Abstract
Background: The benefits of de-escalation of P2Y12 inhibition after percutaneous coronary intervention (PCI) may differ by high bleeding risk (HBR) status.
Aims: We investigated the efficacy and safety of de-escalation from ticagrelor to clopidogrel after PCI by HBR status.
Methods: This is a non-prespecified post hoc analysis of the TicAgrelor Versus CLOpidogrel in Stabilized Patients with Acute Myocardial Infarction (TALOS-AMI) trial. Net adverse clinical events (a composite of cardiovascular death, myocardial infarction, stroke, or Bleeding Academic Research Consortium [BARC] bleeding type 2, 3, or 5) at 1 year post-PCI were compared between the de-escalation (clopidogrel plus aspirin) and the active control (ticagrelor plus aspirin) groups by HBR status, as defined by the modification of the Academic Research Consortium (ARC) criteria.
Results: A total of 2,625 patients in the TALOS-AMI trial were analysed. Of these, 589 (22.4%) met the modified ARC-HBR criteria. The de-escalation group had lower primary endpoint rates than the control group in both HBR (hazard ratio [HR] 0.47, 95% confidence interval [CI]: 0.26-0.84) and non-HBR (HR 0.59, 95% CI: 0.41-0.84) patients. There were no differences in treatment effect for the primary endpoint regardless of HBR status (p for interaction=0.904). BARC bleeding type 3 or 5 was less common in the de-escalation than the control group among HBR patients only (HR 0.24, 95% CI: 0.07-0.84).
Conclusions: In stabilised acute myocardial infarction patients, unguided de-escalation from ticagrelor to clopidogrel was associated with a lower rate of net adverse clinical outcomes irrespective of HBR status. The effect of de-escalation of P2Y12 inhibition on reducing haemorrhagic events was greater in patients with HBR.
Introduction
Ticagrelor and prasugrel are potent P2Y12 inhibitors and are preferable to clopidogrel in patients with acute myocardial infarction (AMI) undergoing percutaneous coronary intervention (PCI), as revealed by pivotal randomised trials12 and subsequent guideline updates34. However, clopidogrel is still recommended when the bleeding risk outweighs the thrombotic risk associated with potent P2Y12 inhibitors35. As both thrombotic and haemorrhagic events are linked to poor clinical outcomes67, various strategies − such as shortening the mandatory duration of dual antiplatelet therapy (DAPT)8, switching from potent P2Y12 inhibitors to clopidogrel9, decreasing the doses of potent P2Y12 inhibitors10, and P2Y12 inhibitor monotherapy (thus dropping aspirin)11121314 − have sought to create trade-offs between thrombosis and haemorrhage.
As time elapses after PCI, both the thrombotic and bleeding risks diminish, and the bleeding risk becomes higher than the ischaemic risk after 1 month15. Thus, the recent TicAgrelor Versus CLOpidogrel in Stabilized Patients with Acute Myocardial Infarction (TALOS-AMI) trial evaluated uniform unguided de-escalation of P2Y12 inhibition (from ticagrelor to clopidogrel) at 1 month in AMI patients with no thrombotic or haemorrhagic events9. This reduced the net adverse clinical outcomes (principally bleeding events) compared to those patients on standard ticagrelor-based 12-month DAPT. We hypothesised that the benefit of de-escalation might be more profound in patients with HBR than those with non-HBR. Therefore, using TALOS-AMI trial data, we explored the efficacy and safety of de-escalation in patients with HBR and those with non-HBR.
Methods
STUDY DESIGN AND POPULATION
This study is a non-prespecified post hoc analysis of the TALOS-AMI trial, which was an open-label, assessor-masked, multicentre, non-inferiority, randomised trial conducted at 32 centres in South Korea between February 2014 and December 2018916. The trial explored whether de-escalation from ticagrelor to clopidogrel 1 month after PCI using drug-eluting stents (DES) in stabilised AMI patients was non-inferior in terms of net ischaemic and bleeding outcomes from 1 to 12 months compared to the active control strategy (maintenance of ticagrelor for 12 months). All patients received aspirin and ticagrelor during the screening period (1 month after PCI), and those without any adverse clinical events were randomised in the outpatient department. The protocol was approved by the institutional review board or ethics committee of each participating centre, and all procedures adhered to the principles of the Declaration of Helsinki. All patients provided written informed consent.
STUDY DEFINITIONS AND ENDPOINTS
The current study investigates whether the benefits of the TALOS-AMI trial were maintained by patients both at HBR and non-HBR. HBR was defined as a modification of the Academic Research Consortium for High Bleeding Risk (ABC-HBR) criteria5, because in the TALOS-AMI trial, i) many HBR patients were excluded at baseline and during the first month after index PCI916 and ii) of 204 patients who were not screened in the first month, bleeding events occurred in 10 patients (Supplementary Table 1). This was not unexpected given the original data (on 43 patients) in terms of the incidence of BARC bleeding type 3 or 59. Also, hidden bleeding events could have occurred in patients who were not followed up during screening, given the high risk of major bleeding events associated with ticagrelor compared to clopidogrel in Korean patients17. Therefore, we modified the ARC-HBR criteria to include as many HBR patients as possible. Supplementary Table 2 lists our major and minor HBR criteria and the differences from the ARC-HBR criteria. Nine major and two minor ARC-HBR criteria that served as exclusion criteria for the TALOS-AMI trial were, thus, not used to define HBR in the present work. Rather, two minor ARC-HBR criteria − age ≥75 years and moderate chronic kidney disease (CKD) − were transferred to the major HBR criteria in the current study, because these were associated with significant bleeding (BARC bleeding type 3 or 5 rates >4%) in several validation studies employing the ARC-HBR criteria1819. Moreover, a combination of age ≥75 years and moderate CKD was associated with a higher incidence of BARC bleeding type 3 or 5 than all other ARC-HBR components combined18. Consequently, we defined HBR when 1 major or 2 minor modified HBR criteria were fulfilled (Supplementary Table 2).
A detailed study protocol (including definitions and endpoints) has been previously published916. Briefly, the primary endpoint was a net adverse clinical event − a composite of cardiovascular death, myocardial infarction, stroke, or BARC bleeding type 2, 3, or 5 − from 1 to 12 months after the index PCI. The key secondary endpoint was BARC bleeding type 3 or 5. Other secondary endpoints included a major adverse cardiac and cerebrovascular event (MACCE; a composite of cardiovascular death, myocardial infarction, or stroke), BARC bleeding type 2, 3, or 5, a composite of MACCE and BARC bleeding type 3 or 5, all-cause death, cardiovascular death, myocardial infarction, stroke, ischaemia-driven revascularisation, and stent thrombosis from 1 to 12 months after the index PCI.
STATISTICAL ANALYSIS
Continuous variables are presented as means±standard deviations and were compared using the unpaired t-test. Categorical variables are expressed as counts with percentages and were compared using Pearson’s chi-square test or Fisher’s exact test. We constructed Kaplan-Meier curves and used the log-rank test to compare the groups in terms of the primary and secondary endpoints (MACCE, BARC bleeding type 3 or 5, and a composite of MACCE and BARC bleeding type 3 or 5). Cox’s proportional hazards models were used to calculate hazard ratios (HR) with 95% confidence intervals (CI). The proportional hazards assumption was evaluated using the log-minus-log plot and the Schoenfeld residual test; all Cox’s proportional hazards models of clinical endpoints satisfied the proportional hazards assumption. A formal interaction test was performed to assess the consistency of the de-escalation effects (compared to those of active control) in patients at HBR and non-HBR.
The primary and secondary endpoints of the 2 groups were compared by HBR and non-HBR status. Sensitivity analysis employed the PREdicting bleeding Complications In patients undergoing Stent Implantation and subsEquent Dual Anti Platelet Therapy (PRECISE-DAPT) scores and the original ARC-HBR criteria520. Of the variables contributing to the PRECISE-DAPT score, previous bleeding, which was excluded in the TALOS-AMI trial, was ignored. Therefore, only 4 variables were used when calculating this score (www.precisedaptscore.com). All analyses were performed on an intention-to-treat principle, all were 2-tailed, and p<0.05 indicated statistical significance. All analyses were performed with the aid of Stata/MP version 16.0 software (StataCorp).
Results
ASSESSMENT OF MODIFIED HBR CRITERIA
Figure 1 shows the study flow. Between February 2014 and December 2018, 2,697 patients were enrolled in the TALOS-AMI trial, of whom 2,625 were analysed in the present study (using the modified HBR criteria) after excluding 72 patients for whom data on at least one of the modified criteria were missing (Supplementary Table 2). Using the modified criteria, 589 patients (22.4%) were at HBR and the other 2,036 (77.6%) were at non-HBR. The prevalence of the modified HBR criteria in the HBR group are shown in Supplementary Figure 1. Age ≥75 years (53.0%) and moderate CKD (48.9%) were the most common major criteria. Of the minor criteria, the prevalence of mild anaemia was 39.6%, but the rate of ischaemic stroke was only 7.0%. The incidence of BARC bleeding type 3 or 5 between 1 and 12 months was ≥4% (major and minor criteria). However, the incidence of major bleeding was <4% in isolation (without other concomitant criteria). Supplementary Figure 2 shows the clinical impacts of multiple HBR criteria. The proportions of multiple HBR criteria were 70.5% (1 criterion), 22.8% (2 criteria), 6.3% (3 criteria), and 0.5% (4 criteria; which included only 3 patients). Increased numbers of HBR criteria modestly predicted the clinical outcome (a composite of MACCE and BARC bleeding type 3 or 5) with incremental prognostic value, but the risk of BARC bleeding type 3 or 5 increased with the number of HBR criteria.
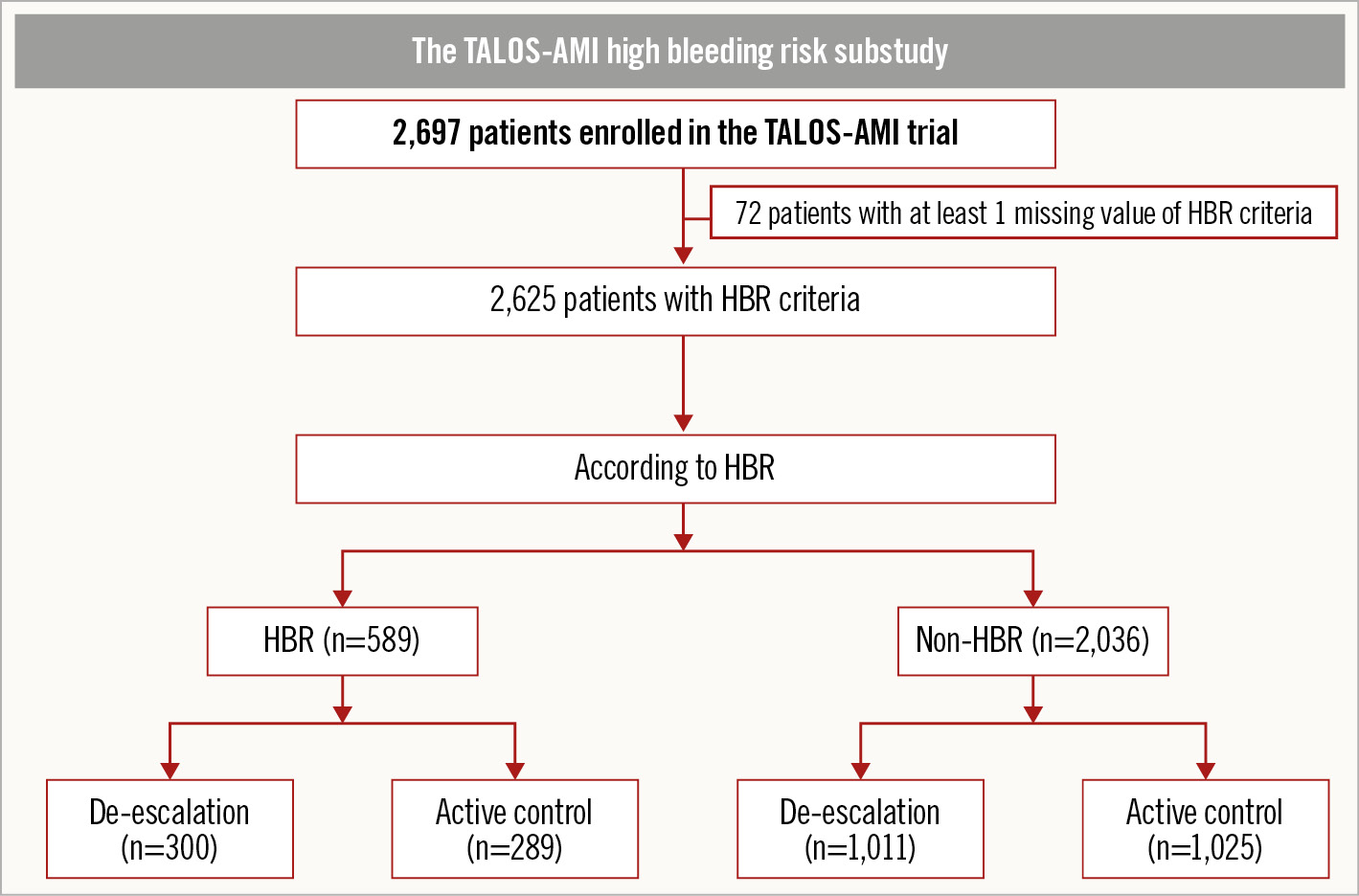
Figure 1. Study flowchart. HBR: high bleeding risk; TALOS-AMI: TicAgrelor Versus CLOpidogrel in Stabilized Patients with Acute Myocardial Infarction
BASELINE CHARACTERISTICS AND CLINICAL OUTCOMES BETWEEN HBR AND NON-HBR BY MODIFIED ARC-HBR CRITERIA
Baseline characteristics and the procedural profiles are shown in Supplementary Table 3. The HBR group contained higher proportions of elderly and female patients. In terms of medical history, the HBR group featured more hypertensive and diabetic patients than the non-HBR group and also those with higher incidences of previous PCI and cerebrovascular accidents. However, the incidences of dyslipidaemia and current smokers were higher in the non-HBR group. In terms of laboratory findings, the creatinine clearance rate, the haemoglobin and platelet levels, and the white blood cell count were lower in the HBR group. The rates of left ventricular ejection fraction <40% were 13.5% in the HBR group and 5.9% in the non-HBR group (p<0.001). There were no between-group differences in terms of clinical presentation or the access site. In both groups, the most frequently infarct-related artery was the left anterior descending artery. Multivessel treatment was performed more in the HBR group than the non-HBR group (33.3 vs 28.7%; p=0.034). The clinical outcomes are shown in Supplementary Table 4 and Figure 2. The primary endpoint incidence was significantly higher in the HBR group (8.7 vs 5.2%, HR 1.75, 95% CI: 1.26-2.45; p=0.001). The key secondary endpoint, BARC bleeding type 3 or 5, was also more common in the HBR group (2.5 vs 1.3%, HR: 2.01, 95% CI: 1.07-3.78; p=0.030). The incidences of other secondary endpoints (MACCE, a composite of MACCE and BARC bleeding type 3 or 5, all-cause mortality, cardiovascular mortality, and spontaneous myocardial infarction) were all higher in the HBR group. There were no between-group differences in the incidences of stroke, ischaemia-driven revascularisation, or stent thrombosis.
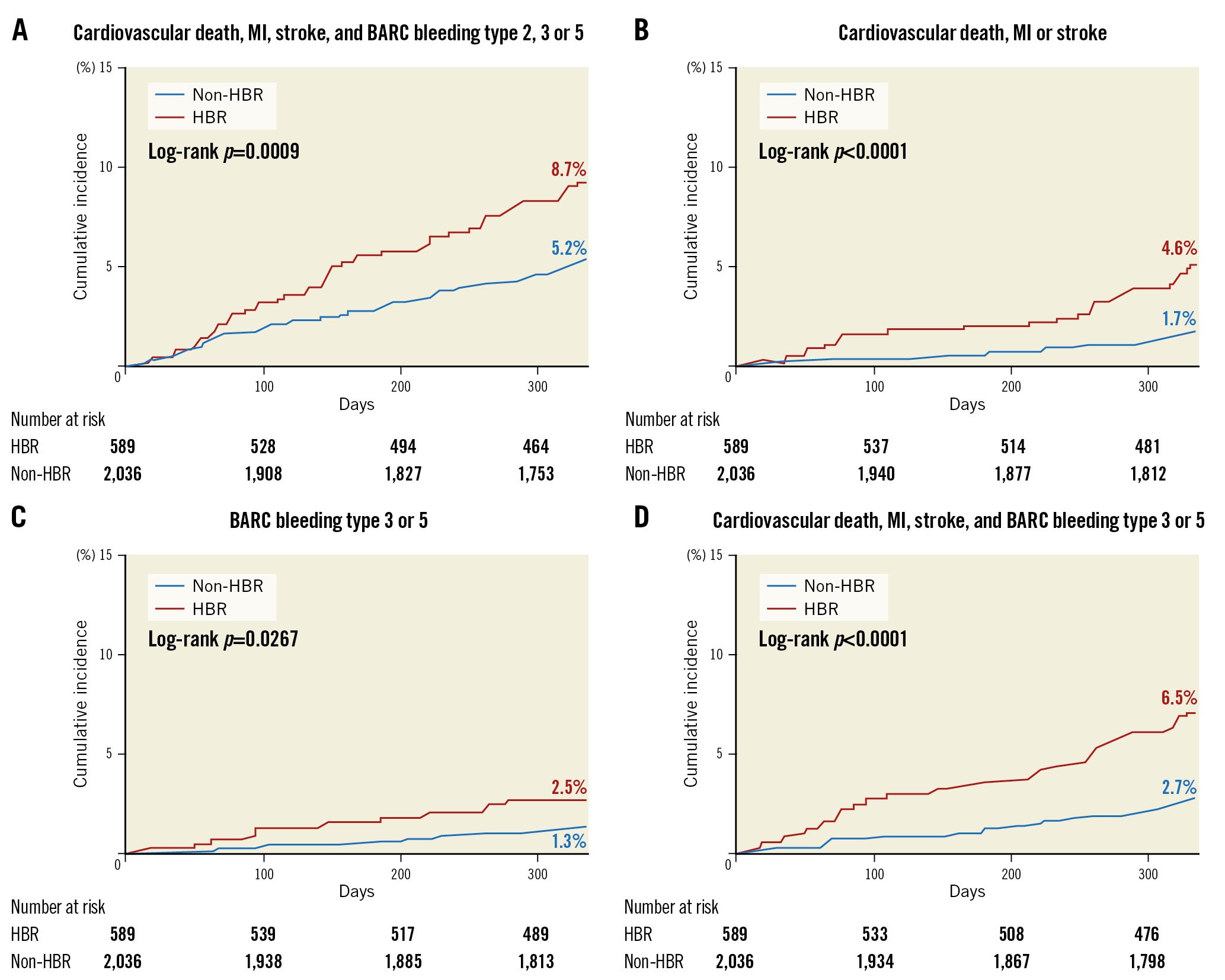
Figure 2. Cumulative incidence of the primary and secondary outcomes by HBR. A) Primary endpoint: a composite of cardiovascular death, myocardial infarction, stroke, or BARC bleeding type 2, 3, or 5. B) Secondary endpoint: a major adverse cardiac and cerebrovascular event (MACCE; a composite of cardiovascular death, myocardial infarction, or stroke). C) Key secondary endpoint: BARC type 3 or 5 bleeding. D) Secondary endpoint: a composite of MACCE and BARC bleeding type 3 or 5. BARC: Bleeding Academic Research Consortium; HBR: high bleeding risk; MI: myocardial infarction
BASELINE CHARACTERISTICS AND CLINICAL OUTCOMES ACCORDING TO HBR BY MODIFIED ARC-HBR CRITERIA AND TREATMENT ARM
For both the HBR and non-HBR groups, the characteristics and outcomes were investigated by the treatment arm (Figure 1). Of the HBR group, 300 were allocated to the de-escalation group and 289 to the active control group. In the non-HBR group, 1,011 patients were in the de-escalation group and 1,025 in the active control group. The baseline and procedural characteristics were balanced between the de-escalation and active control groups for both HBR and non-HBR patients (Table 1). Among the HBR patients, the primary endpoint occurred less frequently in the de-escalation group compared to the active control group (5.7 vs 11.8%, HR 0.47, 95% CI: 0.26-0.84; p=0.011) (Table 2, Figure 3). The incidence of BARC bleeding type 3 or 5 (1.0 vs 4.2%, HR 0.24, 95% CI: 0.07-0.84; p=0.026) and a composite of MACCE and BARC bleeding type 3 or 5 (4.0. vs 9.0%, HR 0.43, 95% CI: 0.22-0.86; p=0.017) were also lower in the de-escalation group. The incidence rates of other secondary endpoints were similar between the groups. In the non-HBR group, the risks of the primary endpoint (4.0 vs 6.4%, HR 0.59, 95% CI: 0.40-0.88; p=0.009) and BARC bleeding type 2, 3, or 5 (HR 0.60, 95% CI: 0.38-0.95; p=0.029) were lower in the de-escalation group (Table 2, Figure 4). However, the incidence of BARC bleeding type 3 or 5 did not differ between the groups (1.2 vs 1.5%, HR 0.79, 95% CI: 0.37-1.69; p=0.542). No interaction was evident between the de-escalation and active control groups for the primary endpoint or other secondary endpoints (Table 2, Central illustration). The adherence rates, assessed by pill count adherence in the HBR subgroup of the intention-to-treat population at 6 months and 12 months after index PCI (5 and 11 months after randomisation), were 97.6% in the de-escalation group and 98.1% in the active control group at 6 months and 97.6% in the de-escalation group and 97.5% in the active control group at 12 months, respectively. The adherence rates in the non-HBR subgroup at 6 months and 12 months after index PCI were 98.7% in the de-escalation group and 97.6% in the active control group at 6 months and 98.7% in the de-escalation group and 97.2% in the active control group at 12 months, respectively. There were no significant differences in adherence.
Table 1. Baseline and procedural characteristics by HBR and treatment arm.
| HBR (n=589) | Non-HBR (n=2,036) | ||||||
|---|---|---|---|---|---|---|---|
| De-escalation (n=300) | Active control (n=289) | p-value | De-escalation (n=1,011) | Active control (n=1,025) | p-value | ||
| Demographics | Age, years | 70.7±10.8 | 71.2±10.2 | 0.522 | 57.0±9.3 | 56.6±9.5 | 0.421 |
| Male | 198 (66.0) | 191 (66.1) | 0.982 | 898 (88.8) | 892 (87.0) | 0.213 | |
| Body mass index, kg/m2 | 24.0±3.3 | 23.5±4.0 | 0.103 | 24.9±3.2 | 24.8±3.4 | 0.832 | |
| Medical history | Hypertension | 191 (63.7) | 190 (65.7) | 0.598 | 443 (43.8) | 453 (44.2) | 0.864 |
| Diabetes | 104 (34.7) | 94 (32.5) | 0.582 | 245 (24.2) | 265 (25.9) | 0.399 | |
| Diabetes treated with insulin | 10 (3.3) | 10 (3.5) | 0.932 | 16 (1.6) | 18 (1.8) | 0.760 | |
| Dyslipidaemia | 115 (38.3) | 102 (35.3) | 0.445 | 427 (42.2) | 443 (43.2) | 0.654 | |
| Current smoker | 92 (30.7) | 79 (27.3) | 0.373 | 557 (55.1) | 576 (56.2) | 0.617 | |
| Impaired renal function* | 160 (53.3) | 144 (49.8) | 0.395 | – | – | – | |
| Past medical history | Previous PCI | 17 (5.7) | 25 (8.7) | 0.159 | 42 (4.2) | 32 (3.1) | 0.213 |
| Previous CABG | 2 (0.7) | 1 (0.3) | 1.000 | 1 (0.1) | – | – | |
| Previous CVA | 23 (7.7) | 18 (6.2) | 0.493 | 27 (2.7) | 30 (2.9) | 0.726 | |
| Clinical presentation | 0.529 | 0.433 | |||||
| STEMI | 150 (50.0) | 152 (52.6) | 560 (55.4) | 550 (53.7) | |||
| NSTEMI | 150 (50.0) | 137 (47.4) | 451 (44.6) | 475 (46.3) | |||
| Laboratory findings | Creatinine clearance†, mL/min/1.73 m2 | 68.2±25.1 | 68.6±26.6 | 0.859 | 91.8±20.7 | 94.1±22.9 | 0.016 |
| Haemoglobin, g/dL | 13.3±1.9 | 13.1±1.9 | 0.248 | 15.0±1.3 | 14.9±1.4 | 0.301 | |
| Platelet, 109/L | 233.8±61.5 | 234.0±64.6 | 0.959 | 242.7±61.0 | 239.7±55.9 | 0.256 | |
| White blood cell count, 109/L | 9.8±3.4 | 9.6±3.4 | 0.348 | 10.4±3.4 | 10.6±3.5 | 0.162 | |
| LVEF <40% | 40/289 (13.8) | 36/273 (13.2) | 0.821 | 59/979 (6.0) | 56/980 (5.7) | 0.769 | |
| Access site | 0.427 | 0.528 | |||||
| Radial | 136 (45.3) | 123 (42.6) | 482 (47.7) | 514 (50.1) | |||
| Femoral | 152 (50.7) | 148 (51.2) | 479 (47.4) | 461 (45.0) | |||
| Glycoprotein IIb/IIIa inhibitor | 74 (24.7) | 67 (23.2) | 0.673 | 234 (23.1) | 241 (23.5) | 0.845 | |
| Infarct-related artery | 0.766 | 0.044 | |||||
| Left main coronary artery | 7 (2.3) | 10 (3.5) | 14 (1.4) | 14 (1.4) | |||
| Left anterior descending artery | 134 (44.8) | 120 (41.8) | 524 (52.3) | 495 (48.7) | |||
| Left circumflex artery | 38 (12.7) | 40 (13.9) | 160 (16.0) | 212 (20.9) | |||
| Right coronary artery | 120 (40.1) | 117 (40.8) | 303 (30.3) | 295 (29.0) | |||
| Number of treated vessels | 1.4±0.6 | 1.4±0.7 | 0.394 | 1.4±0.6 | 1.4±0.6 | 0.830 | |
| Multivessel treatment | 98 (32.7) | 98 (33.9) | 0.749 | 287 (28.4) | 298 (29.1) | 0.733 | |
| Numbers of stents for infarct-related artery | 1.2±0.4 | 1.2±0.4 | 0.765 | 1.2±0.5 | 1.2±0.4 | 0.584 | |
| Total stent length of infarct-related artery, mm | 30.9±13.7 | 30.7±15.3 | 0.915 | 29.7±18.9 | 29.1±13.4 | 0.406 | |
| Stent diameter of infarct-related artery, mm | 3.2±0.4 | 3.2±0.5 | 0.696 | 3.2±0.5 | 3.2±1.0 | 0.613 | |
| Intravascular imaging | Optical coherence tomography | 9 (3.0) | 10 (3.5) | 0.752 | 38 (3.8) | 25 (2.4) | 0.086 |
| Intravascular ultrasonography | 69 (23.0) | 65 (22.5) | 0.883 | 252 (24.9) | 238 (23.2) | 0.368 | |
| Values are expressed as mean±SD, n (%) or n/N (%). *Impaired renal function was defined as an estimated glomerular filtration rate of less than 60 mL/min/1.73 m2 of body surface area at presentation. †Creatinine clearance was calculated by the MDRD (Modification of Diet in Renal Disease) formula: 186 *(serum creatinine)−1.154 *(age)−0.203 *0.742 (for women). CABG: coronary artery bypass graft; CVA: cerebrovascular accident; HBR: high bleeding risk; LVEF: left ventricular ejection fraction; NSTEMI: non-ST-segment elevation myocardial infarction; PCI: percutaneous coronary intervention; SD: standard deviation; STEMI: ST-segment elevation myocardial infarction | |||||||
Table 2. Primary and secondary outcomes by HBR and treatment arm.
| HBR (n=589) | Non-HBR (n=2,036) | p-value for interaction | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| De-escalation (n=300) | Active control (n=289) | Hazard ratio (95% CI) | p-value | De-escalation (n=1,011) | Active control (n=1,025) | Hazard ratio (95% CI) |
p-value | |||
| Primary endpoint* | 17 (5.7) | 34 (11.8) | 0.47 (0.26-0.84) | 0.011 | 40 (4.0) | 66 (6.4) | 0.59 (0.40-0.88) | 0.009 | 0.904 | |
| Secondary endpoints | BARC bleeding type 3 or 5 | 3 (1.0) | 12 (4.2) | 0.24 (0.07-0.84) | 0.026 | 12 (1.2) | 15 (1.5) | 0.79 (0.37-1.69) | 0.542 | 0.413 |
| MACCE† | 11 (3.7) | 16 (5.5) | 0.65 (0.30-1.40) | 0.269 | 14 (1.4) | 20 (2.0) | 0.69 (0.35-1.36) | 0.285 | 0.088 | |
| BARC bleeding type 2, 3 or 5 | 8 (2.7) | 20 (6.9) | 0.38 (0.17-0.85) | 0.019 | 30 (3.0) | 49 (4.8) | 0.60 (0.38-0.95) | 0.029 | 0.226 | |
| BARC bleeding type 2 | 7 (2.3) | 10 (3.5) | 0.67 (0.26-1.76) | 0.417 | 20 (2.0) | 39 (3.8) | 0.51 (0.30-0.87) | 0.013 | 0.593 | |
| BARC bleeding type 3 | 3 (1.0) | 12 (4.2) | 0.24 (0.07-0.84) | 0.026 | 12 (1.2) | 15 (1.5) | 0.79 (0.37-1.69) | 0.542 | 0.413 | |
| BARC bleeding type 5 | 1 (0.3) | – | – | – | – | – | – | – | – | |
| MACCE and BARC bleeding type 3 or 5 | 12 (4.0) | 26 (9.0) | 0.43 (0.22-0.86) | 0.017 | 22 (2.2) | 32 (3.1) | 0.68 (0.39-1.16) | 0.157 | 0.553 | |
| All-cause death | 5 (1.7) | 9 (3.1) | 0.53 (0.18-1.58) | 0.252 | 5 (0.5) | 1 (0.1) | 4.95 (0.58-42.37) | 0.144 | 0.058 | |
| Cardiovascular death | 2 (0.7) | 5 (1.7) | 0.38 (0.07-1.96) | 0.249 | 3 (0.3) | 1 (0.1) | 2.98 (0.31-28.61) | 0.345 | 0.464 | |
| Myocardial infarction | Any myocardial infarction | 5 (1.7) | 8 (2.8) | 0.59 (0.19-1.80) | 0.354 | 7 (0.7) | 11 (1.1) | 0.63 (0.24-1.61) | 0.332 | 0.376 |
| Spontaneous | 5 (1.7) | 6 (2.1) | 0.79 (0.24-2.58) | 0.692 | 4 (0.4) | 7 (0.7) | 0.56 (0.16-1.92) | 0.358 | 0.092 | |
| Periprocedural | – | 2 (0.7) | – | – | 3 (0.3) | 4 (0.4) | 0.74 (0.17-3.30) | 0.692 | – | |
| Target vessel myocardial infarction | 1 (0.3) | 2 (0.7) | 0.47 (0.04-5.19) | 0.538 | 6 (0.6) | 6 (0.6) | 0.98 (0.32-3.05) | 0.975 | 0.588 | |
| Stroke | 4 (1.3) | 4 (1.4) | 0.95 (0.24-3.79) | 0.938 | 4 (0.4) | 8 (0.8) | 0.50 (0.15-1.65) | 0.251 | 0.217 | |
| Ischaemia-driven revascularisation | Target lesion revascularisation | 2 (0.7) | 2 (0.7) | 0.94 (0.13-6.66) | 0.949 | 12 (1.2) | 7 (0.7) | 1.69 (0.67-4.29) | 0.270 | 0.711 |
| Target vessel revascularisation | 3 (1.0) | 4 (1.4) | 0.70 (0.16-3.14) | 0.643 | 14 (1.4) | 13 (1.3) | 1.06 (0.50-2.25) | 0.881 | 0.670 | |
| Any revascularisation | 8 (2.7) | 12 (4.2) | 0.63 (0.26-1.53) | 0.305 | 24 (2.4) | 26 (2.5) | 0.91 (0.52-1.58) | 0.728 | 0.927 | |
| Stent thrombosis | 1 (0.3) | 1 (0.3) | 0.93 (0.06-14.86) | 0.959 | 2 (0.2) | 2 (0.2) | 0.98 (0.14-6.99) | 0.987 | 0.668 | |
| Values are expressed as n (%). *Composite of cardiovascular death, myocardial infarction, stroke, or BARC bleeding type 2, 3, or 5. †Composite of cardiovascular death, myocardial infarction, or stroke. BARC: Bleeding Academic Research Consortium; CI: confidence interval; HBR: high bleeding risk; MACCE: major adverse cardiac and cerebrovascular event | ||||||||||
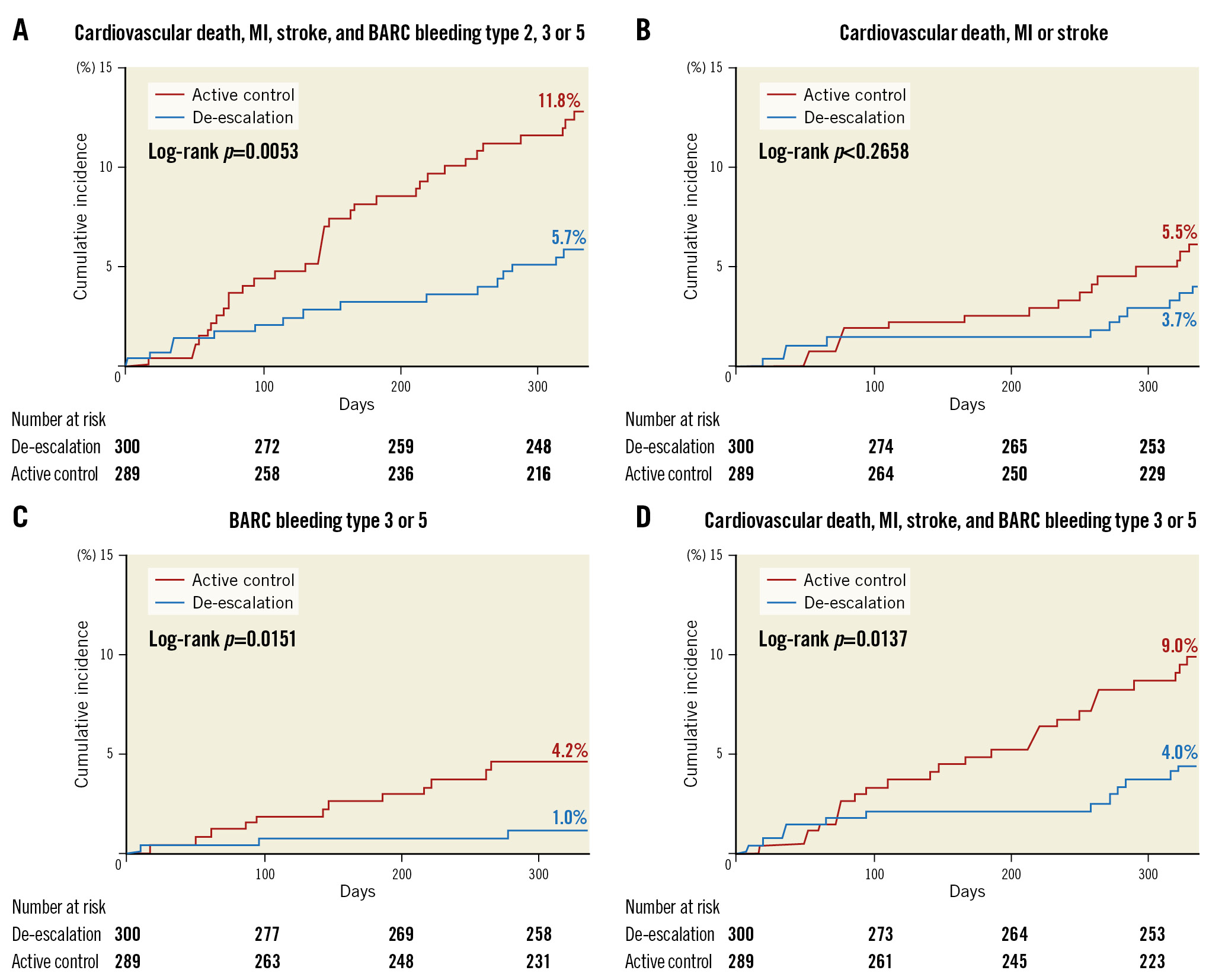
Figure 3. Cumulative incidence of the primary and secondary outcomes by HBR and treatment arm. A) Primary endpoint: a composite of cardiovascular death, myocardial infarction, stroke, or BARC bleeding type 2, 3, or 5. B) Secondary endpoint: a major adverse cardiac and cerebrovascular event (MACCE; a composite of cardiovascular death, myocardial infarction, or stroke). C) Key secondary endpoint: BARC type 3 or 5 bleeding. D) Secondary endpoint: a composite of MACCE and BARC bleeding type 3 or 5. BARC: Bleeding Academic Research Consortium; HBR: high bleeding risk; MI: myocardial infarction
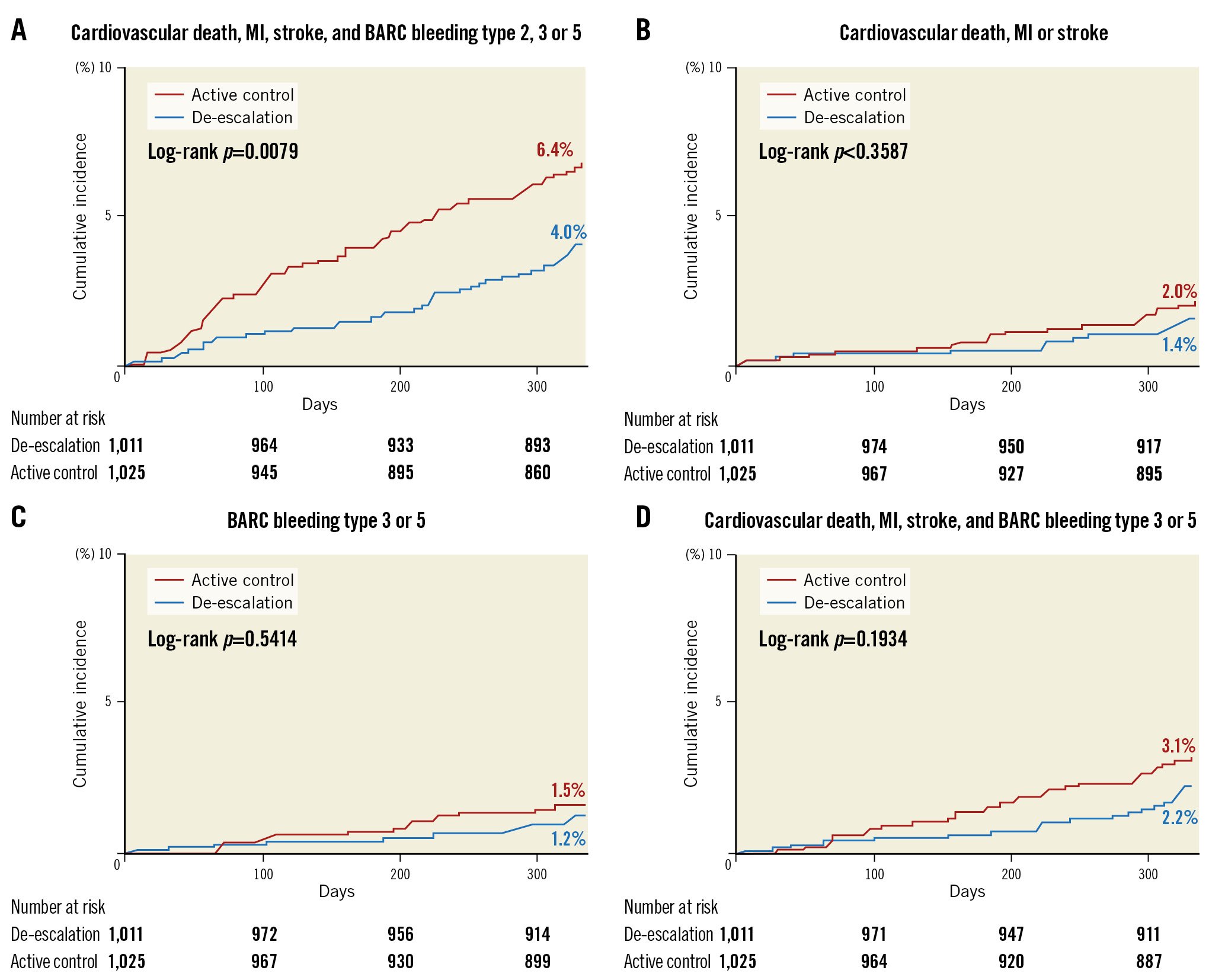
Figure 4. Cumulative incidence of the primary and secondary outcomes by non-HBR and treatment arm. A) Primary endpoint: a composite of cardiovascular death, myocardial infarction, stroke, or BARC bleeding type 2, 3, or 5. B) Secondary endpoint: a major adverse cardiac and cerebrovascular event (MACCE; a composite of cardiovascular death, myocardial infarction, or stroke). C) Key secondary endpoint: BARC type 3 or 5 bleeding. D) Secondary endpoint: a composite of MACCE and BARC bleeding type 3 or 5. BARC: Bleeding Academic Research Consortium; HBR: high bleeding risk; MI: myocardial infarction
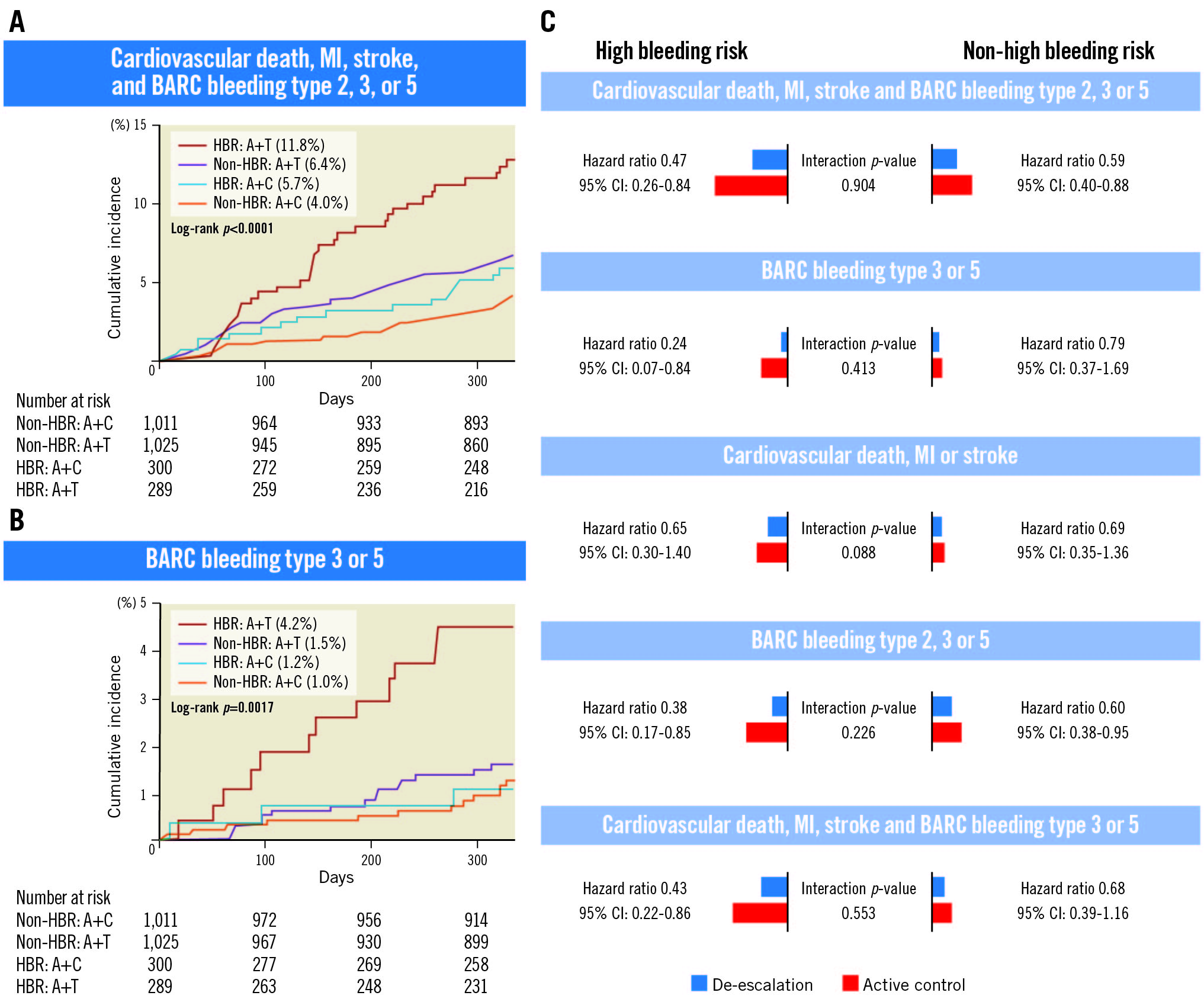
Central illustration. Effects of ticagrelor-based de-escalation in patients with AMI who are undergoing PCI using DES according to high bleeding risk. A) Cumulative incidence of the primary endpoint (net clinical outcome) according to HBR and treatment arm. B) Cumulative incidence of BARC bleeding type 3 or 5 according to the HBR and treatment arm. C) Cumulative event rates between 1 and 12 months in patients with HBR and non-HBR. A: active control; AMI: acute myocardial infarction; BARC: Bleeding Academic Research Consortium; C: clopidogrel; CI: confidence interval; DES: drug-eluting stent; HBR: high bleeding risk; MI: myocardial infarction; PCI: percutaneous coronary intervention; T: ticagrelor; TALOS-AMI: TicAgrelor Versus CLOpidogrel in Stabilized Patients with Acute Myocardial Infarction
VALIDATION BY THE PRECISE-DAPT SCORE AND ARC-HBR CRITERIA
The prevalence of HBR was 13.5% (355 patients) by the PRECISE-DAPT score and 11.5% (303 patients) by the ARC-HBR criteria (Supplementary Figure 3, Supplementary Figure 4). The baseline characteristics and clinical outcomes of HBR and non-HBR patients identified via the PRECISE-DAPT score and ARC-HBR criteria were comparable to those of patients grouped using the modified HBR criteria (Supplementary Table 5, Supplementary Table 6, Supplementary Figure 5, Supplementary Figure 6). The treatment arms according to the PRECISE-DAPT scores and ARC-HBR criteria are shown in Supplementary Figure 3. The baseline and procedural characteristics were well balanced between the de-escalation and active control groups for both HBR and non-HBR patients, as revealed by both the PRECISE-DAPT score and ARC-HBR criteria (Supplementary Table 7, Supplementary Table 8). The PRECISE-DAPT analysis revealed that the incidence of the primary endpoint (HR 0.54, 95% CI: 0.38-0.78; p=0.001) and a composite of MACCE and BARC bleeding type 3 or 5 (HR 0.57, 95% CI: 0.35-0.95; p=0.035) were significantly lower in de-escalation only in the non-HBR group. In the HBR group, although de-escalation somewhat reduced the incidence rate of the primary endpoint (7.2 vs 12.1%, HR 0.59, 95% CI: 0.29-1.17) and BARC bleeding type 3 or 5 (2.2 vs 5.2%, HR 0.42, 95% CI: 0.13-1.38), statistical significance was lacking (Supplementary Table 9, Supplementary Figure 7, Supplementary Figure 8). In the ARC-HBR analysis, the primary endpoint occurred less on de-escalation (compared to control) in both the HBR (6.2 vs 13.4%, HR 0.43, 95% CI: 0.20-0.95; p=0.036) and non-HBR (4.1 vs 6.8%, HR 0.59, 95% CI: 0.41-0.84; p=0.004) groups. There was no significant difference in the incidence of BARC bleeding type 3 or 5 between the treatment arms of either the HBR group or non-HBR group (Supplementary Table 10, Supplementary Figure 9, Supplementary Figure 10).
Discussion
We explored whether the benefit of uniform, unguided de-escalation of the P2Y12 inhibitor from ticagrelor to clopidogrel 1 month after index PCI for AMI patients would be more noticeable in subjects at HBR, using the modified ARC-HBR criteria. The key findings are as follows: 1) de-escalation of DAPT from ticagrelor to clopidogrel reduced the net ischaemic and bleeding events both in patients at HBR and non-HBR, 2) the improved clinical outcomes were principally attributable to reduced haemorrhagic events but with maintenance of anti-ischaemic efficacy, and 3) BARC bleeding type 3 or 5 was less common in the de-escalation group than the ticagrelor-based standard 12-month DAPT group among patients with HBR alone; this was not the case for those with non-HBR.
UNFAVOURABLE PCI OUTCOMES IN AN HBR POPULATION
DAPT duration, the choice of P2Y12 inhibitor (with aspirin as the bedrock), and a decision on aspirin or P2Y12 monotherapy after DAPT are determined via the thorough assessment of individual thrombotic and bleeding risks, because post-PCI clinical outcomes are influenced by a complex interplay between the risks42122. Bleeding per se is closely linked to increased thrombosis via multiple mechanisms such as discontinuation of antiplatelet agents, nitric oxide-depleted blood transfusions, and bleeding-induced enhancement of inflammation and thrombosis22. Patients aged ≥75 years (a minor ARC-HBR criterion) are associated with many cardiometabolic comorbidities such as diabetes, CKD, and extensive polyvascular disease, all of which increase thrombotic risk. Moreover, reduced creatinine clearance is both a high thrombotic and bleeding risk35. For these reasons, patients at HBR exhibited a poorer prognosis than those with non-HBR723. In the present study, we found that HBR status by the modified ARC-HBR criteria was linked to increased BARC bleeding type 3 or 5 and more ischaemic events.
DE-ESCALATION OF DAPT STRATEGIES IN AMI PATIENTS AT HBR
The current standard DAPT regimen for AMI patients undergoing PCI consists of aspirin and a P2Y12 inhibitor, such as ticagrelor or prasugrel in preference to clopidogrel34. Recently, various modified strategies have emerged to create a trade-off between thrombosis and haemorrhage. Such strategies include a shortened DAPT period8, a reduced dose (5 mg) of prasugrel10, de-escalation of the P2Y12 inhibitor from ticagrelor to clopidogrel9, and P2Y12 inhibitor monotherapy11121314. The benefits of these strategies were particularly marked in patients at HBR823. Our results show that net adverse clinical outcomes occurred less frequently in the de-escalation group than in the active control group, regardless of the HBR status. However, the incidence of BARC bleeding type 3 or 5 events was lower in the de-escalation group among only HBR, not non-HBR, patients.
Notably, unguided DAPT de-escalation from ticagrelor to clopidogrel did not increase the incidence of thrombotic events even in AMI patients at high thrombotic risk, in line with the results of previous trials that alternated the standard DAPT89101112142425. Any benefit of prolonged DAPT in terms of decreasing ischaemic events is offset by more haemorrhagic events when a high thrombotic risk and an HBR coexist26. In such a situation, the HBR may dominate. Over time (approximately 1 month after an acute coronary syndrome), the bleeding risk surpasses the ischaemic risk15. Therefore, in AMI patients who are stabilised 1 month after PCI, de-escalation of the DAPT strategy from ticagrelor to clopidogrel, reducing the dose of prasugrel from 10 to 5 mg, or ticagrelor monotherapy (thus dropping aspirin) are viable alternatives to ticagrelor- or prasugrel-based 12-month DAPT, especially for those at HBR.
GUIDED AND UNGUIDED DE-ESCALATION DAPT STRATEGIES IN HBR PATIENTS
The current study is a post hoc analysis of the TALOS-AMI trial which evaluated an unguided ticagrelor-based de-escalation strategy in HBR patients; the POPular Genetics and TROPICAL-ACS trials evaluated guided de-escalation of DAPT strategy in this population2728. In the individual patient-level meta-analysis, which included the 3 randomised trials mentioned above and the HOST REDUCE POLYTECH ACS trial, ischaemic and bleeding endpoints were significantly lower in the de-escalation strategy compared to the standard antiplatelet strategy1029. Notably, bleeding endpoints were more reduced with unguided de-escalation than with the guided de-escalation strategy, and this was proved in another meta-analysis30. This prominent reduction of bleeding endpoints with the unguided de-escalation strategy might be associated with several factors, such as study population (the trials regarding unguided de-escalation strategy only enrolled Asian patients) or the timing of de-escalation. The current post hoc analysis of the TALOS-AMI trial shows the benefits of a ticagrelor-based de-escalation strategy in HBR patients. As far as we know, there are only a few studies which have investigated the efficacy and safety of guided de-escalation strategy in HBR patients.
In the ESC Guidelines for the management of acute coronary syndromes, DAPT de-escalation may be considered as an alternative treatment regimen based on whether it is guided or unguided by platelet function test or CYP2C19 genotyping (Class IIb, Level of Evidence A)3. However, this recommendation was based on the results of the POPular Genetics and TROPICAL-ACS trials, both of which evaluated guided de-escalation strategies2728; the results of large trials which evaluated unguided de-escalation strategies (the TALOS-AMI and HOST REDUCE POLYTECH ACS trials) were only introduced after this. Therefore, the next guidelines (2023 ESC Guidelines for ACS) may reflect the evidence from these more recent trials, and de-escalation of DAPT strategy may be recommended more, especially in patients with HBR.
Limitations
Several limitations exist in the present study. First, this is a post hoc analysis using data from the TALOS-AMI trial. Our findings only generate hypotheses, and research validation is essential. Second, patients at HBR in our study do not reflect our daily PCI practice, as subjects with HBR and those having actual bleeding episodes or coagulopathy were excluded from the study at enrolment. Third, we arbitrarily modified the definition of ARC-HBR, because we lacked data on some ARC-HBR criteria. We considered that age ≥75 years and moderate CKD (an ARC-HBR minor criterion) were important in terms of the HBR. Both factors were individually associated with more bleeding episodes in the present study and in previous trials1819. However, major bleeding only occurred in less than 4% of patients in isolation of major criteria and without any other coexisting criteria, and 70.5% of the distribution of HBR subgroups corresponds to only one HBR definition. Nevertheless, a sensitivity analysis using the original ARC-HBR definition and the PRECISE-DAPT scores yielded findings consistent with our principal results. Compared to the ticagrelor-based 12-month DAPT, the de-escalation strategy tended to lower the incidence of bleeding events in patients with HBR using the ARC-HBR definition and the PRECISE-DAPT score. Fourth, there were no data for CYP2C19 genotyping. However, the TALOS-AMI study aimed to evaluate the benefits of an unguided de-escalation of DAPT strategy. Fifth, we could not check the prescription rate of proton pump inhibitors (PPI), which have been associated with reduced gastrointestinal bleeding. In other trials regarding antiplatelet strategies performed in South Korea, the prescription rate for PPI was about 18%, and we think the rate of PPI prescription in this subgroup analysis may have been similar to that of the abovementioned trials10. Finally, the interaction p-values for all outcomes are>0.05, which indicates that there were no statistically significant differences in de-escalation treatment effects between the HBR and non-HBR groups despite large differences in the hazard ratios in some outcomes, such as major bleeding. This finding suggests that the current study had inadequate power; thus, the results should be interpreted cautiously.
Conclusions
Uniform unguided de-escalation of the P2Y12 inhibitor from ticagrelor to clopidogrel 1 month after AMI was safe and efficacious in terms of decreasing the rate of net adverse clinical outcomes, regardless of HBR status by the modified ARC-HBR criteria. The effect of de-escalation of DAPT strategy on reductions in BARC bleeding type 3 or 5 was profound for patients at HBR. De-escalating DAPT from ticagrelor to clopidogrel might be a reasonable option in stabilised AMI patients with HBR.
Impact on daily practice
Compared to ongoing ticagrelor-based DAPT, for AMI patients undergoing PCI with DES, switching from ticagrelor to clopidogrel after 1 month reduced haemorrhage while maintaining protection against thrombotic events in both HBR and non-HBR patients. Future studies should investigate the impact of the de-escalation of DAPT strategy from ticagrelor to clopidogrel in AMI patients with HBR at earlier timepoints after PCI.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

