
Abstract
Aims: In patients with non-ST-elevation acute coronary syndromes (NSTE-ACS) treated with PCI, high (H) platelet reactivity (PR) significantly affects one-year outcome. The aim of this report was to analyse the relationships between HPR, the SYNTAX score (SS) and one-year major adverse cardiac events (MACE: cardiac death, myocardial infarction, stent thrombosis) according to diabetes mellitus (DM) status in patients included in the GEne Polymorphism, Platelet REactivity, and the Syntax Score (GEPRESS) study.
Methods and results: PR was measured using the vasodilator-stimulated phosphoprotein (VASP) assay at three time points (before PCI, at hospital discharge and at one month after PCI), with HPR defined as >50% PR index in 1,042 patients treated with aspirin and clopidogrel for one year after PCI. Patients with DM and an SS ≥15 had the highest MACE rate between one month and one year, further increased by the presence of HPR (16.4%). On the other hand, among all patients with an SS <15, MACE rates remained low (<3%), irrespective of DM status and PR.
Conclusions: Among NSTE-ACS patients treated with PCI, the combination of DM, an SS ≥15 and HPR characterised a cohort with the highest MACE rate from one month to one year. In such high-risk patients, careful clinical monitoring and implementation of secondary prevention measures, including the use of potent P2Y12 inhibitors, are strongly advised.
Introduction
Diabetes mellitus (DM) is an independent predictor of outcome and cardiovascular mortality in patients with coronary artery disease1,2, including those with non-ST-elevation acute coronary syndromes (NSTE-ACS)3,4. Platelets of DM patients show dysregulation of both receptor and intracellular signalling pathways, leading to increased platelet reactivity5,6. This can play a role not only in the higher proportion of DM patients with inadequate response to antiplatelet agents compared with non-DM subjects7-9 but also in their worse outcomes despite compliance with recommended antiplatelet treatment regimens7-9. Among DM patients with coronary artery disease, the presence of a high (H) platelet reactivity (PR) despite chronic treatment with aspirin and clopidogrel is associated with an over threefold increase in two-year cardiovascular event rates compared with those without HPR10,11.
We recently reported the results of the GEne Polymorphism, Platelet REactivity, and the Syntax Score (GEPRESS) study12, showing that, in patients with NSTE-ACS treated with percutaneous coronary interventions (PCI) on dual antiplatelet therapy with aspirin and clopidogrel for one year, HPR was associated with an increased number of cardiac events only in the presence of a high SYNTAX score (SS), an index of extent and complexity of coronary artery disease13. The aim of the present report was to analyse how DM status interplays with PR and SS in determining outcome among patients included in the GEPRESS study.
Patients and methods
PATIENTS AND STUDY DESIGN
The GEPRESS study is a prospective, multicentre study designed to determine the impact of platelet reactivity and the SS in NSTE-ACS patients treated with PCI and followed for one year while on dual antiplatelet therapy with aspirin and clopidogrel. Details of the study have been previously reported12. In brief, inclusion criteria were a diagnosis of NSTE-ACS with at least one coronary stenosis >50% requiring PCI, with no allergy to aspirin or clopidogrel. PCI was performed according to the standard of care. All patients received a loading dose of 300 mg or 600 mg of clopidogrel and, following the procedure, were treated with aspirin indefinitely, while clopidogrel was recommended for one year. Exclusion criteria were concomitant therapy with oral anticoagulants, cardiogenic shock, any contraindication to dual antiplatelet therapy for one year, therapy with prasugrel or ticagrelor, or major comorbidities associated with life expectancy less than one year. The study was approved by the local ethics committee at each participating centre, and all patients provided written informed consent.
PLATELET FUNCTION TESTING
Platelet reactivity was measured using the vasodilator-stimulated phosphoprotein (VASP) assay (BioCytex, Marseille, France), using the flow cytometric technique as previously described14, expressed as platelet reactivity index (PRI). HPR was defined as a PRI >50% as previously reported to be associated with ischaemic recurrences and in agreement with expert consensus15. The VASP assay was used to measure platelet reactivity because results are not affected by the use of glycoprotein IIb/IIIa inhibitors, which are commonly used in patients with NSTE-ACS, particularly among enrolling centres in this study. The PRI was determined at three time points: before PCI, at hospital discharge, and at one month after PCI. The incidence of the CYP2C19*2 polymorphism was calculated for the DM and non-DM groups. The detailed protocol for the genotypic analysis has been described in a prior manuscript12.
OBJECTIVE AND DEFINITIONS
The primary objective of this study was to investigate the association between one-month HPR and the SS for the risk of cardiac events in the period between one month and one year in the DM and non-DM cohorts of the GEPRESS study. Major adverse clinical events (MACE) were defined as the composite of cardiac death, MI, and stent thrombosis. Any death in which a cardiac cause could not be excluded was also adjudicated as cardiac. Periprocedural MI was defined if new Q-waves or an increase of CPK >2 times the upper normal levels with the MB fraction >10% was documented. After hospital discharge, MI was defined as the occurrence of typical chest pain associated with an increase in troponin levels above the upper normal levels. Renal dysfunction was defined as a calculated creatinine clearance by the Cockcroft-Gault equation of <60 mL/minute. Stent thrombosis was defined according to the Academic Research Consortium (ARC)16. Bleeding was defined according to the Bleeding ARC (BARC) definition17. A high SS was defined as ≥15, a measure that characterised the upper tertile of the study population.
STATISTICAL ANALYSIS
Categorical variables were displayed as count (percentage) and compared with the χ² test. Continuous variables were expressed as mean±standard deviation and compared with the Wilcoxon rank-sum test. The longitudinal effect of DM on HPR was tested by repeated measures ANOVA18. The Wald test statistic was calculated for testing the null hypothesis. Time-to-event was explored by Kaplan-Meier analysis, and differences in outcome were compared using the log-rank test. The hazard ratio (HR) and 95% confidence interval (CI) of DM patients were estimated by fitting a Cox proportional hazards regression model. The association between DM and MACE was adjusted for potential confounders and established risk factors using the Cox regression. The following univariate predictors were used for adjustment: age, male gender, baseline renal failure, NSTEMI at presentation, LV ejection fraction, HPR and SS ≥15. Sensitivity analysis using case deletion was used to address the role of missingness. Proportionality risk assumption was assessed by Schoenfeld residuals analysis. A two-sided probability value ≤0.05 was considered significant. Data were analysed in R version 3.1.2 software environment19, “Survival”, and “ggplot2” packages.
Results
CLINICAL AND ANGIOGRAPHIC CHARACTERISTICS
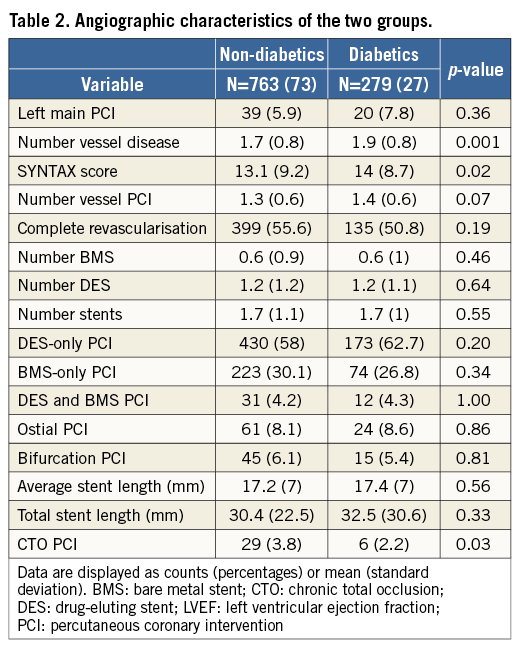
In 1,042 patients included in the GEPRESS study, 283 patients (~27%) had DM. For the present analysis, 279 patients were considered because results of platelet function tests were inconclusive in four subjects. As shown by Table 1, diabetics were older, more frequently had hypertension, prior coronary bypass surgery and a greater body mass index than non-DM patients. The CYP2C19*2 polymorphism was more frequently found in non-DM than in DM patients. Moreover, diabetics were less likely to be smokers and had a lower baseline haemoglobin than non-diabetics. No difference was found in medication use during hospitalisation except for the use of antidiabetic drugs in the DM group (including insulin in 21% of DM patients). Angiography revealed a greater number of diseased vessels and a higher SS in diabetics than in non-diabetic patients (Table 2). No difference was found in the number of treated vessels and of implanted stents per patient or in the complexity of the PCI procedures (treatment of ostial lesions, bifurcations), yet chronic total occlusions were more frequently addressed in non-diabetics than in diabetics.
ASSESSMENT OF PR AND ONE-YEAR OUTCOME
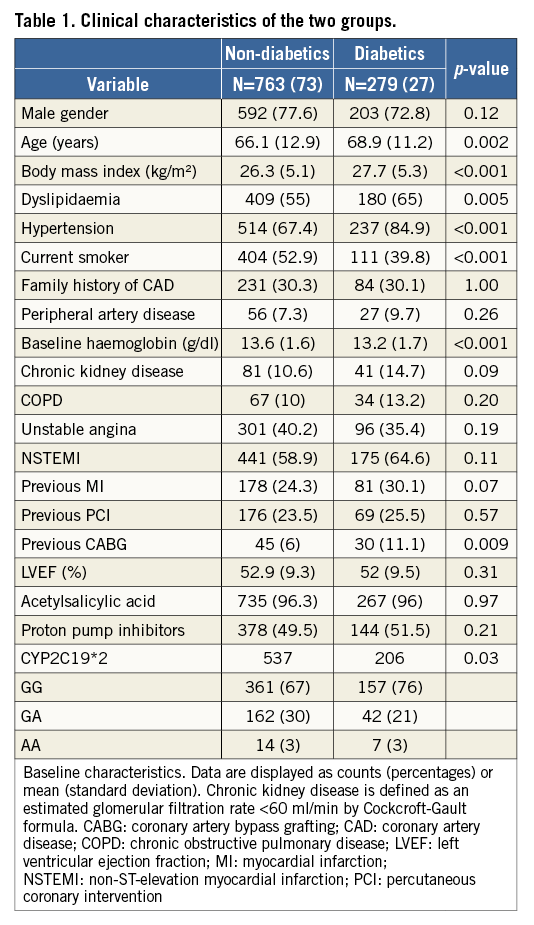
Platelet response to clopidogrel assessed by VASP at different time points was significantly lower in DM than in non-DM patients (Figure 1). A post hoc comparison showed that this effect was mainly driven by less potent platelet inhibition occurring between discharge and one-month follow-up in DM patients than in non-DM patients.
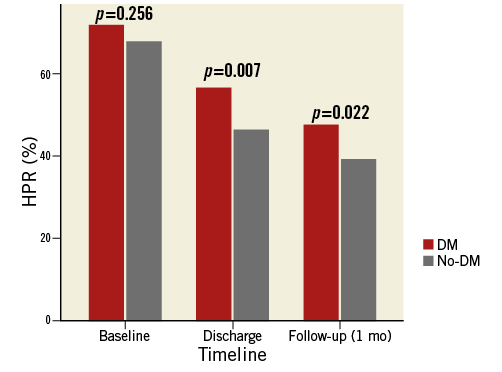
Figure 1. Percentage of patients with HPR at baseline, at discharge and at one month. Red columns represent diabetic patients (DM), the grey ones the non-diabetic patients (No-DM).
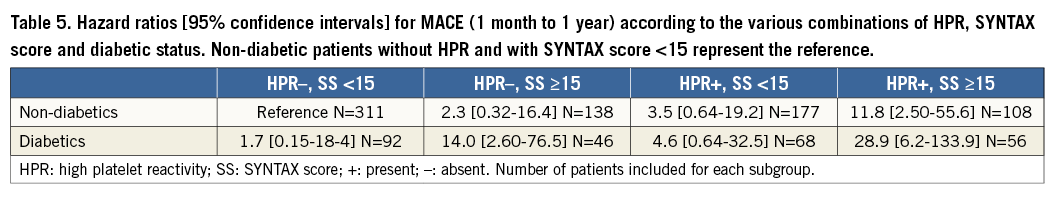
MACE were significantly higher in diabetics than in non-diabetics from one month to one year, the primary endpoint of the study (Figure 2). Table 3 shows the adverse events occurring between one month and one year. No difference was found between the two groups in the total number of bleeding events. After adjusting for potential confounders by multivariable analysis, age, DM, HPR and an SS ≥15 were independently associated with MACE events between one month and one year (Table 4). Figure 3 shows the relationship between DM, SS, PR and outcome. Patients with DM and an SS ≥15 had the highest MACE rates between one month and one year, further increased by the presence of HPR (16.4%). On the contrary, among all patients with an SS <15 MACE rates remained low (<3%) irrespective of DM status and PR. Table 5 presents the HR and 95% CI of the various combinations of SS values (SS ≥15 or <15) and PR in the DM and non-DM patients, as compared to the reference represented by non-DM patients without HPR and SS <15. A combination of HPR, SS ≥15 and DM characterised a cohort with the highest hazard ratio for MACE (HR 28.9, 95% CI: 6.2-133.9).
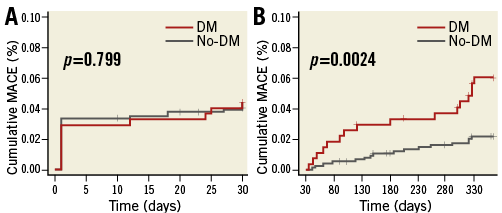
Figure 2. MACE rates in diabetic (red lines) and non-diabetic patients (grey lines) between admission and one month (A) and between one month and one year (B).
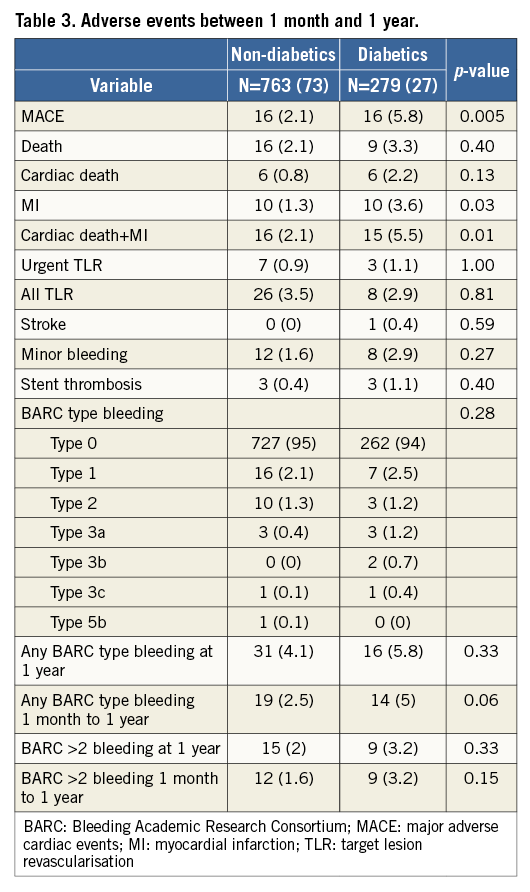
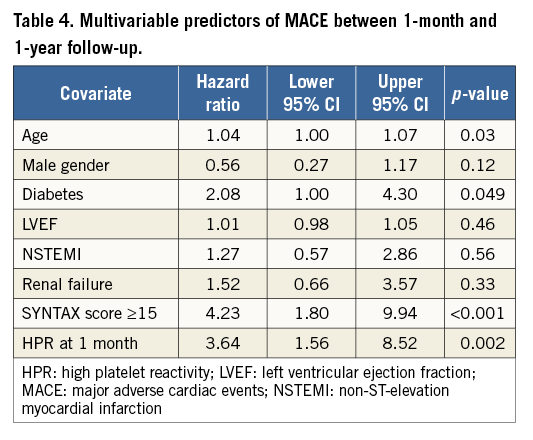
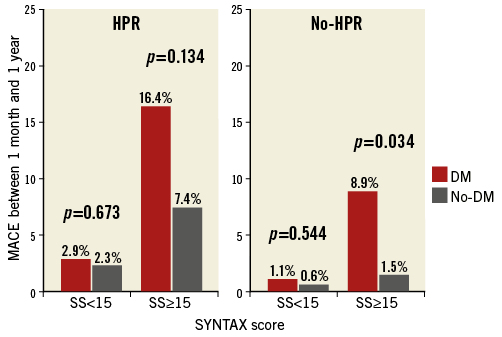
Figure 3. MACE rates in patients with or without high platelet reactivity (HPR). Diabetics are shown by the red histograms. Percentages refer to MACE rates between one month and one year.
Discussion
The main finding of the present analysis is that, among NSTE-ACS patients treated with PCI and on dual antiplatelet therapy with aspirin and clopidogrel, the highest MACE rates between one month and one year were observed when DM status was associated with an SS ≥15, particularly when HPR was also present. On the other hand, MACE rates were below 3% when SS was <15 also in DM patients and irrespective of the presence or not of HPR.
Recent data may explain the reason why the association of DM status with an SS ≥15, an index not only of the atherosclerotic burden but also of lesion complexity, is a powerful prognostic indicator in NSTE-ACS patients. Analysis of volumetric plaque composition of the coronary arterial tree using virtual histology-intravascular ultrasound imaging has shown that the atherosclerotic plaques of DM patients have larger amounts of necrotic core and more thin-cap fibroatheromas than non-DM patients, histologic characteristics implying high vulnerability and rapid lesion progression that may lead to clinical instability20. Moreover, in a recent analysis including patient-level data from 18 prospective trials, DM status was a risk factor for repeat revascularisation only in patients with complex lesions21.
Several investigations have shown that patients with DM treated with clopidogrel have an impaired response to the drug, enhancing the atherosclerotic risk associated with that clinical condition7,8,10,22,23. In our study, about 50% of DM patients had HPR after one month following PCI, a percentage significantly higher than that observed in non-DM patients, despite the fact that in our series CYP2C19*2 polymorphism was more frequently found in non-DM than in DM patients. Recently, Angiolillo et al24 found that such an impaired response is mediated by a less favourable pharmacokinetic profile, leading to low levels of clopidogrel’s active metabolite formation rather than being secondary to a pharmacodynamic dysfunction of the P2Y12 signalling pathways.
We assessed platelet function by flow cytometric analysis of the phosphorylation status VASP, which is a specific measure of the degree of blockade of the P2Y12 receptor. It is possible that DM could affect other signalling pathways of platelets. Generation of thrombin, a link between plasmatic and cellular components of the thrombotic process and a potent agonist of platelet aggregation, has been shown to be enhanced in patients with DM25. Moreover, platelet turnover, represented by a high number of reticulated hyperreactive platelets, has been described as being associated with a DM status26.
The GEPRESS data show that HPR after one month in NSTE-ACS patients while on treatment with clopidogrel significantly affects outcome only when associated with an SS ≥1512. The present analysis expands those data by showing that when HPR was found in patients with an SS <15 the MACE rates between one month and one year were below 3% in both DM and non-DM patients. On the other hand, HPR doubled the risk for MACE in DM patients with a high SS. Therefore the impact of HPR on outcome calls for a strong indication for the use of powerful P2Y12 receptor blockers in this clinical condition. Recent trials have shown that prasugrel and ticagrelor reduce the number of clinical adverse events as compared to clopidogrel in NSTE-ACS patients, particularly in those with DM27-29.
Study limitations
Our study has several limitations. We acknowledge that, in the current analysis, 283 patients with diabetes were further subdivided into four strata according to SYNTAX score and HPR, limiting the power of the analysis. Moreover, included patients were all treated with clopidogrel, whereas in the ACS setting current guidelines recommend using prasugrel and ticagrelor30. However, clopidogrel is still largely used in this setting, as shown by recent registries in ACS patients31,32.
In this observational study diabetic patients are a high-risk subset of patients and there may be confounders associated with both HPR and outcome. We used a Cox regression analysis to adjust the risk between DM and outcome. In this regard, age and renal failure were forced and kept in the model. Smoking status was not significantly associated with outcome (HR 1.82, 95% CI: 0.79-4.22), and we decided to leave it out of the model. Likewise, we did not adjust for the polymorphism CYP2C19*2 which was not significantly related to outcome in the univariate analysis and had several missing observations.
In our DM patients we did not assess the glycaemic control by measuring HbA1c and we did not report separate analyses for insulin-dependent patients. However, further subdivision of our population would have resulted in lack of statistical power.
Conclusions
Among NSTE-ACS patients treated with PCI, the combination of DM, an SS ≥15 and HPR characterised the cohort with the highest MACE rates from one month to one year. In such patients, careful clinical monitoring, and implementation of secondary prevention measures, including the use of potent P2Y12 inhibitors, are strongly advised.
| Impact on daily practice Diabetes mellitus is associated with poor outcome in patients with coronary artery disease. High platelet reactivity is frequently found in diabetic patients, leading to increased major adverse clinical events. The study shows that, in non-ST-elevation acute coronary syndrome patients undergoing PCI and treated with clopidogrel, diabetes and SYNTAX score >15 are associated with higher event rates between 30 days and one year (8.9%), which was increased twofold by high platelet reactivity (16.4%). On the contrary, diabetic patients with a SYNTAX score <15, with or without high platelet reactivity, showed an event rate consistently below 3%. Our study may help clinicians to use novel, more powerful P2Y12 receptor blockers in diabetic patients with higher SYNTAX scores, regardless of platelet reactivity. |
Conflict of interest statement
The authors have no conflicts of interest to declare.

