or residual thrombus post PCI. Group 3 comprised 118 patients without identifiable cause of Tn elevation. Group 4 comprised 653 patients without abnormal troponin elevation post PCI. Mean PRU values in each group were 220.6±101.8, 227.6±103.9, 224.0±109.3, 206.33±97.3 and the percentage of patients with high residual platelet activity to clopidogrel was 50.4%, 50.8%, 40.9% and 44.4% in Group 1, Group 2, Group 3 and Group 4, respectively (p: NS).
SAFETY ENDPOINT
Major bleeding occurred in six patients: access-site bleeding in three, haemorrhagic stroke in two and gastrointestinal bleeding in one. Five of these patients had PRU values >230 (clopidogrel non-responders). At one-month follow-up, the rate of drug discontinuation was 4.4% for aspirin and 13.4% for clopidogrel. Only six patients interrupted both drugs. No patient had to stop antiplatelet treatment because of bleeding or haemorrhagic complications, and reasons for clopidogrel discontinuation were not reported. Among patients who stopped clopidogrel, one had a repeat PCI of a non-culprit vessel at 30 days.
Discussion
In this prospective multicentre study, we addressed the value of PR testing before PCI in a large number of patients undergoing PCI for stable angina pectoris and who were pretreated according to the current standard with aspirin 500 mg plus clopidogrel 600 mg at least 12 hours before the procedure. A high PR as assessed before intervention by a point-of-care assay does not predict procedural ischaemic clinical events or a worse clinical outcome up to one month. Reactivity to clopidogrel shows a wide interindividual variability yielding 50.2% non-responders to clopidogrel, while PR to aspirin is more predictable with only 6.7% non-responders to aspirin according to suggested definitions12,16,17. We also found that response to clopidogrel diminishes with advancing age and increasing body mass index. Glycaemic control, haemoglobin and fibrinogen are also linked to clopidogrel resistance.
Results of the ARMYDA-PRO study indicate that high pre-PCI PR measured by the VerifyNow assay might predict 30-day events in stable patients loaded with 600 mg clopidogrel. In this study, the primary endpoint was more frequent in patients with preprocedural PRU levels in the fourth quartile versus those in the first quartile, and this was essentially due to periprocedural MI22. There was, however, a substantial overlap in PRU values between groups and no difference was found when troponin elevation was used to define periprocedural MI. Hochholzer et al21 showed that the incidence of ischaemic events increased gradually from the first to the fourth quartile (p=0.03) among stable patients undergoing stent implantation. However, the low incidence of events in 15 out of 802 patients reduces the power of this observation, and quartiles of platelet aggregation were not associated with periprocedural elevations in troponin. Our study extends and clarifies these observations. First, we confirm the very low incidence of major clinical events at 30 days in stable patients undergoing PCI with the current standard of care. Second, we prove that the variability of response to clopidogrel is large and not associated with periprocedural myonecrosis, even when analysis is performed according to quartiles of platelet aggregation (Table 2).
In multivariate analysis, neither the absolute level of platelet inhibition in response to clopidogrel nor the response to aspirin emerged as an independent predictor of the composite endpoint of in-hospital periprocedural myonecrosis, stent thrombosis, non-fatal myocardial infarction, stroke and death. The combined endpoint for thromboembolic and ischaemic events was predicted by the total length of stented segment, GFR <60 ml/min, white blood cell count and diabetes. Conversely, a history of previous PCI was associated with a risk of fewer events. Recent observations also showed the lack of benefit in increasing the dose of clopidogrel among non-responders12 and the futility of a more potent P2Y12 inhibitor13 to reduce the incidence of death from cardiovascular causes, non-fatal MI or stent thrombosis at 30 days and six months. However, a trend towards fewer cardiovascular events at six months was observed in patients with very low PR (PRU <208)14. These findings are consistent with previous observations23 and indicate that clinical and procedural characteristics play a more important role than PR in predicting early outcomes following PCI in patients with stable angina pectoris when treated by aspirin and clopidogrel according to current recommendations. However, it has been observed that patients with multivessel disease have a higher chance of being non-responders than patients with single-vessel disease, and this translated into a higher incidence of periprocedural infarctions7. Conversely, treatment with GP IIb/IIIa inhibitors in those patients significantly improved outcomes23. We therefore cannot exclude that platelet reactivity plays an independent role in sicker patients with extensive multivessel disease. On the contrary, in patients presenting with an acute coronary syndrome, inflammatory reaction and various mechanisms that induce platelet activation are predominant and explain the clinical benefit of a potent platelet inhibition, particularly during the first months following PCI.
Troponin three times greater than normal occurred in 19% of our patients undergoing PCI for stable angina. In the meta-analysis of Feldman et al24 including series published between 1998 and 2009, the rate of troponin elevation following PCI ranged from 26% to 34%. We may speculate that the lower incidence of myonecrosis observed in our study could be attributed to a better inhibition of the PR before PCI. Administration of clopidogrel before PCI was frequently omitted in these series or indeed given without adequate loading dose.
Patient, lesion and procedural factors have been associated with periprocedural myocardial infarction25,26. In our study, we sorted out mechanisms responsible for troponin elevation post PCI in 194 patients. Procedure-related factors (main vessel or side branch occlusion, occlusive dissection, emergency bypass surgery, use of rotational atherectomy, etc.) were identified in 54 patients (28%). Thromboembolisation and slow flow unexplainable by a procedural cause were noted in 22 patients (11%), while in 118 patients no procedure or lesion-related factor could be identified. No difference was noted between each group with respect to PR. Specifically, residual high PR was not identified among patients with slow flow post PCI. This suggests that mechanisms other than platelet aggregation or thrombus formation, such as plaque component embolisation or vasoactive reaction, play an important role in slow flow occurring post PCI in stable patients.
Besides periprocedural myonecrosis, the rate of cardiovascular events observed in this study was low and similar to that described in similar populations of patients undergoing PCI. The frequency of major bleeding events was also very low and not associated with a high PR. To address the impact of PR on hard clinical and safety endpoints in this low-risk population, a large number of patients and/or a long-term follow-up observation are therefore required.
Our study evaluated PR to aspirin concomitantly with P2Y12 reactivity. As expected from previous observations17,27, response to aspirin was highly predictable, and resistance to aspirin was noted in 6.7% of this population. This emphasises the importance of aspirin treatment as the cornerstone of antiplatelet therapy. Our results also confirmed the wide individual variability in the platelet response to clopidogrel. According to point-of-care results, only 49.8% of this population of patients with stable coronary artery disease achieved an adequate platelet inhibition. The rate of clopidogrel responders was 59.3% in the GRAVITAS trial12. Assessment of PR 12 to 24 hours after PCI may explain the higher rate of response observed in this trial. As mentioned before, the unpredictable and poor efficacy of clopidogrel does not affect early outcomes and seems to be sufficient to reduce the rate of periprocedural myonecrosis as compared to earlier reports. Overall, resistance to aspirin and clopidogrel was recognised in 4% of the population. No clinical or demographic data characterised this small subgroup of 34 patients whose outcome did not differ from the rest of the population. However, a larger series of non-responders to both aspirin and clopidogrel is needed before definitive conclusions on the immediate and late consequences of this situation can be drawn.
Numerous studies have shown that individual responsiveness to clopidogrel is not uniform. Besides pharmacogenetic factors linked to CYP enzymes, cellular and clinical factors may also contribute to the reduction of platelet responsiveness to clopidogrel28. The clinical and procedural characteristics of our population assessed according to PRU quartiles confirmed that increased platelet reactivity following clopidogrel administration is linked to body mass index29. The incidence of diabetes mellitus was significantly more frequent from the first to the fourth quartile, in particular the mean glycaemia as expressed by HbA1c. We identified other clinical factors which might influence clopidogrel response. Elevated plasma fibrinogen level is increased in relation to increased PRU. Fibrinogen level has been found to predict a suboptimal response to eptifibatide30, and may also play a role in the level of platelet inhibition achieved with clopidogrel or other P2Y12 inhibitors. Other clinical factors such as advancing age, renal failure or anaemia may also play a role and deserve further investigation.
Conclusion
In conclusion, the results of the present study confirm that the level of PR immediately before elective PCI in patients pretreated with 600 mg clopidogrel and 500 mg aspirin is not correlated with early clinical outcome after the procedure. Applied to large patient populations undergoing elective PCI, the absolute level of PR as assessed by the VerifyNow point-of-care has little clinical utility in routine practice to predict early clinical outcomes. These results do not support the use of platelet testing in patients undergoing PCI for stable coronary artery disease.
| Impact on daily practice Evaluation of platelet reactivity before PCI in patients undergoing angioplasty for stable coronary artery disease, pretreated with aspirin and clopidogrel according to guidelines, is futile. Reactivity to aspirin and/or clopidogrel, as assessed by point-of-care VerifyNow, does not predict up to 30-day clinical events, nor does it correlate with periprocedural myonecrosis. |
Conflict of interest statement
The authors have no conflicts of interest to declare.

