
Abstract
Aims: The aim of this study was to evaluate the impact of 23-month ticagrelor monotherapy following one-month dual antiplatelet therapy (DAPT) after percutaneous coronary intervention (PCI) on the rates of patient-oriented composite endpoints (POCE) and net adverse clinical events (NACE).
Methods and results: The rates of site-reported Academic Research Consortium (ARC)-2 defined POCE (all-cause death, any stroke, any myocardial infarction or any revascularisation) and NACE (POCE or bleeding type 3 or 5 according to the Bleeding ARC [BARC]) were reported up to two years by intention-to-treat principle in the randomised, multicentre, open-label GLOBAL LEADERS study comparing two antiplatelet strategies in 15,991 patients undergoing PCI. The experimental strategy consisted of aspirin with ticagrelor for one month followed by ticagrelor monotherapy for 23 months, whereas the reference treatment consisted of 12-month DAPT followed by 12-month aspirin monotherapy. At two years, POCE occurred in 1,050 (13.2%) patients in the experimental group and in 1,131 (14.2%) in the reference group (HR 0.93, 95% CI: 0.85-1.01, p=0.085). NACE occurred in 1,145 (14.4%) patients in the experimental group and in 1,237 (15.5%) patients in the reference group (HR 0.92, 95% CI: 0.85-1.00, p=0.057). In pre-specified subgroup analyses, no significant treatment-by-subgroup interactions were found for either POCE or NACE at two years.
Conclusions: The experimental treatment strategy of one-month DAPT followed by 23 months of ticagrelor alone did not result in a significant reduction in the rates of site-reported POCE or NACE, when compared to the reference treatment. ClinicalTrials.gov Identifier: NCT01813435
Introduction
Acetylsalicylic acid-free antiplatelet strategies consisting of more potent P2Y12 receptor antagonists have been advocated to ensure an increased net benefit for individual patients, given the expected reduction in the bleeding risk without a trade-off in anti-ischaemic efficacy1,2.
In the randomised open-label all-comers GLOBAL LEADERS study, the experimental treatment strategy of ticagrelor monotherapy, following one-month dual antiplatelet therapy (DAPT) with aspirin, was not superior to standard DAPT in reducing the primary endpoint consisting of all-cause mortality or core lab-adjudicated new Q-wave myocardial infarction (MI) among patients undergoing percutaneous coronary interventions (PCI)3. However, no excess safety signal attributable to this treatment strategy was detected, given the fact that the upper boundary of the 95% confidence interval (CI) of the primary endpoint was close to unity3.
According to the recent Academic Research Consortium (ARC)-2 consensus, the overall cardiovascular outcomes from the patient perspective, including all-cause death, any type of stroke, MI, and any repeat revascularisation, namely patient-oriented composite endpoints (POCE), should constitute the basis for evaluating novel coronary devices or pharmacotherapeutic agents4. The combination of clinically relevant events linked by common elements of pathophysiology may be expected to provide additional statistical power4. It has been argued that composite endpoints built on site-reported events may allow detection of benefit and risk signals with potentially superior sensitivity5,6,7.
Furthermore, given the strong association between bleeding and mortality risk, attempts to quantify the net adverse clinical events (NACE) incorporating safety-related bleeding events aside from ischaemic complications, may provide incremental insights into the understanding of the benefit-risk ratio of an evaluated treatment strategy.
Given this background, and to complement the interpretation of the GLOBAL LEADERS study results, we analysed the impact of the experimental treatment strategy on the rates of ARC-2 defined site-reported POCE and NACE up to two years after PCI in this randomised, multicentre, open-label study, being so far the first trial comparing two antiplatelet treatment strategies with randomisation done at the time of PCI1,3.
Methods
STUDY DESIGN AND POPULATION
GLOBAL LEADERS was an open-label, randomised, multicentre, superiority trial that enrolled a total of 15,991 patients at 130 sites in 18 countries1. The rationale, design and methodology of the GLOBAL LEADERS study (ClinicalTrials.gov number NCT01813435) have been detailed elsewhere1.
In brief, the study population comprised patients scheduled to undergo PCI for stable coronary artery disease (CAD) or acute coronary syndromes (ACS), who required DAPT, on condition that oral anticoagulation was not indicated. There were no restrictions on the number of treated lesions or vessels, on lesion length or number of stents used. All types of lesion were allowed, including left main, bifurcations, chronic total occlusions, interventions on grafted vessels, etc.1. Detailed inclusion and exclusion criteria have been described previously1 and are presented in Supplementary Appendix 1.
Patients were randomly allocated either to an experimental strategy of one-month aspirin and ticagrelor, followed by 23 months of ticagrelor alone, or to the reference strategy with one-year DAPT consisting of 75-100 mg aspirin daily in combination with either 75 mg clopidogrel daily (for patients with stable CAD) or 90 mg ticagrelor twice daily (for patients with ACS), followed by 75-100 mg aspirin alone for another 12 months1. Antiplatelet therapy was initiated before or at the time of the index procedure. PCI was standardised by uniform implantation of biodegradable polymer-based biolimus A9-eluting stents and bivalirudin administration, the periprocedural anticoagulation by default, except for those countries (Bulgaria, Poland) where the drug was not available (Supplementary Appendix 2).
The primary endpoint was a composite of all-cause death or new Q-wave MI within 730 days of the index procedure. Deaths from any cause were ascertained without adjudication but a search for vital status was conducted through public domains in national and municipal registries among the 18 countries involved in the trial. Q-wave MI was centrally adjudicated and described according to the Minnesota classification (new major Q-QS wave abnormalities) or by the appearance of a new left bundle branch block in conjunction with abnormal biomarkers3.
In the present exploratory analysis, we evaluated the efficacy and safety of the experimental versus the reference treatment strategy in reducing the rates of the composite endpoints of POCE and NACE up to two years after PCI in the overall GLOBAL LEADERS study cohort, as well as across different patient subgroups pre-specified in the study protocol.
COMPOSITE ENDPOINT DEFINITIONS
Composite endpoints analysed in the present investigation involved site-reported secondary clinical endpoints1,3. POCE were defined as a composite of all-cause mortality, any stroke, any MI or any revascularisation, as specified by the ARC-2 consensus, whereas NACE included POCE and bleeding type 3 or 5, according to the Bleeding Academic Research Consortium (BARC) definitions. A detailed description of the study endpoint definitions, follow-up and study overview has been provided in Supplementary Appendix 3. Composite endpoints were reported up to two years according to the time-to-first-event principle. In addition, the pre-specified landmark analyses were performed corresponding to the planned dates of discontinuation of aspirin at 30 days in the experimental group or of a P2Y12 receptor antagonist at one year in the reference group after the index procedure.
STATISTICAL ANALYSIS
Statistical analyses for the primary and secondary endpoints in the GLOBAL LEADERS study have been described in detail previously1,3.
For the purpose of the current post hoc analysis, the composite endpoints of POCE and NACE involving the secondary efficacy and safety endpoints were evaluated using the Mantel-Cox log-rank method up to the time point when the first event occurred, reporting hazard ratios (HR) with 95% CI. Two landmark analyses at 30 days and one year after randomisation were performed.
In addition, we performed predefined subgroup analyses of the POCE and NACE at two years with interaction tests for treatment effect.
All analyses were performed following the intention-to-treat definition using SPSS software, Version 25 (IBM Corp., Armonk, NY, USA). A two-sided p-value of <0.05 was considered statistically significant.
Results
Between July 2013 and November 2015 the GLOBAL LEADERS study enrolled and randomised 15,991 patients3. Except for 31 patients (0.19%: 23 patients who withdrew consent and formally requested their data deletion and eight patients with survival information missing despite additional search through public domains in national and municipal registries), there were data from 7,980 patients in the experimental group and 7,988 in the reference group available for analysis (Figure 1).
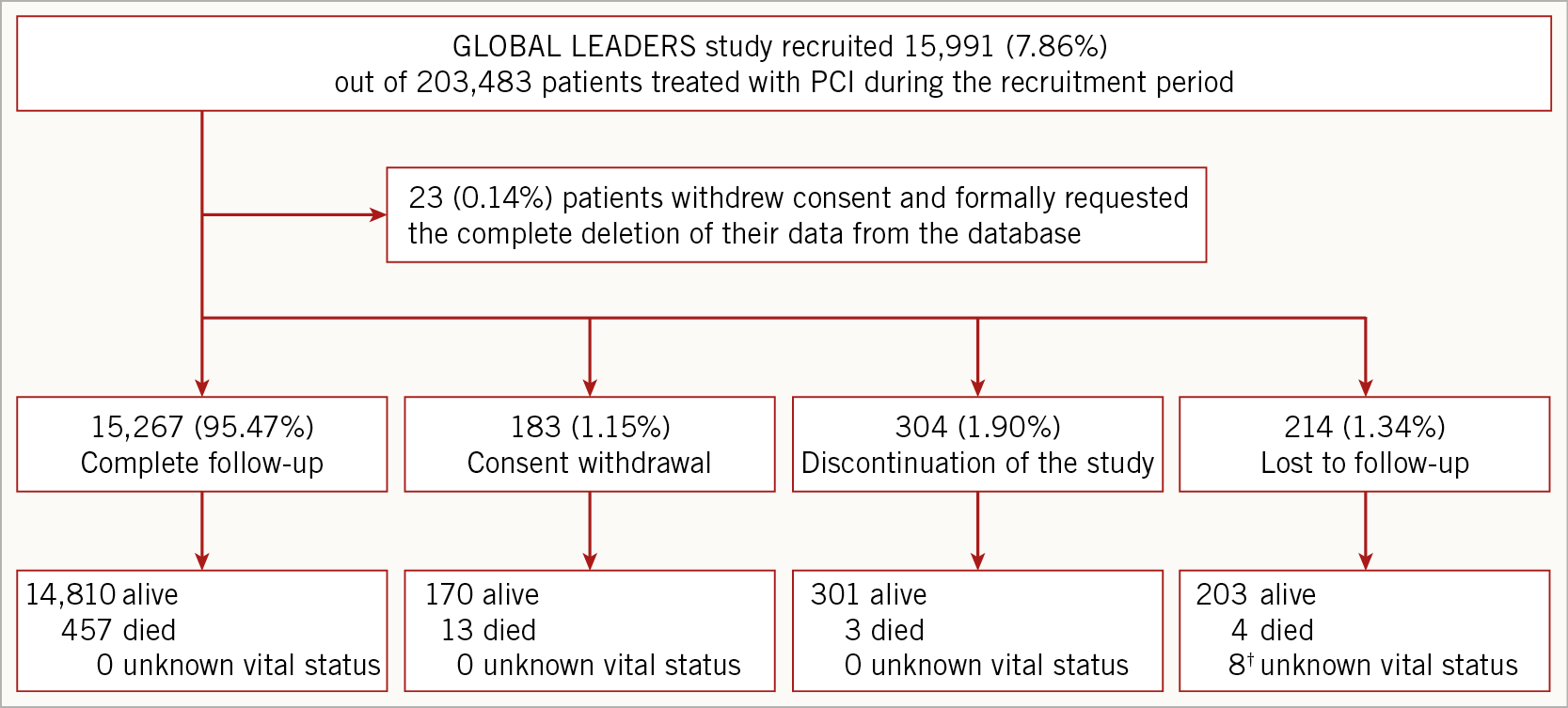
Figure 1. The GLOBAL LEADERS study flow diagram. ? Four patients were in the experimental group and the others were in the reference group.
The baseline clinical and procedural characteristics were well balanced between the experimental and reference treatment strategy (Supplementary Table 1, Supplementary Table 2).
At two-year follow-up, POCE occurred in 1,050 (13.2%) patients in the experimental group and in 1,131 (14.2%) patients in the reference treatment strategy group (HR 0.93, 95% CI: 0.85-1.01, p=0.085 (Figure 1).
NACE had occurred in 1,145 (14.4%) patients in the experimental group and in 1,237 (15.5%) in the reference group (HR 0.92, 95% CI: 0.85-1.00, p=0.057) (Figure 2, Supplementary Table 3).
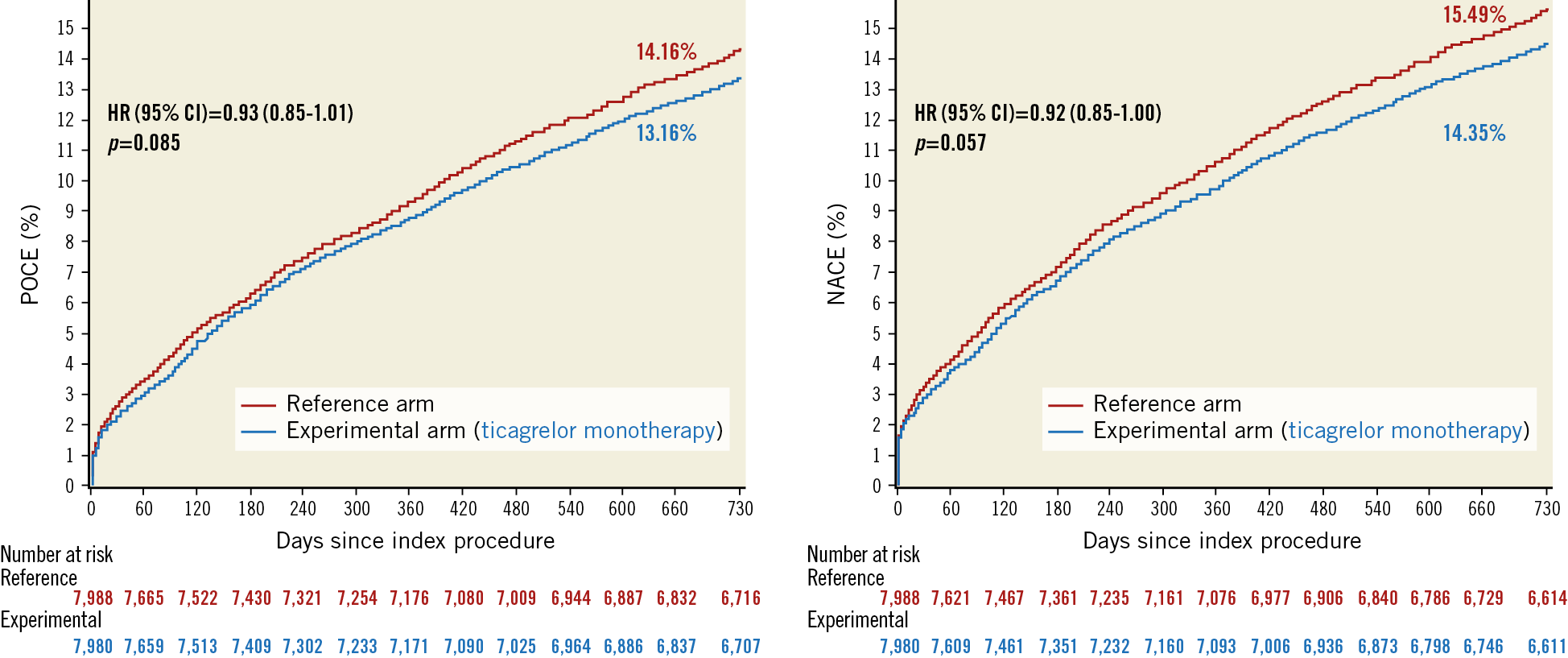
Figure 2. Kaplan-Meier estimate of patient-oriented composite endpoints (POCE) and net adverse clinical events (NACE) at two years. CI: confidence interval; HR: hazard ratio
There were no between-group differences in each individual component of the composite endpoints, all-cause mortality, any stroke, any MI, any revascularisation, or bleeding BARC 3 or 5 type (Supplementary Table 3).
LANDMARK ANALYSES
Following landmark analysis at 30 days, there were no significant differences between the experimental and the reference group in the rates of POCE (11.2% vs 11.8%, HR 0.95, 95% CI: 0.86-1.04, p=0.249) and NACE (12.0% vs 12.8%, HR 0.94, 95% CI: 0.86-1.02, p=0.149) occurring between 30 days and two years post randomisation.
Similarly, following landmark analysis at one year, there were no significant differences between the treatment groups in the rates of POCE (4.9% vs 5.4%, HR 0.91, 95% CI: 0.78-1.05, p=0.180) and NACE (5.2% vs 5.6%, HR 0.93, 95% CI: 0.81-1.08, p=0.341) occurring between one and two years post randomisation (Figure 3, Supplementary Table 4).
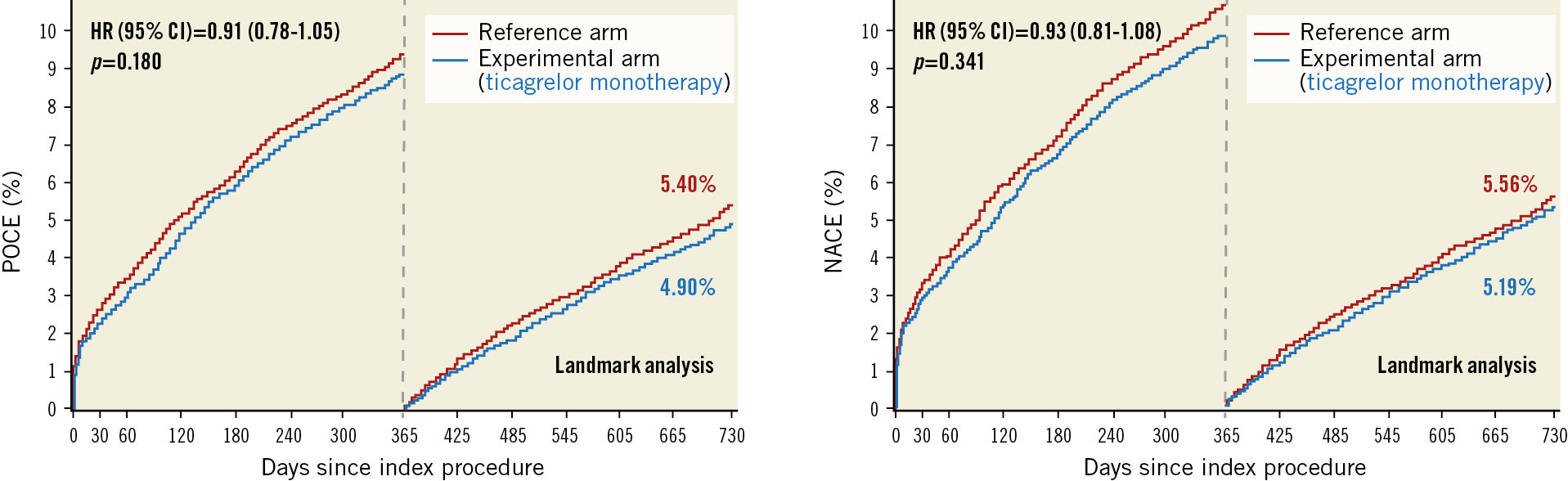
Figure 3. Landmark analysis at one year for patient-oriented composite endpoints (POCE) and net adverse clinical events (NACE). CI: confidence interval; HR: hazard ratio
SUBGROUP ANALYSES
At two-year follow-up, among ACS patients POCE occurred in 492 (13.1%) patients in the experimental group and in 519 (13.9%) patients in the reference treatment strategy group (HR 0.94, 95% CI: 0.83-1.07, p=0.336), whereas in stable CAD patients POCE occurred in 557 (13.2%) patients in the experimental group and in 613 (14.4%) patients in the reference group (HR 0.91, 95% CI: 0.81-1.02, p=0.108) (p for interaction=0.700).
Among ACS patients, NACE occurred in 531 (14.2%) patients in the experimental group and in 578 (15.5%) in the reference group (HR 0.91, 95% CI: 0.81-1.02, p=0.110), whereas in stable CAD patients NACE occurred in 613 (14.5%) patients in the experimental group and in 660 (15.5%) patients in the reference group (HR 0.93, 95% CI: 0.83-1.04, p=0.205) (p for interaction=0.754).
The remaining subgroup analyses according to pre-specified clinical characteristics and type of treatment also did not demonstrate significant treatment effects, as statistically ascertained by non-significant tests for treatment effect for POCE (Figure 4, Supplementary Table 5) and NACE two years after PCI (Figure 5, Supplementary Table 6).
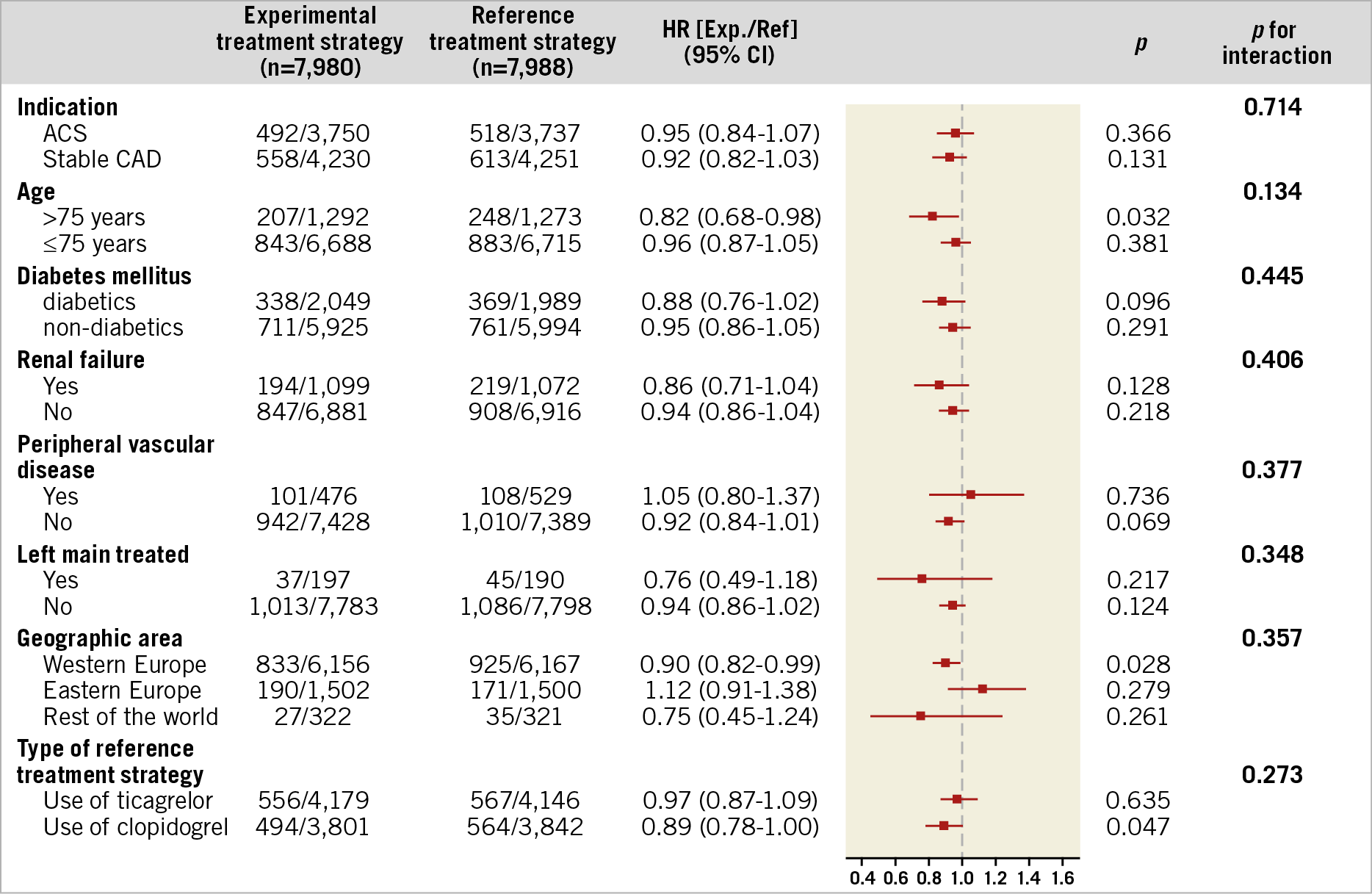
Figure 4. Subgroup analysis for patient-oriented composite endpoints (POCE) at two years, according to the pre-specified baseline variables. Number of first events and percentages are reported. ACS: acute coronary syndrome; CAD: coronary artery disease; HR: hazard ratio; CI: confidence interval
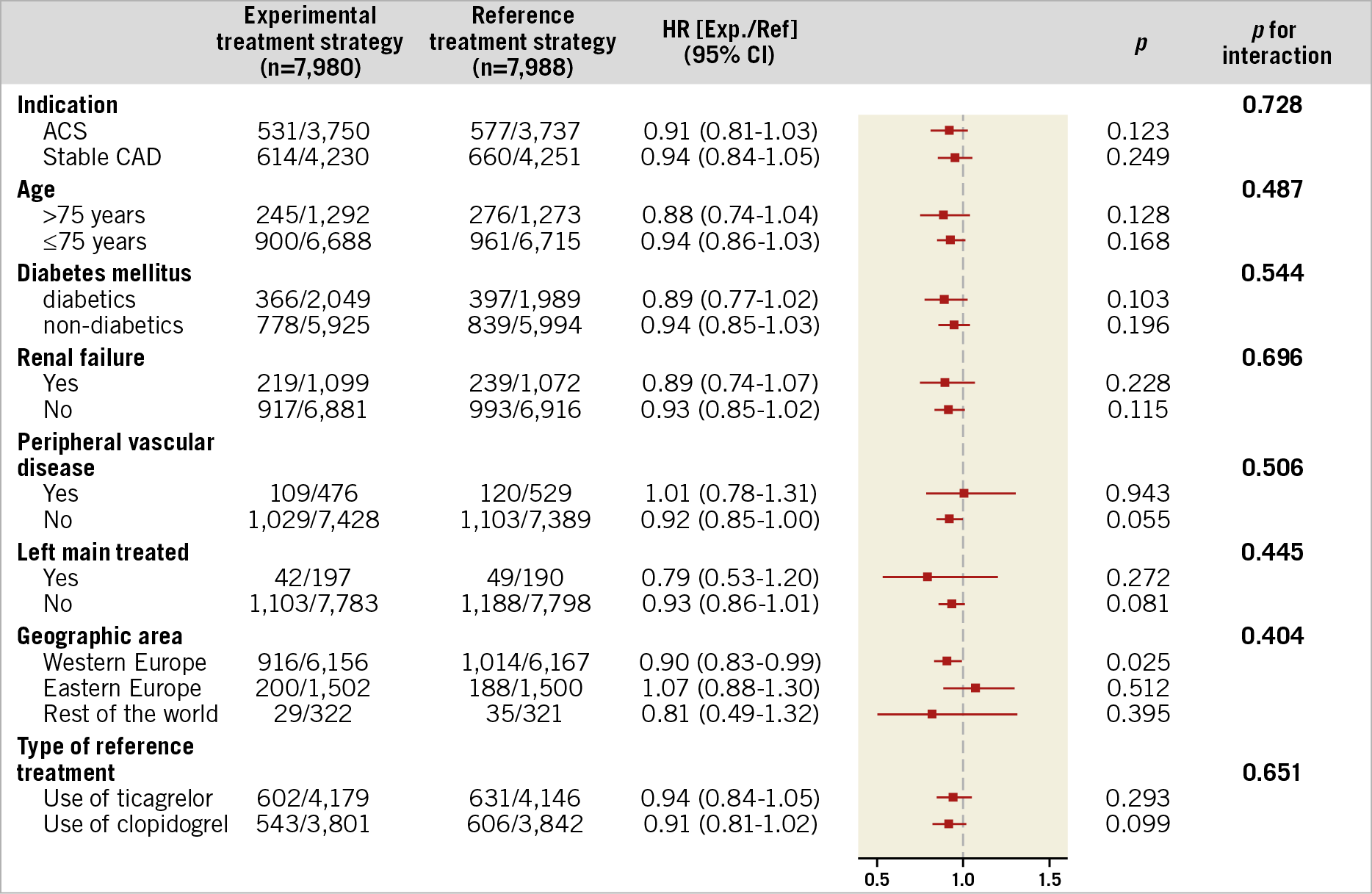
Figure 5. Subgroup analysis for net adverse clinical events (NACE) at two years, according to the pre-specified baseline variables. Number of first events and percentages are reported. ACS: acute coronary syndrome; CAD: coronary artery disease; HR: hazard ratio; CI: confidence interval
In the experimental versus the reference arm, the rates of patients who adhered to the randomised treatment regimen at discharge, 3, 12, and 24 months were, respectively: 97.6% vs 97.2%, 86.0% vs 93.6%, 81.7% vs 89.3% and 77.6% vs 93.1%.
Discussion
This is the first contemporary all-comers PCI trial reporting site-reported POCE rates according to the new ARC-2 definition which, at variance with the previously reported three-component endpoint (all-cause death, any MI, any revascularisation), also includes stroke as a highly relevant clinical outcome from the individual patient’s perspective4,8.
The experimental strategy did not reduce the risk of POCE when compared to the reference treatment. In addition, no significant differences were seen in the rates of NACE between the treatment groups (p=0.057), though numerical NACE were less frequently observed in the experimental arm. There was no treatment effect for any of the pre-specified subgroups. These findings, in line with the primary study results, do not support a change in clinical practice consisting of standard DAPT followed by aspirin monotherapy.
These observations, however, need to be interpreted in light of the significantly lower treatment adherence in the experimental versus the reference group, that was different in particular between the first and the second year of observation. Although any plausible explanation for these findings would be considered speculative, it cannot be excluded that the lower adherence to the experimental treatment could have partially contributed to the loss of statistical significance for the difference in the rates of the primary endpoint between the two treatment groups at two years, compared with the rates found at one year. In the GLOBAL LEADERS adherence substudy, conducted among 8,545 consecutive patients in whom both the stop and restart dates of the allocated treatment have been recorded after the modification of the electronic case report form (eCRF), the main reasons for non-adherence at two years were dyspnoea (233 [26%] vs 8 [3%], p=0.001), bleeding (191 [21%] vs 44 [16%], p=0.070) and repeat PCI (128 [14%] vs 50 [18%], p=0.102) in the experimental versus the reference group, respectively3.
SITE-REPORTED vs CEC-ADJUDICATED ENDPOINTS
In the GLOBAL LEADERS study no formal clinical events committee (CEC) procedures were performed for the following reasons. First, the study aimed to be a pragmatic trial to increase generalisability of results and facilitate the adoption of new treatment strategies in clinical practice. Second, limited resources of this investigator-initiated study precluded formal event adjudication in the overall cohort. In addition, the main component of the primary endpoint – all-cause mortality – by definition does not require adjudication and was available in all but eight patients out of 15,991 patients enrolled in the study. The new Q-wave MIs were adjudicated by a dedicated core laboratory using the Minnesota Classification. The study was monitored for event underreporting and event definition consistency, with as many as seven on-site monitoring visits carried out at individual sites; one fifth of events underwent verification against the source documentation.
The role of an independent CEC has been challenged on several occasions with the suggestion that site-reported events may provide sufficient accuracy for reporting5,6,9,10, especially in pharmacological trials. Both the rate of events and therapeutic effects have been shown to vary after adjudication; a meta-analysis of trials with cardiovascular outcomes failed to detect any effect of event adjudication on study conclusions6,9,11,12,13.
Beyond MI, the clinical endpoint of bleeding has been considered one of those outcomes that requires central adjudication in order to avoid potential event misclassification by investigators. Nevertheless, the superiority of such an approach over the investigator-reported events has not been sufficiently documented14. “Objective” adjudication of bleeding may not change the overall trial results, though it is associated with a significant increment in study cost, as demonstrated in the PROTECT study14.
Use of site-reported endpoints is a valid methodology in clinical research, especially involving large cohorts and with highly reliable endpoints (e.g., all-cause mortality). The degree of concordance in detailed or complex endpoints (e.g., universal definition of MI or BARC-defined bleeding categories) between sites and a central CEC is an area of ongoing research, where reproducibility of adjudication has been given increasing attention6,7. Of note, well-defined and restricted categories within a classification (e.g., BARC-defined bleeding type 3 to 5 as compared with types 1 and 2) are expected to provide higher concordance among sites and a central CEC, as well as a higher reproducibility. In addition, the risk of missing a serious adverse event by the CEC due to non-comprehensive triggering or lack of full site monitoring has been emphasised5. Therefore, public reporting of both site-reported and adjudicated data is an approach that might become the best practice to present and interpret trial results6,7.
GLOBAL LEADERS STUDY EVENT ADJUDICATION: OPPORTUNITIES AND RISKS OF THE GLASSY SUBSTUDY
At the present time, it is still unclear whether central adjudication or inclusion of all investigator-reported events in the primary composite outcome might have increased or decreased the power and the sensitivity of the trial. To that end, the GLOBAL LEADERS Adjudication Sub-Study – GLASSY – has been designed (ClinicalTrials.gov Identifier: NCT03231059). The GLASSY study aims to re-evaluate, with the use of standard CEC adjudication procedures, the clinical outcomes in a selection of patients recruited in the highest recruiting centres in the GLOBAL LEADERS study. The study will assess the non-inferiority of the experimental versus reference treatment strategy with respect to CEC-adjudicated death, non-fatal MI, non-fatal stroke or urgent target vessel revascularisation (TVR) in addition to the superiority in preventing the adjudicated BARC 3 or 5 bleeding (as a co-primary safety endpoint) at two years. The scope of the study resembles previous attempts at trial event re-evaluation5. Importantly, the design of the GLASSY study has been published in the public domain prior to completion of the GLOBAL LEADERS trial, and the database was locked prior to the final results being known. Efforts have been made to ensure blinded endpoint analysis by the CEC members. Some inherent limitations of adjudication of the site-reported endpoints in the highest-recruiting centres of the GLOBAL LEADERS cohort potentially reside in the foreknowledge of the main study results, and in the reduction of the total number of patients involved in this major analysis, thereby potentially affecting the statistical power1,15. Furthermore, additional search strategies and enquiries sent to the participating sites may trigger additional endpoint reporting which may also interfere with the final study results and contrast with the originally reported study outcomes. Notwithstanding these risks, the adjudication of secondary endpoints in the GLASSY study will provide valuable data for the clinical community.
ASPIRIN-FREE ANTIPLATELET STRATEGIES IN THE FUTURE
To prove the mechanistically sound concept of aspirin-free strategies, any future randomised clinical trials will have to design novel treatment schemes and specific study endpoints2.
The true two-year all-cause mortality (2.99%) observed in the present trial was markedly lower than the assumed all-cause mortality (4.5%) derived from the LEADERS trial, potentially underpowering the trial3. It appears that additional patient selection and enrichment of the cardiovascular risk in the evaluated population may be needed to detect treatment effects, i.e., with the use of simple prediction tools such as the PARIS or the PRECISE DAPT score16,17.
Sole ticagrelor use after three months of DAPT comprising ticagrelor and aspirin is currently being evaluated in the TWILIGHT study (ClinicalTrials.gov Identifier: NCT02270242). In contrast to the GLOBAL LEADERS study, TWILIGHT has selected bleeding BARC 2, 3 or 5 type as the composite primary endpoint for the superiority analysis and the composite secondary endpoint of ischaemic events (all-cause death, non-fatal MI, or stroke) for the non-inferiority analysis. An even more drastic approach to acetylsalicylic acid elimination from the antiplatelet regimen is being tested in the ASET trial in which, after successful and optimal PCI for stable CAD, patients receive three-month prasugrel monotherapy followed by aspirin monotherapy (or DAPT). This proof-of-concept, feasibility and safety study is not regulated by formal statistics but conducted in accordance with a stopping rule based on the limited occurrence of definite stent thrombosis – the trial will be terminated if more than three patients experience a definite stent thrombosis within four months of follow-up (ClinicalTrials.gov Identifier: NCT03469856).
Limitations
First and foremost, no central independent adjudication of clinical events was implemented in this open-label study and the currently reported composite endpoints solely involved the site-reported events, carrying the risk of event misclassification. The methodology of reported endpoints based on the electronic report form with description and definition of them in the eCRF could have limited this latter bias. Notwithstanding, with meticulous and continuous monitoring of event underreporting and event definition consistency including as many as seven onsite monitoring visits carried out at individual sites and 20% of reported events checked against the source documents, the clinical events reported by investigators represent a source of valuable data for the global cardiology community.
Secondly, the composite clinical endpoints reported in the present study, though advocated by leading academic and regulatory authorities4,18, were not pre-specified in the study protocol as formal secondary endpoints. Nevertheless, every component of these clinically relevant endpoints was pre-specified in the study protocol and meaningful. Thirdly, revascularisation has been included in the composite endpoint to increase the sensitivity to detect hidden stent thrombosis or MI, which could have potentially been reported by investigators as only repeat revascularisation. However, it has to be acknowledged that the revascularisation component could also have introduced some noise which is not directly attributable to the treatment effect of antiplatelet therapy. Fourthly, MI has been reported by investigators in this trial according to the third universal definition, applicable at the time of the study design and conduct. Therefore, it cannot be excluded that MI reported according to the new MI definition could be somewhat different.
Finally, the definitions and methodology for the most accurate assessment of net clinical benefit have still not been standardised, and the optimal approach for the benefit-risk balance measurement in an attempt to conclude which therapy may be pursued and which abandoned still remains to be established.
Conclusions
The experimental treatment consisting of one-month DAPT, aspirin and ticagrelor, followed by 23 months of ticagrelor alone, was not associated with statistically significant differences in terms of POCE – the composite of all-cause mortality, any MI, any stroke, and any revascularisation – and NACE – the composite of POCE and any BARC 3 or 5 type bleeding – at two years, when compared to the reference treatment comprising 12-month DAPT, followed by aspirin monotherapy for 12 months. Adjudication of the trial may bring new insights into the tested antiplatelet regimens, though judicious interpretation of these results would be warranted.
|
Impact on daily practice The novel antiplatelet regimen of 23-month ticagrelor monotherapy following one-month dual antiplatelet therapy (DAPT) after percutaneous coronary intervention (PCI) did not reduce the risk of POCE or NACE when compared to the reference treatment strategy. Although these findings, in line with the primary study results, do not support a change in clinical practice consisting of standard DAPT followed by aspirin monotherapy, further studies evaluating aspirin-free antiplatelet strategies after PCI are warranted. |
Appendix. Authors’ affiliations
1. NHLI, Imperial College London, London, United Kingdom; 2. Erasmus Medical Center, Erasmus University, Rotterdam, the Netherlands; 3. First Department of Cardiology, Medical University of Warsaw, Warsaw, Poland; 4. Amsterdam UMC, University of Amsterdam, Amsterdam, the Netherlands; 5. Division of Cardiology, Department of Internal Medicine, Faculty of Medicine, Prince of Songkla University, Songkhla, Thailand; 6. Department of Internal Medicine, Cardiology Division, University of Campinas (UNICAMP), Campinas, Brazil; 7. Cardialysis Core Laboratories and Clinical Trial Management, Rotterdam, the Netherlands; 8. Royal Victoria Hospital, Belfast, Northern Ireland, United Kingdom; 9. University of Leicester and University Hospitals Leicester, Leicester, United Kingdom; 10. Royal Sussex County Hospital, Brighton, United Kingdom; 11. Department of Cardiology, University of Debrecen, Debrecen, Hungary; 12. University of Groningen, University Medical Centre Groningen (UMCG), Groningen, the Netherlands; 13. Charité – Universitätsmedizin Berlin, Department of Internal Medicine and Cardiology, Campus Virchow-Klinikum, Berlin, Germany; 14. Royal Blackburn Hospital, Blackburn, United Kingdom; 15. FACT (French Alliance for Cardiovascular Trials), Université Paris Diderot, Hôpital Bichat, Assistance Publique - Hôpitaux de Paris, and INSERM U-1148, Paris, France; 16. University of Giessen, Giessen, and Campus Kerckhoff, Bad Nauheim, Germany; 17. Applied Health Research Centre (AHRC), Li Ka Shing Knowledge Institute of St. Michael’s Hospital, Department of Medicine and the Institute of Health Policy, Management and Evaluation at the University of Toronto, Toronto, Canada; 18. Department of Cardiology and Critical Care Medicine, Hartcentrum Hasselt, Jessa Ziekenhuis, Hasselt, Belgium; 19. Department of Cardiology, Onze Lieve Vrouwe Gasthuis (OLVG), Amsterdam, the Netherlands. The list of the GLOBAL LEADERS trial investigators is presented in Supplementary Appendix 4.
Guest Editor
This paper was guest edited by Alec Vahanian, MD, PhD; Department of Cardiology, Hôpital Bichat-Claude Bernard, and University Paris VII, Paris, France.
Funding
GLOBAL LEADERS was sponsored by the European Clinical Research Institute, which received unrestricted grants from Biosensors International, AstraZeneca, and The Medicines Company.
Conflict of interest statement
P.W. Serruys has received personal fees from AstraZeneca, Biotronik, Cardialysis, GLG Research, Medtronic, Sino Medical Sciences Technology, Société Europa Digital & Publishing, Stentys France, Svelte Medical Systems, Philips/Volcano, St. Jude Medical, Qualimed, and Xeltis, outside the submitted work. E. Spitzer reports institutional grants from the European Cardiovascular Research Institute, during the conduct of the study. M. Tomaniak has received lecture fees from AstraZeneca. P. Chichareon has received a research grant from Biosensors. R. Modolo has received research grants from Biosensors. D. Adlam reports grants from the British Heart Foundation, grants from the NIHR Rare Diseases Translational Research Collaboration, grants and non-financial support from NIHR Leicester Biomedical Research Centre, grants from Beat SCAD, grants from Abbott Vascular, and non-financial support from AstraZeneca, during the conduct of the study. J. Tijssen reports personal fees from Cardialysis, for DSMB membership of the GLOBAL LEADERS trial during the conduct of the study. P.G. Steg reports grants and personal fees from Bayer/Janssen, Merck, Sanofi, Amarin, and Servier, personal fees from Amgen, Bristol-Myers Squibb, Boehringer-Ingelheim, Pfizer, Novartis, Regeneron, Lilly, and AstraZeneca, outside the submitted work. C. Hamm reports personal fees from AstraZeneca during the conduct of the study and personal fees from AstraZeneca outside the submitted work. P. Jüni has received research grants to the institution from AstraZeneca, Biotronik, Biosensors International, Eli Lilly and The Medicines Company, outside the submitted work, and is a Tier 1 Canada Research Chair in Clinical Epidemiology of Chronic Diseases (the GLOBAL LEADERS study was completed in part, with funding from the Canada Research Chairs Programme). P. Jüni serves as unpaid member of the steering group of trials funded by AstraZeneca, Biotronik, Biosensors, St. Jude Medical and The Medicines Company. P. Vranckx has received personal fees from AstraZeneca and The Medicines Company during the conduct of the study and personal fees from Bayer HealthCare, Terumo, and Daiichi Sankyo outside the submitted work. Y. Onuma has received consultancy fees from Abbott Vascular outside the submitted work. F. Verheugt reports personal fees from AstraZeneca during the conduct of the study. The other authors have no conflicts of interest to declare. The Guest Editor is a consultant for Edwards Lifesciences.

