
Abstract
Aims: A guided de-escalation of P2Y12 inhibitor treatment is considered an alternative treatment strategy in ACS patients undergoing PCI. However, the safety and efficacy of this strategy may differ in diabetic vs non-diabetic patients. The aim of this study was to compare the outcomes of platelet function testing (PFT)-guided de-escalation of dual antiplatelet therapy (DAPT) in ACS patients with and without diabetes mellitus.
Methods and results: The TROPICAL-ACS trial randomised 2,610 biomarker-positive ACS patients 1:1 to either standard treatment with prasugrel for 12 months (control group) or PFT-guided DAPT de-escalation. The association and interaction of diabetes on clinical endpoints across treatment groups and on platelet reactivity was investigated. In diabetic patients (n=527, 20.2%), the overall event rates were high and the one-year incidence of the primary endpoint (cardiovascular death, myocardial infarction, stroke or bleeding ≥grade 2) did not differ between guided de-escalation and control group patients (12.5% vs 10.8%; HR 1.17, 95% CI: 0.71–1.93, p=0.55). In non-diabetic patients (n=2,083, 79.8%), the one-year incidence of the primary endpoint was lower in the guided de-escalation vs control group (6.1% vs 8.5%; HR 0.71, 95% CI: 0.52–0.99, p=0.04, pint=0.10). Diabetic patients showed higher platelet reactivity levels in both control (=on prasugrel, p=0.01) and guided de-escalation group (=on clopidogrel, p=0.005) patients.
Conclusions: Although diabetic status did not significantly interfere with the treatment effects of guided DAPT de-escalation, our results suggest that this approach might be safe and effective in non-diabetic patients. Further investigation is definitely warranted in diabetic patients.
Introduction
Current guidelines1 recommend potent P2Y12 receptor inhibition with ticagrelor or prasugrel for up to one year in invasively managed acute coronary syndrome (ACS) patients. Landmark analyses elaborated that the greatest ischaemic benefits of potent platelet inhibition are seen early, whereas the risk for bleeding complications is a major issue during maintenance treatment2,3. Such time-dependent risk patterns as well as clinical and socio-economic circumstances4 have strengthened interest in dual antiplatelet therapy (DAPT) de-escalation strategies5, with a preference for treatment with potent P2Y12 inhibitors only during the first weeks after percutaneous coronary intervention (PCI). Indeed, switching antiplatelet agents including a strategy of DAPT de-escalation is commonly practised4,6,7; however, smaller studies6,8 have provided conflicting results on outcomes of ACS patients following a uniform and non-guided approach of DAPT de-escalation. The clinical relevance and importance of switching P2Y12 inhibitors, including a strategy of DAPT de-escalation, was highlighted recently in an International Expert Consensus document9.
In the randomised TROPICAL-ACS trial10, we identified and established a platelet function testing (PFT)-guided DAPT de-escalation with an early switch from prasugrel to clopidogrel as an effective, safe and alternative treatment strategy in invasively managed ACS patients. Subsequently, the 2018 ESC/EACTS Guidelines on myocardial revascularisation have included a new recommendation on guided DAPT de-escalation as a treatment concept that may be considered as an alternative DAPT strategy for ACS patients1. The concept of DAPT de-escalation was also incorporated in an update on the product label of clopidogrel11. With respect to DAPT strategies in diabetic patients, several studies investigating the effect of DAPT prolongation found no significant heterogeneity between patients with and those without diabetes concerning the reduction of ischaemic risk, while extended DAPT increased the risk of bleeding in both populations12. In line with these studies, current guideline recommendations do not consider diabetes status per se as a driver for prolonged DAPT. Despite a lack of heterogeneity for DAPT duration, the presence of diabetes mellitus, which is associated with a prothrombotic milieu13, a high ischaemic risk14 and increased levels of platelet reactivity15, may impact on treatment effects of DAPT de-escalation (DAPT potency). In line with this, prasugrel was found to be of particular benefit in patients with diabetes mellitus16. Thus, dedicated analyses are mandatory. In this pre-specified substudy from the TROPICAL-ACS trial, we aimed to assess whether patient outcomes following guided de-escalation of antiplatelet treatment may differ in the setting of diabetes mellitus.
Methods
STUDY DESIGN AND PATIENTS
TROPICAL-ACS was an investigator-initiated and randomised multicentre trial in ACS patients undergoing PCI. The trial was conducted at 33 sites in Europe (ClinicalTrials.gov Identifier: NCT01959451) and more details on the study design have been published previously10. In brief, this trial enrolled biomarker-positive ACS patients (aged ≥18 and ≤80 years) after PCI. For this specific substudy, which was pre-specified in the study protocol, the study cohort was stratified into diabetic and non-diabetic patients. The cohort of diabetic patients included patients on insulin, oral antidiabetics and/or on dietary diabetes management. Details on randomisation and study procedures, study endpoints and statistical analysis are provided in Supplementary Appendix 1-Supplementary Appendix 3,10,14,17,18.
Results
STUDY POPULATION
TROPICAL-ACS enrolled 2,610 ACS patients after PCI. Of these, 1,306 patients were randomised to the control group and 1,304 to the guided de-escalation group. Overall, 527 patients (20.2%) were diabetic patients and 2,083 (79.8%) were non-diabetic patients. Diabetic patients were subdivided into 368 patients (69.8%) with non-insulin-dependent diabetes (NIDDM), among whom 67 patients were treated by dietary management only, and a subgroup of insulin-dependent diabetes mellitus (IDDM) patients consisting of 159 subjects (30.2%). Figure 1 illustrates the study flow and the subgroups investigated in this analysis. Table 1 lists the baseline characteristics of all study patients stratified by diabetes status and study group. Overall, angiographic and procedural characteristics were equally distributed across the stratified study groups, except for more PCIs performed on coronary artery bypass grafts in the non-diabetic control group (Supplementary Table 1). The overall adherence rate to the per protocol mandated treatment was 92.7% in the diabetes group (control 93.8% vs guided de-escalation 91.4%) and 94.7% in the non-diabetes group (control 94.3% vs guided de-escalation 95.1%).
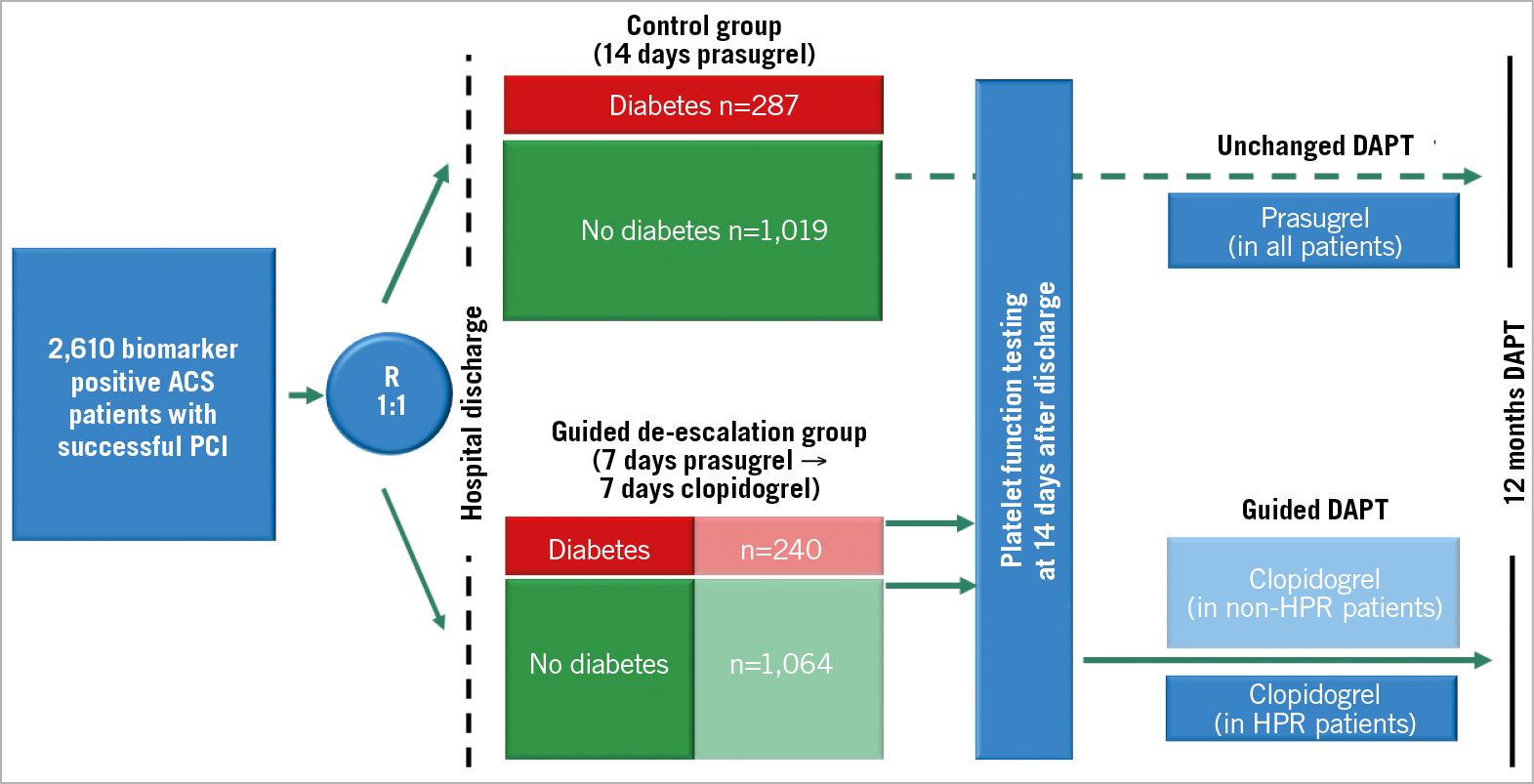
Figure 1. Study design and groups. ACS: acute coronary syndrome; DAPT: dual antiplatelet therapy; HPR: high platelet reactivity; PCI: percutaneous coronary intervention
PLATELET FUNCTION AND DIABETES
Platelet function data were available for 2,527 patients (97%). In prasugrel-treated control group patients with platelet function data (n=1,261), adenosine diphosphate (ADP)-induced platelet aggregation (median [IQR]) was significantly higher in diabetic vs non-diabetic patients (29.0 U [20.0-40.0] vs 25.3 U [17.0-37.0], p=0.01). This difference for diabetic vs non-diabetic patients was even more pronounced in clopidogrel-treated (n=1,266) guided de-escalation group patients (44.0 U [31.0-65.0] vs 38.0 U [25.0-59.0], p=0.005). Figure 2 illustrates platelet function data in diabetic vs non-diabetic patients and for the study groups. Figure 3 shows the HPR rates, which were significantly higher in diabetic patients in the guided de-escalation group and numerically higher in the control group.
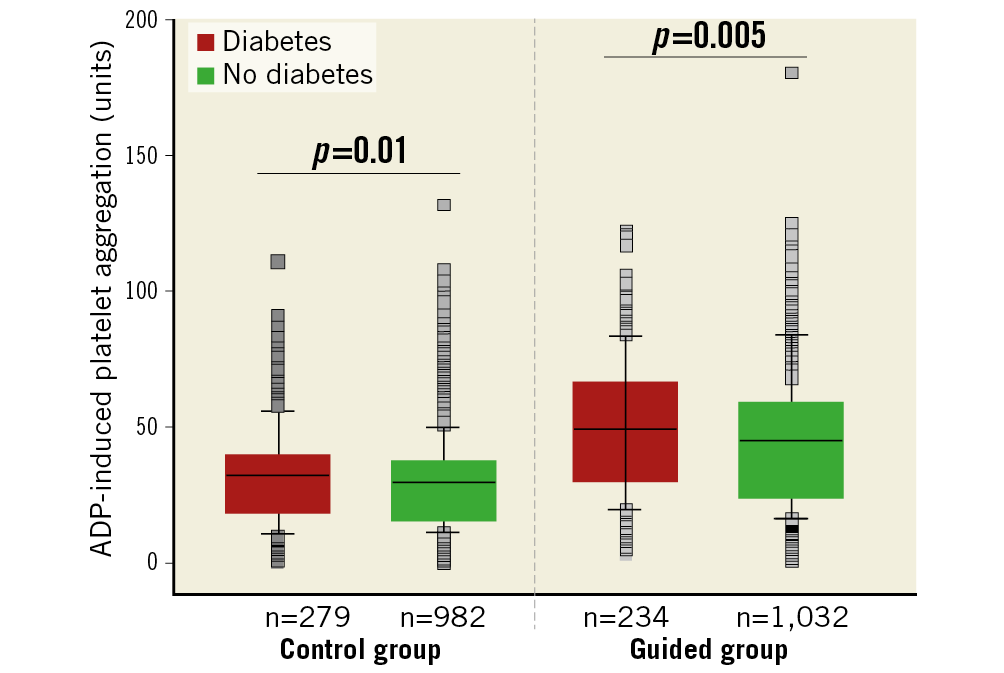
Figure 2. Box plots for ADP-induced platelet aggregation values according to the pre-specified study and diabetes subgroups. The black line denotes the median, boxes denote the upper and lower quartile of values and whiskers denote the 10th and 90th percentile. Outliers are shown as grey boxes.
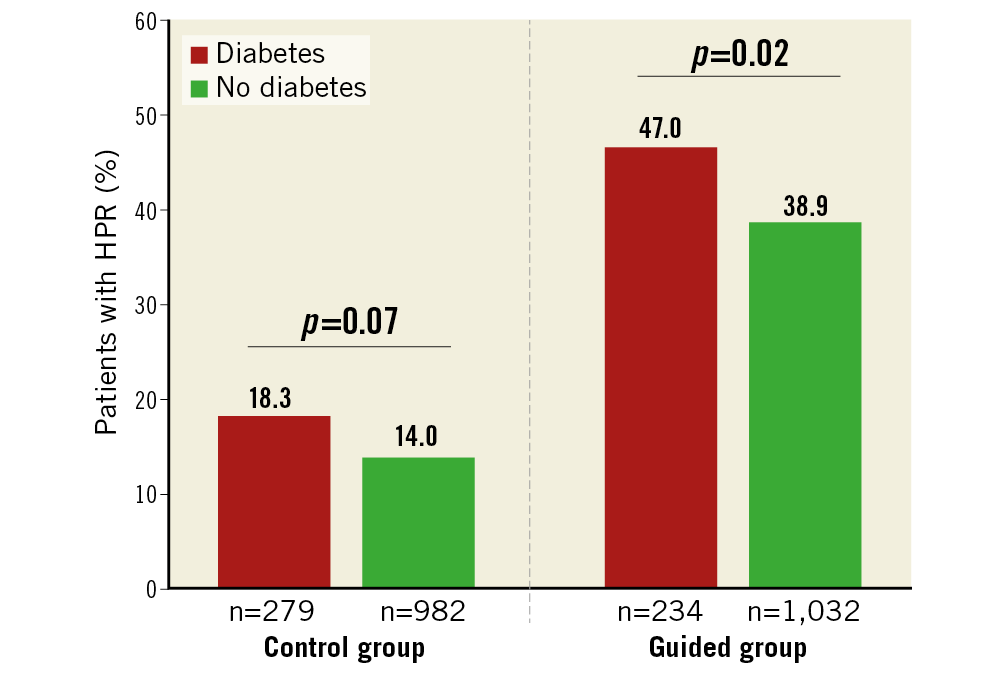
Figure 3. Bar charts for the percentage of patients with HPR according to the pre-specified study and diabetes subgroups. The boxes denote the rate of HPR in the various subgroups. HPR: high platelet reactivity
CLINICAL OUTCOMES AND DIABETES
In the 527 diabetic patients, the overall event rates were high, and the one-year incidence of the primary endpoint did not differ between guided de-escalation and control group patients (12.5% vs 10.8%; HR 1.17, 95% CI: 0.71–1.93, p=0.55). The incidence of BARC ≥2 bleedings in diabetic patients was 6.3% in de-escalation vs 5.6% in the control group (HR 1.14, 95% CI: 0.56–2.30, p=0.75). All-cause mortality at one year was 2.9% (7 events) in the guided de-escalation group vs 1.4% (4 events) in the control group (p=0.23). Table 2 shows the entire clinical outcome data for diabetic patients and Figure 4 illustrates time-to-event analysis for the primary endpoint, the combined ischaemic endpoint and bleeding events. Supplementary Table 2 provides additional information on outcomes for IDDM and NIDDM patients. Both ischaemic and bleeding event rates were high in IDDM patients. While no statistically significant differences were found for diabetes types across study groups, we have to acknowledge that combined ischaemic event rates were nearly twice as high in IDDM patients in the guided de-escalation vs control group (13.7% vs 7.0%, p=0.17).
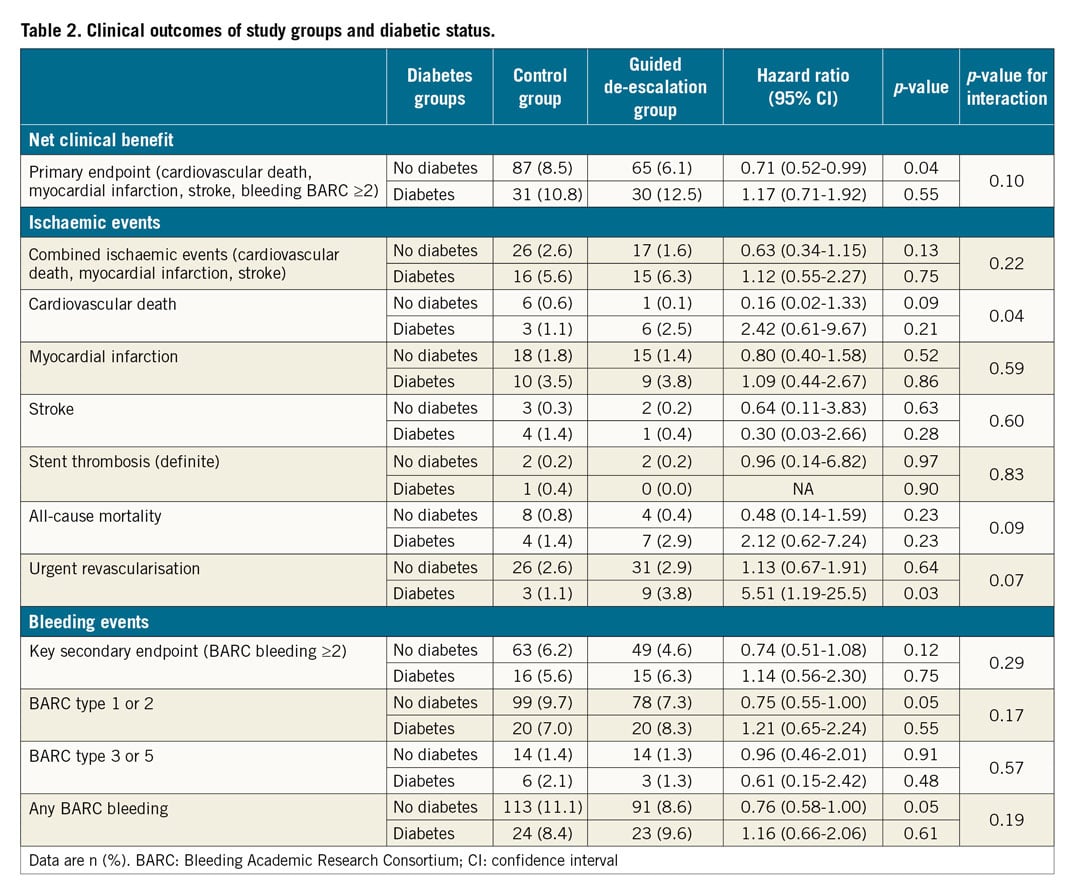
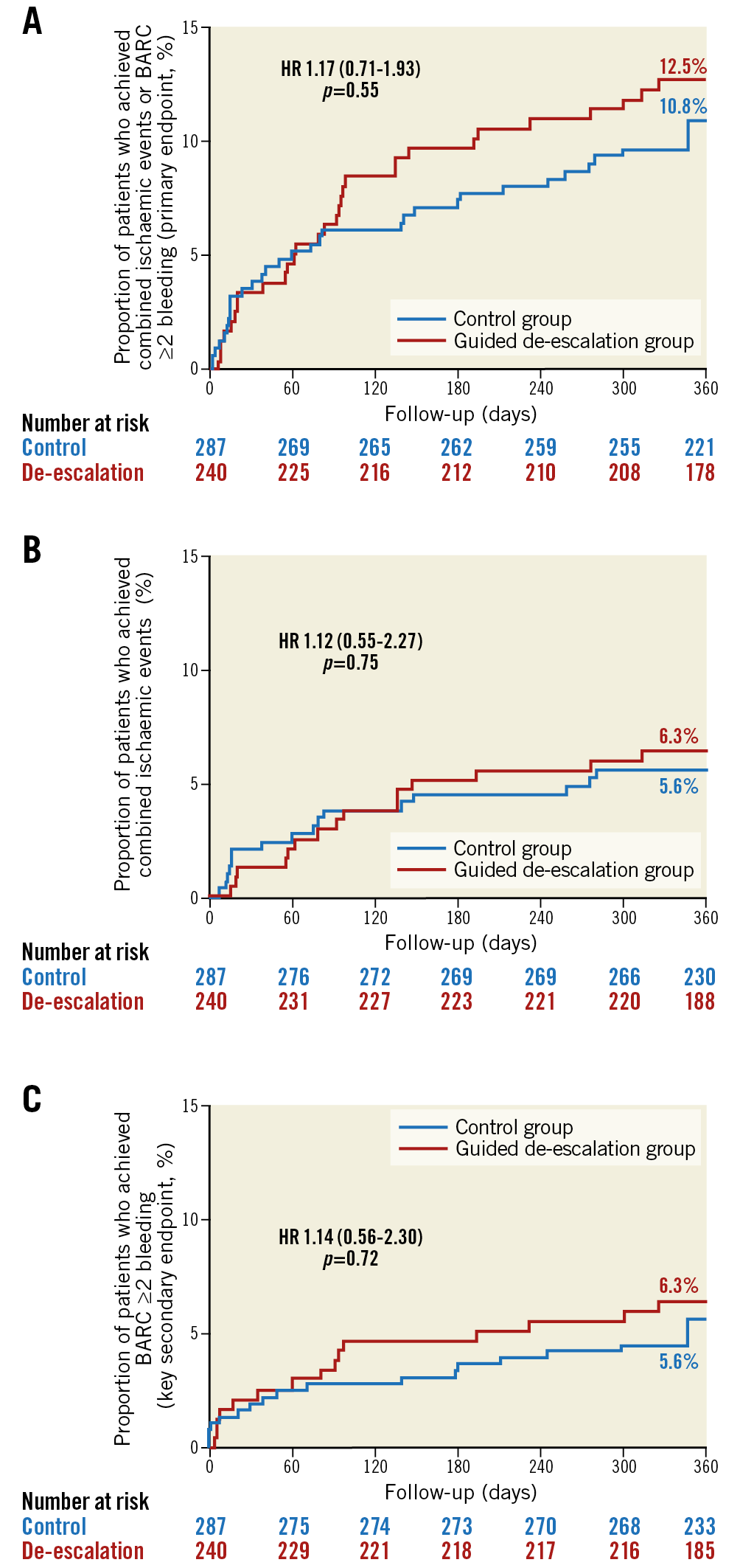
Figure 4. Kaplan-Meier curves for key endpoints in diabetic patients. Primary endpoint (A), combined ischaemic events (B) and the key secondary endpoint (C) in diabetic patients at 12-month follow-up.
In the 2,083 non-diabetic patients, the one-year incidence of the primary endpoint was lower in the guided de-escalation vs control group (6.1% vs 8.5%; HR 0.71, 95% CI: 0.52-0.99, p=0.04, p-value for interaction of diabetes on treatment effects=0.10). The incidence of BARC ≥2 bleedings in non-diabetic patients was 4.6% in de-escalation vs 6.2% in the control group (HR 0.74, 95% CI: 0.51-1.08, p=0.12). All-cause mortality at one year was 0.4% (4 events) in the guided de-escalation group vs 0.8% (8 events) in the control group (p=0.23). Table 2 shows the entire clinical outcome data for non-diabetic patients and Figure 5 illustrates time-to-event analysis for the primary endpoint, the combined ischaemic endpoint and bleeding events.
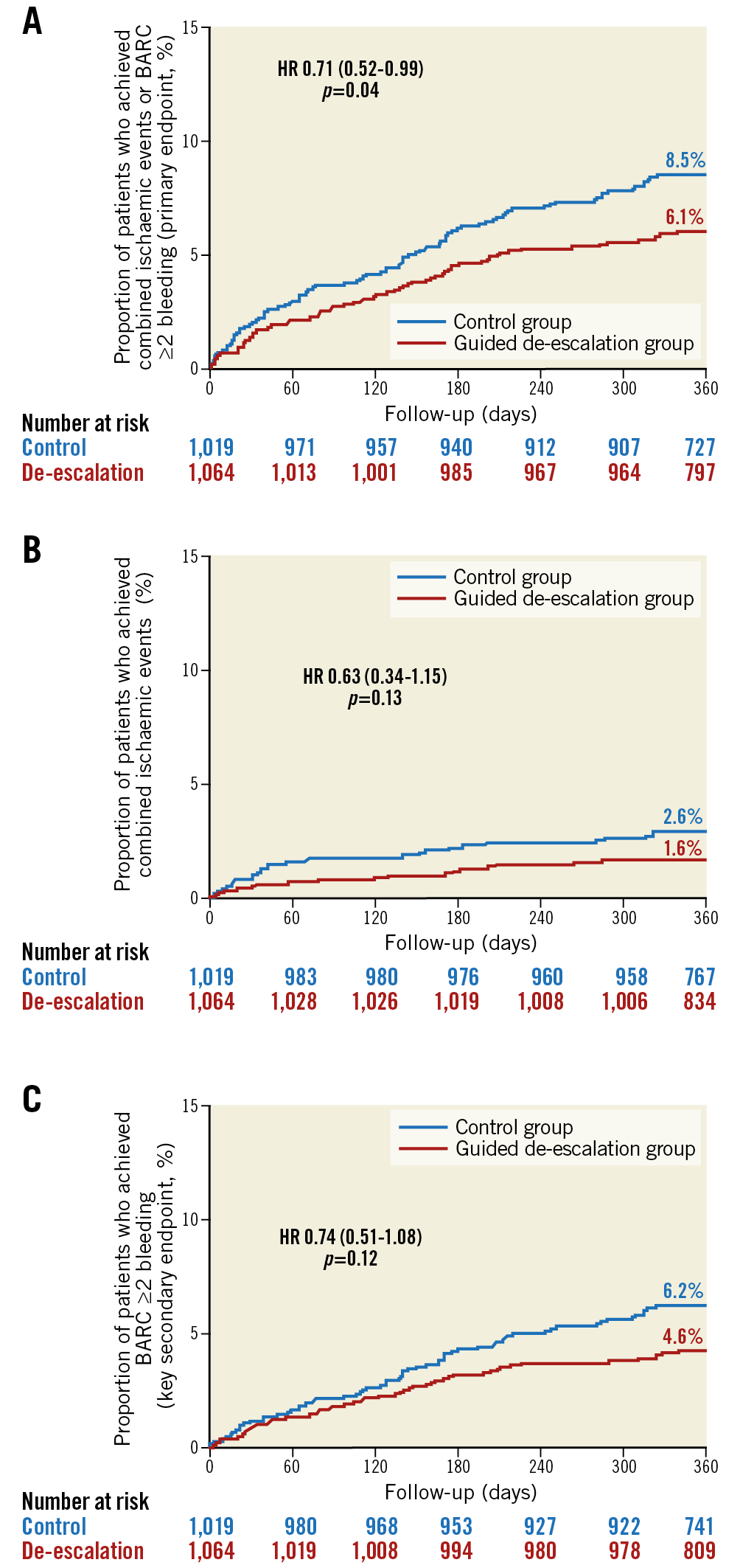
Figure 5. Kaplan-Meier curves for key endpoints in non-diabetic patients. Primary endpoint (A), combined ischaemic events (B) and the key secondary endpoint (C) in non-diabetic patients at 12-month follow-up.
Discussion
To the best of our knowledge, the present pre-specified analysis from the randomised TROPICAL-ACS trial is the first investigation on platelet function and clinical outcome data in diabetic vs non-diabetic patients who are scheduled for DAPT de-escalation treatment. Key findings from our study include that diabetic patients showed comparable outcomes including their overall ischaemic risk for a guided DAPT de-escalation vs a uniform prasugrel treatment approach. In the larger cohort of non-diabetic patients, we observed a net clinical benefit for a guided DAPT de-escalation approach, which was mainly driven by a reduction in bleeding events. Our analysis is the first comparative PFT assessment in prasugrel- vs clopidogrel-treated patients from a randomised trial in invasively managed diabetic patients with ACS. A key result in that respect is that diabetic patients showed higher platelet reactivity levels in both control (=on prasugrel) and guided de-escalation group (=on clopidogrel) patients.
There is recognition that diabetic patients with an ACS are at higher risk for subsequent ischaemic events16,19. Underlying mechanisms include a higher frequency of other cardiac risk factors20, a greater burden of atherosclerotic disease, systemic inflammation21 and, most importantly, a heightened baseline22 and on-treatment platelet reactivity23. The results of our study in diabetic patients confirm and extend these important observations by showing a significantly higher risk for ischaemic events in diabetic vs non-diabetic patients. In particular, the subset of IDDM patients exhibited the highest ischaemic risk and –although not statistically significant– ischaemic event rates were nearly twice as high for guided de-escalation vs control group patients. This circumstance alone mandates further studies in IDDM patients. Furthermore, a significant interaction was found for treatment effects with diabetes status and cardiovascular death. It must be emphasised, however, that event rates are very low for this individual endpoint and the findings may be a play of chance since the study was not powered sufficiently to detect differences for individual endpoints. Of note, on-treatment platelet reactivity levels in diabetic patients were markedly increased both on clopidogrel and also on prasugrel treatment. To the best of our knowledge, this is the largest data set from a randomised controlled trial of invasively managed ACS patients which shows that even the potent P2Y12 inhibitor prasugrel is not yet strong enough to achieve similar levels of on-treatment platelet reactivity in diabetic vs non-diabetic patients. Further dedicated trials in diabetic patients who exhibit HPR on prasugrel are warranted. These studies may include potent i.v. antiplatelet agents such as cangrelor24 or GP IIb/IIIa receptor inhibitors to reduce the burden of HPR in ACS patients receiving potent P2Y12 inhibitors further. While we have to acknowledge that our subset of diabetic patients is comparatively small and comprises only about one fifth of the entire TROPICAL-ACS population, results suggest that a DAPT de-escalation strategy in diabetic patients is not linked to a significant increase in adverse events presupposing that platelet function testing confirmed responsiveness to clopidogrel. Indeed, the present results are gaining further importance, considering that the recent guidelines1 have included a new recommendation on guided DAPT de-escalation as a treatment concept that may be considered as an alternative DAPT strategy for ACS patients. Further, a mostly unguided DAPT de-escalation is commonly practised4,9 and certainly includes a relevant number of diabetic patients in a real-life setting. It is obvious that especially the diabetic patients – if deemed necessary – might be more safely de-escalated to clopidogrel when DAPT de-escalation is guided by PFT. In contrast, de-escalation from potent P2Y12 inhibitors to clopidogrel without PFT guidance, especially in diabetics and early after ACS, might expose patients to a substantially increased ischaemic risk. However, to corroborate our results, especially in the subset of patients with diabetes, further studies with and without guidance of treatment are urgently needed.
The group of non-diabetic ACS patients constituted the majority (approx. 80%) of enrolled subjects. In this large subset that comprises >2,000 patients from the trial, DAPT could be safely de-escalated as combined ischaemic events were similar and even numerically lower when compared with uniform prasugrel treatment. Indeed, we even observed a net clinical benefit of a guided DAPT de-escalation strategy in these patients. This benefit for the primary combined endpoint of our study was mainly driven by a relative risk reduction of >25% for bleeding events with BARC grade ≥2. In particular, minor and minimal bleeding events were reduced in non-diabetic patients, whereas major (BARC 3 or 5) bleeding complications were comparable between the two study groups. However, even minor bleeding events are associated with poor treatment compliance and increased healthcare costs related to hospital readmissions or consultation of a primary care physician25. Thus, DAPT strategies that come along with a risk reduction for minor or minimal bleeding events should have a positive impact on patients’ compliance, outcomes, and treatment costs. In general, non-diabetic vs diabetic patients were found to be younger and included a higher proportion of STEMI patients. Indeed, both a younger age26 and presence of STEMI vs NSTEMI10 were explored in prior analyses as variables that favoured the treatment effects of a guided DAPT de-escalation. Results of our substudy here are in line with these observations, as the treatment effects of guided DAPT de-escalation were found to be more favourable in non-diabetic vs diabetic patients. Of note, reflecting the smaller sample size of certain subgroups (here: diabetic patients), such associations and interactions within a certain subset of patients would require investigations in larger cohorts of ACS patients. With respect to the interaction of diabetes mellitus on treatment effects across the study groups in our trial, we have to acknowledge that formal interaction testing remained non-significant with a borderline p-value for interaction of 0.10 for the primary endpoint. Again, this emphasises that further studies are needed that should include various approaches of unguided and guided DAPT de-escalation strategies.
Limitations
The TROPICAL-ACS trial was a non-inferiority and not a superiority trial and the pre-specified subgroup of diabetic patients in this trial was relatively small. Thus, results obtained in subgroups must be considered as hypothesis-generating. The small subgroup of diabetic patients leads to broader confidence intervals for the studied combined and individual endpoints. Inclusion of BARC bleeding type 2 softens the primary safety endpoint. Nevertheless, even minor bleeds may have an important impact on treatment compliance and outcomes25. Furthermore, our study protocol mandated choosing prasugrel; it remains unclear to what extent our findings can be extrapolated to ticagrelor. Finally, IDDM patients must be considered a subset of patients that carries a very high ischaemic risk and heightened platelet reactivity. In TROPICAL-ACS, they comprised only a small group of patients; further studies in this cohort are certainly warranted.
Conclusions
Although diabetic status did not significantly interfere with the treatment effects of guided DAPT de-escalation, our results suggest that this approach might be safe and effective in non-diabetic patients. Further investigation is definitely warranted in diabetic patients.
|
Impact on daily practice For various reasons (intended major surgery, socioeconomic factors, side effects) and for certain patient subsets (elderly, patients with high bleeding risk), DAPT including a potent P2Y12 inhibitor is not feasible or safe. In these scenarios, guided de-escalation may be considered since previous data indicated its safety and efficiency. The results of the current analysis further suggest that especially non-diabetic patients may obtain a net clinical benefit from a PFT-guided DAPT de-escalation. Further research is warranted in diabetic patients. |
Funding
TROPICAL-ACS is an independent, investigator-initiated trial with an academic sponsor (Klinikum der Universität München). The trial was financially supported by a research grant from Roche Diagnostics (Rotkreuz, Switzerland). Prasugrel purchase, drug delivery and related logistics were kindly supported by Eli Lilly and Company and Daiichi Sankyo Company. Funders of this study had no role in the study design, collection of data and data analysis, or writing of the manuscript.
Appendix. Study collaborators
Tobias Geisler, MD, University Hospital of Tübingen, Department of Cardiology, Tübingen, Germany; Kurt Huber, MD, Wilhelminen Hospital Vienna, Vienna, Austria; Ferenc T. Nagy, MD, University of Szeged, First Department of Internal Medicine, Szeged, Hungary; Csaba A. Dézsi, MD, Petz Aladár County Teaching Hospital, Department of Cardiology, Győr, Hungary; Lukasz Koltowski, MD, Medical University of Warsaw, 1st Department of Cardiology, Warsaw, Poland; Harald Mudra, MD, Department of Cardiology, Klinikum Neuperlach, Munich, Germany; Anja Löw, MD, and Sabine Deuschl, MD, Ludwig-Maximilians University, Department of Cardiology, Munich, Germany; DZHK (German Centre for Cardiovascular Research), Munich Heart Alliance, Munich, Germany.
Conflict of interest statement
D. Sibbing reports grants from Roche Diagnostics and Daiichi Sankyo, and personal fees from Bayer AG, Daiichi Sankyo, Eli Lilly, Roche Diagnostics, MSD, Pfizer, AstraZeneca and Sanofi Aventis. Z. Huczek has received lecturing honoraria and proctoring fees from Medtronic and Abbott outside the submitted work. L. Koltowski has received institutional research grants from Reva Medical Inc., and personal fees from Abbott and Boston Scientific, outside the submitted work. D. Aradi reports receiving personal fees from Roche Diagnostics, DSI/Lilly, AstraZeneca, Pfizer, Bayer AG, and MSD Pharma. D. Trenk reports receiving personal fees from AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, Eli Lilly, other from Otsuka, and Sanofi, outside the submitted work. T. Geisler reports receiving personal fees from AstraZeneca, Pfizer, MSD, and Boehringer Ingelheim, grants and personal fees from Bayer Healthcare, Daiichi Sankyo, Eli Lilly, The Medicines Company, and grants from Siemens Healthcare, and Spartan Bioscience, outside the submitted work. B. Merkely reports receiving personal fees from MSD Pharma, Bayer AG, Boehringer Ingelheim, Pfizer, and AstraZeneca, outside the submitted work. The other authors have no conflicts of interest to declare.

