
In patients with an acute coronary syndrome (ACS) undergoing percutaneous coronary intervention (PCI), one-year dual antiplatelet therapy (DAPT) with low-dose aspirin plus a platelet P2Y12 receptor antagonist is the mainstay strategy for the prevention of recurrent atherothrombotic complications1. Among the P2Y12 inhibitors, prasugrel and ticagrelor are preferred over clopidogrel1. However, the observation that the greatest anti-ischaemic benefits of more potent P2Y12 inhibitors are seen early after an acute coronary event, while bleeding complications continue to accrue over time during long-term treatment with these drugs, has set the rationale for studies of switching (i.e., de-escalating) the intensity of P2Y12 inhibition after the early high ischaemic risk phase with the objective of reducing bleeding without compromising efficacy2. Results of the TROPICAL-ACS (Testing Responsiveness to Platelet Inhibition on Chronic Antiplatelet Treatment For Acute Coronary Syndromes) trial3 have led to updates of current guidelines and an expert consensus document, which indicate that de-escalation of P2Y12 inhibition guided by platelet function testing (PFT) may be considered as an alternative DAPT strategy, especially for ACS patients deemed unsuitable for 12 months of potent platelet inhibition4,5. Such restricted indication for de-escalation (class IIb level of evidence B) is due to the lack of powered evidence on how this strategy could affect ischaemic events, raising concerns for some of the subgroups at high thrombotic risk, such as those with diabetes mellitus (DM).
In this issue of EuroIntervention, Hein et al6 report the results of a pre-specified sub-analysis of the TROPICAL-ACS trial evaluating clinical outcomes associated with a PFT-guided P2Y12 inhibitor de-escalation strategy in ACS patients stratified according to DM status.
In brief, the TROPICAL-ACS trial randomised a total of 2,610 biomarker-positive ACS patients undergoing PCI to either a guided de-escalation regimen (prasugrel for one week after ACS/PCI followed by one-week clopidogrel and a subsequent PFT-guided maintenance therapy with clopidogrel or prasugrel from day 14 after hospital discharge) or standard of care with 12 months of prasugrel treatment4. One year after PCI, PFT-guided de-escalation was shown to be non-inferior to standard treatment on the primary net clinical endpoint (composite of cardiovascular death, myocardial infarction [MI], stroke or Bleeding Academic Research Consortium [BARC] ≥2). Although there was no significant reduction in bleeding between the groups (probably due to the fact that many patients in the PFT-guided group needed to switch back to prasugrel), there was no apparent increase in ischaemic events with PFT-guided P2Y12 inhibitor de-escalation. In the current TROPICAL-ACS sub-analysis6, outcomes were assessed among non-DM (n=2,083, 79.8%) and DM (n=527, 20.2%) patients; in the DM cohort, 159 (30.2%) had insulin-dependent diabetes mellitus (IDDM). The one-year rate of the primary net clinical endpoint was significantly lower in the PFT-guided de-escalation compared with the standard of care group in the non-DM cohort (6.1% vs 8.5%, p=0.04), while this was numerically higher, although not significantly different, in the PFT-guided de-escalation group among DM patients (12.5% vs 10.8%, p=0.55). The interaction p-value for the primary endpoint was 0.10, which, despite not reaching statistical significance and thus indicating consistency of the study findings with the overall TROPICAL-ACS study results, warrants some attention in light of the directional trends of the observations.
The reduction of the primary endpoint associated with the PFT-guided de-escalation strategy within the non-DM population was driven by lower BARC ≥2 events (albeit the difference was not statistically significant). On the other hand, rates of bleeding were quite comparable between the two treatment arms among DM patients. There were no differences in the composite and individual ischaemic endpoints between the two treatment arms among non-DM patients. However, cardiovascular mortality and urgent revascularisation tended to be higher with PFT-guided de-escalation compared with standard-of-care treatment among DM patients, with interaction p-values for these endpoints (0.04 and 0.07, respectively) at the limits of statistical significance. Moreover, numerically higher one-year rates of the composite ischaemic endpoint of cardiovascular death, MI or stroke were observed in the PFT-guided de-escalation versus standard of care among IDDM patients (13.7% vs 7%, p=0.17).
The authors should be commended for reporting this analysis, which represents the first investigation assessing the effects of DM status on clinical outcomes associated with an early PFT-guided de-escalation strategy after an ACS/PCI. Consistent with the overall TROPICAL-ACS trial, the reported data support that PFT-guided selection of P2Y12 inhibiting therapy represents a safe alternative approach to a standard strategy of 12-month prasugrel use among non-DM patients. However, although formal statistics showed that DM status did not interfere with the treatment effects of the de-escalation strategy, this strategy did not appear to be very reassuring among DM patients. Indeed, while it should be acknowledged that findings within the DM cohort, especially within the IDDM group, could be attributed to the play of chance due to the small sample size and low number of events, the numerically higher event rates observed with the de-escalation strategy in this high-risk population deserve caution and further investigation. Therefore, the reported sub-analysis is of utmost relevance as it warns about the need to put de-escalation in DM patients with ACS/PCI under close scrutiny for several reasons, as elucidated below (Figure 1).
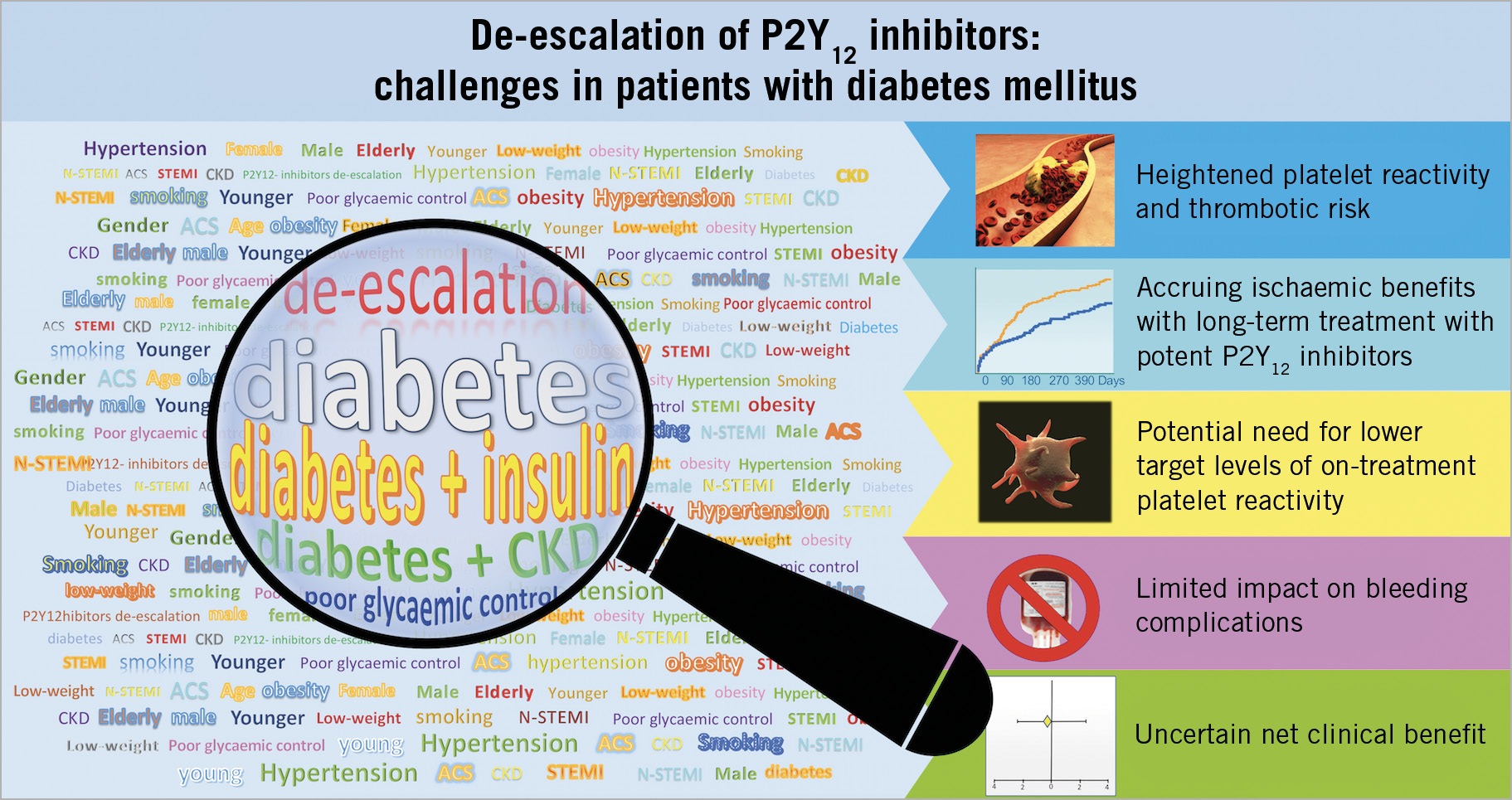
Figure 1. Challenges to a P2Y12 inhibitor de-escalation strategy in patients with diabetes mellitus experiencing an acute coronary syndrome and undergoing percutaneous coronary intervention. CKD: chronic kidney disease
ACS patients with DM, especially those with IDDM, concurrent chronic kidney disease (CKD) or poor glycaemic control, have a heightened risk of cardiovascular events that can, at least in part, be explained by their higher platelet reactivity, as shown by the greater benefits derived from more potent antiplatelet regimens in these populations7,8,9. In particular, prasugrel compared with clopidogrel achieved the greatest reductions in ischaemic events in the DM cohort of ACS patients undergoing PCI7. Moreover, the net clinical benefit of prasugrel versus clopidogrel continued to grow over time in the DM cohort, while this late advantage of prasugrel was not apparent after 30 days from the index event among non-DM patients7. This pattern of consistent but greater absolute reductions in recurrent atherothrombotic events among DM patients was also observed for ticagrelor, which compared with clopidogrel achieved enhanced and sustained benefits among ACS patients with DM, in particular those with concomitant CKD, compared with those without these risk factors8,9. The greater magnitude of benefits associated with the use of more potent P2Y12 inhibitors in ACS patients with DM could be explained by the multifactorial higher baseline atherothrombotic risk but also by their high rate of clopidogrel non-responsiveness10,11. Moreover, among patients with DM, those treated with insulin or having CKD have even higher rates of clopidogrel non-responsiveness12,13. The substantial proportion of clopidogrel non-responders among DM patients can potentially impact negatively on outcomes associated with P2Y12 inhibitor de-escalation, particularly if early and not guided. However, the evidence on non-guided de-escalation is very limited, as the only study comparing unguided P2Y12 inhibitor de-escalation at one month after ACS versus conventional 12-month treatment with prasugrel or ticagrelor included a very small number of DM patients (n=177, 27%), in whom no significant differences in the net clinical composite endpoint were observed between the two compared antiplatelet treatment regimens14. A PFT-guided de-escalation strategy of P2Y12 inhibitors as assessed in TROPICAL-ACS could help to prevent ischaemic events by minimising high on-treatment platelet reactivity status by switching back from clopidogrel to a more potent antiplatelet agent. However, the heightened thrombotic risk of DM patients, together with the fact that these patients also have multiple other risk factors for atherothrombotic complications, may suggest that these patients may require lower target levels of on-treatment platelet reactivity compared to non-DM patients.
Finally, PFT-guided de-escalation could have a smaller effect on bleeding among DM patients due to the fact that a high proportion of these patients have high on-clopidogrel platelet reactivity (47% in TROPICAL-ACS) and thus required escalation to prasugrel therapy, reducing the chances of observing a safety benefit. In addition, a potentially limited safety effect of all de-escalation approaches among DM patients can be attributed to their reduced rates of low platelet reactivity, which was strongly associated with bleeding in the TROPICAL-ACS trial15. Overall, these observations associated with the aforementioned higher thrombotic risk, which has been shown to be attenuated by more intense and sustained platelet inhibition, may lead to less convincing evidence to support de-escalation, even if guided, among DM patients.
Conflict of interest statement
D.J. Angiolillo declares that he has received consulting fees or honoraria from Amgen, Aralez, AstraZeneca, Bayer, Biosensors, Boehringer Ingelheim, Bristol-Myers Squibb, Chiesi, Daiichi Sankyo, Eli Lilly, Haemonetics, Janssen, Merck, PLx Pharma, Pfizer, Sanofi, and The Medicines Company, and has received payments for participation in review activities from CeloNova and St. Jude Medical. He declares that his institution has received research grants from Amgen, AstraZeneca, Bayer, Biosensors, CeloNova, CSL Behring, Daiichi Sankyo, Eisai, Eli Lilly, Gilead, Idorsia, Janssen, Matsutani Chemical Industry Co., Merck, Novartis, Osprey Medical, and Renal Guard Solutions. The other author has no conflicts of interest to declare.

