Abstract
Aims: We assessed prospectively the association between occurrence of post-discharge non-CABG-related TIMI major and minor bleeding and post-treatment platelet reactivity in patients with non-ST elevation acute coronary syndrome (NSTE ACS).
Methods and results: Five hundred and ninety-seven consecutive patients admitted with NSTE ACS were prospectively included. Between hospital discharge and one month follow-up, we observed 16 (2.7%) non-CABG-related TIMI haemorrhagic complications including five (0.84%) major and 11 (1.8%) minor bleeds. Patients with bleeding had significantly lower post-treatment values of ADP-induced aggregation (43±14% versus. 56±19%, p=0.002) and platelet reactivity index VASP (43±14% versus 54±23%; p=0.04) and a trend for lower values of arachidonic acid-induced aggregation (2.4±5.4 versus 13±21; p=0.27). After stratification by quartiles based on post-treatment ADP-induced platelet aggregation, we identified patients in the first quartile as hyper-responders with very low post-treatment platelet reactivity, below <40%. The risk of TIMI major and minor bleeding was significantly higher in the first quartile of hyper-responders than in the others quartiles: 10 (6.6%) versus six (1.4%), p=0.001.
Conclusions: Our results suggest that assessment of post-treatment platelet reactivity might be used to detect hyper-responders to antiplatelet therapy with higher risk of non-CABG related bleeding and tailor antiplatelet therapy according to both ischaemic and bleeding risk.
Introduction
Platelet inhibition with aspirin and clopidogrel plays an important role in the management of cardiac patients. Dual platelet inhibition significantly reduces recurrent ischaemic events after non-ST-segment elevation acute coronary syndrome (NSTE ACS)1,2. In addition, the risk of recurrent ischaemic events subsequent to percutaneous coronary intervention (PCI) is associated with the extent of platelet inhibition achieved, as assessed by light transmittance aggregometry (LTA)3,4. Current evidence suggests an inverse association between the level of platelet inhibition and ischaemic risk3,4. This concept has been confirmed with the recently published TRITON study, which demonstrated a lower thrombotic risk with a more effective P2Y12 receptor antagonist5,6. However, this benefit was counterbalanced with a higher risk of bleeding complications. A number of other randomised trials and registries have also highlighted the importance of haemorrhagic complications associated with dual antiplatelet therapy, demonstrating a connection between episodes of major bleeds and mortality7-10. However, it is unclear whether testing for platelet reactivity subsequent to antiplatelet therapy can be used to assess the risk of haemorrhagic complications.
In a previous prospective study, by assessing response to both aspirin and clopidogrel using LTA, we demonstrated that a low response to antiplatelet therapy was associated with a higher risk of recurrent ischaemic events11,12. In this study, we collected the incidence of non-coronary artery bypass surgery (CABG) related thrombolysis in myocardial infarction (TIMI) major and minor haemorrhagic complications occurring between hospital discharge and at one month follow-up and analysed the association between bleeding complications and level of post-treatment platelet reactivity.
Methods
Consecutive patients admitted with a NSTE ACS were recruited to this prospective study. NSTE ACS was defined as clinical symptoms compatible with acute myocardial ischaemia within 12 hours of admission to hospital and at least one of the following: new onset of ST-segment depression >0.05 mV, transient (<20 min) ST-segment elevation >0.1 mV, T-wave inversion >0.3 mV in at least two contiguous leads, elevation in biochemical markers of myocardial injury (troponin I >0.4 ng/mL) or coronary artery disease as documented by a past history of revascularisation or myocardial infarction. The exclusion criteria included: a history of bleeding diathesis, persistent ST-segment elevation, New York Heart Association (NYHA) class IV heart failure, percutaneous coronary intervention (PCI) or CABG surgery in the previous three months, contraindications to antiplatelet therapy, platelet count <100 g/L, creatinine clearance <30 mL/min), use of glycoprotein IIbIIIa antagonists before the procedure and patients receiving chronic oral anticoagulation. Patients received a 600 mg loading dose of clopidogrel and 250 mg of aspirin at least 12 hours before coronary angiography and PCI as indicated. Loading doses of antiplatelet medication were administrated under nurse supervision to avoid any problems with compliance. Use of glycoprotein IIbIIIa antagonists during cardiac catheterisation was left to physician’s discretion. The study protocol was approved by the ethics committee of our institution and all patients gave written informed consent for participation.
Blood samples were drawn after cardiac catheterisation, at least 12 hours after the loading dose of clopidogrel and aspirin, light transmittance aggregometry was used as described previously11,12,14. Aggregation was expressed as the maximal % change in light transmittance from baseline with PPP as reference. The coefficients of variation for maximal intensity of platelet aggregation with ADP and AA were 6.5% and 6% respectively. In a healthy population, the normal range for ADP-induced aggregation is 69% to 104% and for AA-induced aggregation 26% to 99%. As previously described, non-response to aspirin and clopidogrel was defined as AA-Ag >30% and ADP-Ag >70% respectively3,4,11,12.
To determine the VASP phosphorylation state of whole blood, we used a standardised flow cytometric assay (Platelet VASP®; Diagnostica Stago [Biocytex], Asnières, France) as previously described13,14. A platelet reactivity index VASP (PRI VASP) was calculated from the median fluorescence intensity (MFI) of samples incubated with PGE1 or PGE1 and ADP according to the following formula: PRI VASP=[(MFI (PGE1)-MFI (PGE1+ADP)/MFIPGE1] x 100. Non-response to clopidogrel was defined as PRI VASP >53% as previously described14.
The primary endpoint of the study was the occurrence of non-CABG related TIMI major and minor haemorrhagic complications as already defined15 between hospital discharge and one month follow-up. In-hospital and procedure-related bleeding complications were excluded from the analysis. The events were assessed by a clinical visit one month after hospital discharge. The association between non-CABG related TIMI major and minor bleeding and platelet parameters were analysed.
Statistical analysis was performed using graphpad prism software (version 4.0, GraphPad Software, Inc., La Jolla, CA, USA). Continuous variables are expressed as mean ±SD. Categorical variables are expressed as frequencies and percentages. The Wilcoxon rank-sum test was used to compare continuous variables in individuals with and without bleeding events. Comparison between categorical variables were performed using the Chi 2-test or the Fischer’s exact test when frequencies were below five. P<0.05 was considered significant.
Results
A total of 597 patients with NSTE ACS who fulfilled the inclusion criteria were recruited to the study. Baseline characteristics of patients are summarised in Table 1.
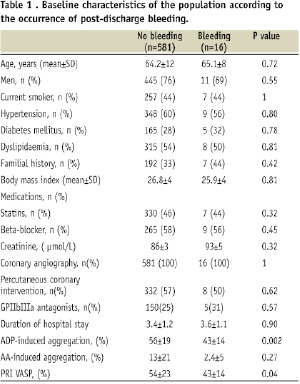
All patients received 600 mg of clopidogrel and 250 mg of aspirin at least 12 hours before coronary angiography / PCI. All patients remained on dual antiplatelet therapy with 75 mg of aspirin and clopidogrel for one month post-PCI.
AA-Ag and ADP-Ag were obtained in all patients and PRI VASP was obtained in 495 patients. There was high inter-individual variability of all platelet parameters. Maximal intensity of AA-Ag ranged between 0 to 86 % with mean value of 13.1±21%. Maximal intensity of ADP-Ag ranged between 0 to 100 % with mean value of 56.3±19%. PRI VASP was also variable ranging between 1 to 95% with mean value of 53.7±23.2%. There was a significant correlation between ADP-Ag and PRI VASP, both assessing platelet response to clopidogrel (in 495 patients r=0.61; P<0.001).
In the one month period, post-discharge from the hospital, there were 16 (2.7%) non-CABG related TIMI haemorrhagic complications including five (0.84%) major and 11 (1.8%) minor bleeds. Major haemorrhagic complications included three gastrointestinal (0.50%) and two intracranial (0.34%) bleeds including one fatal (0.17%). Platelet parameters of the five patients suffering a major haemorrhagic complication are summarised in Table 2.
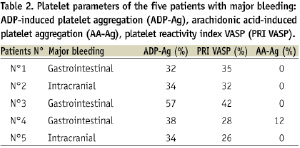
None of the five patients were classified as non-responder to aspirin and/or clopidogrel. In addition, there was no significant difference in clinical, biological or angiographic data between patients who did / did not suffer a major haemorrhagic complication, including individuals at a high-risk of bleeding (based on a previous history of bleeding, history of stroke, renal failure, diabetes mellitus and advanced age) (Table 1). In the entire study cohort, patients who suffered a haemorrhagic complication (both major and minor) had significantly lower values of ADP-Ag (43±14% versus 56±19%; P=0.002) and PRI VASP (43±14% versus 54±23%; P=0.04) (Figures 1 and 2).
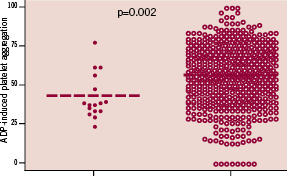
Figure 1. ADP-induced platelet aggregation among patients with bleeding and controls.
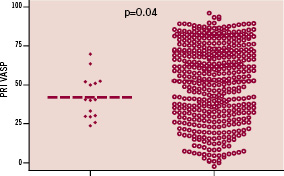
Figure 2. Platelet reactivity index VASP (PRI VASP) among patients with bleeding and controls.
There was also a trend toward lower values of AA-Ag (2.4±5% versus 13±21%; P=0.27). Patients in the entire study population were divided into quartiles on the basis of ADP-induced platelet aggregation. Patients in the lower quartile of ADP-induced platelet aggregation were identified as hyper-responders. The cut-off value isolating this quartile was <40%. There was no significant difference in terms of clinical, biological or angiographic data between patients in the first quartile and other quartiles. The risk of non-CABG related TIMI major and minor bleeding was significantly higher in the first of hyper-responders than in the others quartiles: 10 (6.6%) versus six (1.4%), p=0.001 (Figure 3).
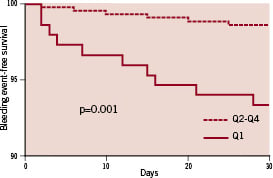
Figure 3. Occurrence of bleeding according to ADP-induced platelet aggregation (ADP-Ag). Quartiles: quartile 1 of hyper-responders (ADP-Ag<40%) vs quartiles 2 to 4.
Discussion
The present study suggests that the level of platelet response to antiplatelet agents can be used to assess both the risk of haemorrhagic as well as ischaemic complications in patients with NSTE ACS and/or undergoing PCI. To date, variability in response to antiplatelet therapy has primarily been used to identify low responders to antiplatelet agents with increased thrombotic risk3,4,11,12,14. However, attempts to define a threshold value to identify such patients, has not been successful due to methodological differences between different studies. As a result, there is currently no consensual definition of non-response to aspirin and/or clopidogrel. On the other hand, haemorrhagic complications are the most frequently encountered non-ischaemic complications in NSTE ACS patients. It is estimated that the frequency of major bleeds is between 2-8% across a spectrum NSTE ACS patients and depends on the type and dose of both the antithrombotic and antiplatelet therapies administered. Results from a number of randomised trials and registries highlight the clinical importance haemorrhagic complications by demonstrating an association between occurrence of a major bleed and increased mortality7-10. In addition, Ndrepepa et al have recently shown bleeding complications, myocardial infarction and urgent revascularisation have comparable discriminatory power for predicting 1-year mortality16. Multiple factors are thought to contribute to the poor outcome associated with bleeding. These include renal failure, haemodynamic instability and potential deleterious effects of blood transfusion. In addition, bleeding can trigger a prothrombotic and pro-inflammatory state. The requirement to discontinue antiplatelet / antithrombotic medication is another risk, as this can result in further ischaemic complications by creating a rebound prothrombotic milieu. Haemorrhagic and ischaemic complications are closely related. This has been highlighted in the development of new more potent P2Y12 antagonists such as prasugrel, which have higher levels of platelet inhibition than clopidogrel5. In clinical studies, the lower risk of ischaemic complications associated with prasugrel is paralleled by a higher risk of bleeding6. In this present study, we did not include in-hospital bleeding complications as part our analysis. The majority of in-hospital bleeding episodes are the direct result of procedural complications, often in patients receiving concurrent anticoagulants and/or glycoprotein IIb-IIIa antagonists. In addition, procedural bleeds are also influenced by many co-factors such as vascular access, sheath size, operator experience and timing of sheath removal. Thus, to assess the link between platelet reactivity after dual oral antiplatelet therapy and major haemorrhagic complications, we investigated the post-discharge incidence of non-CABG related TIMI major bleeds. In contrast to non-responders to antiplatelet therapy who are known to be at increased risk of thrombotic complications, our results may suggest that assessment of post-treatment platelet reactivity could be used to detect hyper-responders with higher risk of bleeding complications. These preliminary findings suggested a need for a “therapeutic range” of platelet inhibition for a good balance between efficacy (ischaemic events) and safety (bleeding). This concept will probably be highlighted with the arrival of new more potent oral antiplatelet drugs such as prasugrel or ticagrelor.
Study limitations
The main limitations of the present study include its small sample size and number of events. Moreover, this is a preliminary study with no definite clinical consequences at this stage. Larger sample size studies will be required to draw definite conclusions. Finally, this study used LTA with a cut-off value of 40% as a marker of post-treatment platelet reactivity. However, before including platelet testing in daily clinical practice for assessing bleeding risk, a consensual definition of “hyper-response” will be required, and this will probably prove to be as difficult to arrive at as the definition of “non-response”, which remains in question.
Acknowledgements
This work was supported by the Assistance Publique Hôpitaux of Marseille, France. The assistance of our teams of nurses and technicians in executing this study are gratefully acknowledged.

